Abstract
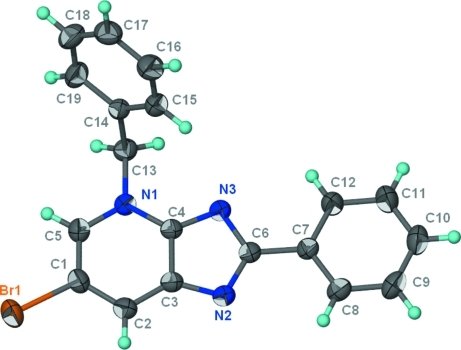
The imidazopyridine fused ring in the title compound, C19H14BrN3, is almost coplanar with the phenyl ring at the 2-position of the five-membered ring [dihedral angle = 2.4 (1). The crystal structure features short Br⋯Br contacts [3.562 (1) Å].
Related literature
For the synthesis of imidazo[4,5-b]pyridines, see: Aridoss et al. (2006 ▶); Benham et al. (1995 ▶); Cundy et al. (1997 ▶); Kale et al. (2009 ▶); Walsh et al. (1994 ▶); Zaki & Proença (2007 ▶).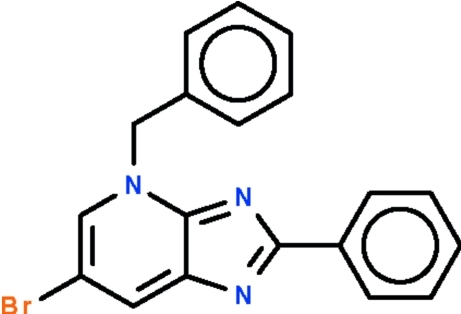
Experimental
Crystal data
C19H14BrN3
M r = 364.24
Monoclinic,
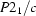
a = 8.6613 (6) Å
b = 19.7631 (13) Å
c = 9.3683 (6) Å
β = 99.647 (3)°
V = 1580.93 (18) Å3
Z = 4
Mo Kα radiation
μ = 2.60 mm−1
T = 293 K
0.28 × 0.24 × 0.20 mm
Data collection
Bruker X8 APEXII diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.529, T max = 0.624
57936 measured reflections
4613 independent reflections
3492 reflections with I > 2σ(I)
R int = 0.035
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.098
S = 1.00
4613 reflections
208 parameters
H-atom parameters constrained
Δρmax = 0.63 e Å−3
Δρmin = −0.51 e Å−3
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053681001038X/pk2232sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681001038X/pk2232Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Université Sidi Mohammed Ben Abdallah, Université Mohammed V-Agdal and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
Imidazo[4,5-b]pyridines are a class of sedative drugs exemplified by Zolpidem, Alpidem, Saripidem and Necopidem. There is intense interest in designing new synthetic routes; for example, an eco-friendly synthesis by oxidation in aqueous medium has been claimed (Kale et al., 2009). Other methods require more than one step (Aridoss et al., 2006; Benham et al., 1995; Cundy et al., 1997; Walsh et al., 1994; Zaki & Proença, 2007).
We have been able to react 6-bromo-2-phenyl-1H-imidazo[4,5-b]pyridine with benzyl chloride in the presence of a catalytic quantity of tetra-n-butylammonium bromide under mild conditions to furnish the title compound (Scheme I, Fig. 1). The imidazopyridine fused-ring in C19H14BrN3 is co-planar with the phenyl ring at the 2-position [dihedral angle 2.4 (1) °]. In the five-membered imidazo portion, the carbon–nitrogen bond whose carbon atom is also connected to the pyridine nitrogen atom is predominantly a double bond [1.329 (2) Å], whereas the carbon–nitrogen bond whose atom is connected to the pyridine carbon atom is predominantly a single bond [1.372 (2) Å].
Experimental
To a solution of the 6-bromo-2-phenyl-1H-imidazo[4,5-b]pyridine (0.30 g, 1.09 mmol), potassium carbonate (0.20 g, 1.42 mmol) and tetra-n-butylammonium bromide (0.04 g (0,1 mmol) in DMF (15 ml) was added benzyl chloride (0.15 ml, 1.31 mmol). Stirring was continued at room temperature for 12 hours. The salt was removed by filtration and the filtrate concentrated under reduced pressure. The residue was separated by chromatography on a column of silica gel with ethyl acetate/hexane (1/1) as eluent. Brown crystals were isolated when the solvent was allowed to evaporate.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.93–0.97 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2U(C).
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of C19H14BrN3 at the 50% probability level. Hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C19H14BrN3 | F(000) = 736 |
| Mr = 364.24 | Dx = 1.530 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 9876 reflections |
| a = 8.6613 (6) Å | θ = 2.4–27.2° |
| b = 19.7631 (13) Å | µ = 2.60 mm−1 |
| c = 9.3683 (6) Å | T = 293 K |
| β = 99.647 (3)° | Prism, brown |
| V = 1580.93 (18) Å3 | 0.28 × 0.24 × 0.20 mm |
| Z = 4 |
Data collection
| Bruker X8 APEXII diffractometer | 4613 independent reflections |
| Radiation source: fine-focus sealed tube | 3492 reflections with I > 2σ(I) |
| graphite | Rint = 0.035 |
| φ and ω scans | θmax = 30.1°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −12→11 |
| Tmin = 0.529, Tmax = 0.624 | k = −27→27 |
| 57936 measured reflections | l = −13→13 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.032 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.098 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0518P)2 + 0.5269P] where P = (Fo2 + 2Fc2)/3 |
| 4613 reflections | (Δ/σ)max = 0.001 |
| 208 parameters | Δρmax = 0.63 e Å−3 |
| 0 restraints | Δρmin = −0.50 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.10158 (3) | 0.475089 (12) | 0.85824 (2) | 0.06483 (10) | |
| N1 | 0.29485 (16) | 0.43405 (7) | 0.49901 (15) | 0.0385 (3) | |
| N2 | 0.26154 (17) | 0.60722 (7) | 0.40338 (16) | 0.0424 (3) | |
| N3 | 0.35446 (17) | 0.50867 (7) | 0.31224 (15) | 0.0387 (3) | |
| C1 | 0.1792 (2) | 0.48667 (9) | 0.68312 (19) | 0.0454 (4) | |
| C2 | 0.1807 (2) | 0.55060 (9) | 0.62007 (19) | 0.0458 (4) | |
| H2 | 0.1429 | 0.5886 | 0.6616 | 0.055* | |
| C3 | 0.2408 (2) | 0.55460 (8) | 0.49376 (18) | 0.0392 (3) | |
| C4 | 0.29944 (19) | 0.49489 (8) | 0.43321 (17) | 0.0367 (3) | |
| C5 | 0.2352 (2) | 0.43003 (9) | 0.62362 (18) | 0.0437 (4) | |
| H5 | 0.2322 | 0.3884 | 0.6694 | 0.052* | |
| C6 | 0.32810 (19) | 0.57716 (8) | 0.30027 (17) | 0.0379 (3) | |
| C7 | 0.37246 (19) | 0.61435 (8) | 0.17772 (18) | 0.0390 (3) | |
| C8 | 0.3423 (2) | 0.68335 (9) | 0.1595 (2) | 0.0459 (4) | |
| H8 | 0.2938 | 0.7067 | 0.2261 | 0.055* | |
| C9 | 0.3845 (2) | 0.71728 (10) | 0.0425 (2) | 0.0547 (5) | |
| H9 | 0.3644 | 0.7634 | 0.0310 | 0.066* | |
| C10 | 0.4557 (3) | 0.68322 (11) | −0.0569 (2) | 0.0574 (5) | |
| H10 | 0.4829 | 0.7062 | −0.1357 | 0.069* | |
| C11 | 0.4869 (3) | 0.61517 (11) | −0.0399 (2) | 0.0624 (5) | |
| H11 | 0.5353 | 0.5922 | −0.1069 | 0.075* | |
| C12 | 0.4459 (3) | 0.58097 (10) | 0.0773 (2) | 0.0538 (5) | |
| H12 | 0.4679 | 0.5350 | 0.0888 | 0.065* | |
| C13 | 0.3544 (2) | 0.37271 (8) | 0.43491 (19) | 0.0427 (3) | |
| H13A | 0.4041 | 0.3433 | 0.5121 | 0.051* | |
| H13B | 0.4331 | 0.3859 | 0.3778 | 0.051* | |
| C14 | 0.22599 (19) | 0.33413 (8) | 0.34017 (17) | 0.0380 (3) | |
| C15 | 0.1392 (2) | 0.36407 (9) | 0.21824 (19) | 0.0481 (4) | |
| H15 | 0.1605 | 0.4084 | 0.1945 | 0.058* | |
| C16 | 0.0214 (3) | 0.32854 (11) | 0.1321 (2) | 0.0574 (5) | |
| H16 | −0.0379 | 0.3494 | 0.0522 | 0.069* | |
| C17 | −0.0082 (3) | 0.26217 (11) | 0.1643 (2) | 0.0585 (5) | |
| H17 | −0.0859 | 0.2380 | 0.1050 | 0.070* | |
| C18 | 0.0769 (3) | 0.23206 (10) | 0.2833 (3) | 0.0598 (5) | |
| H18 | 0.0570 | 0.1873 | 0.3047 | 0.072* | |
| C19 | 0.1930 (2) | 0.26789 (9) | 0.3728 (2) | 0.0517 (4) | |
| H19 | 0.2487 | 0.2473 | 0.4548 | 0.062* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.08695 (19) | 0.06436 (15) | 0.05128 (13) | 0.00593 (10) | 0.03507 (11) | 0.00630 (9) |
| N1 | 0.0419 (7) | 0.0355 (6) | 0.0382 (7) | 0.0001 (5) | 0.0074 (5) | 0.0003 (5) |
| N2 | 0.0515 (8) | 0.0338 (6) | 0.0437 (7) | −0.0007 (6) | 0.0131 (6) | −0.0028 (5) |
| N3 | 0.0443 (7) | 0.0337 (6) | 0.0394 (7) | −0.0009 (5) | 0.0104 (5) | −0.0012 (5) |
| C1 | 0.0501 (10) | 0.0498 (10) | 0.0386 (8) | −0.0024 (7) | 0.0140 (7) | 0.0004 (7) |
| C2 | 0.0535 (10) | 0.0417 (9) | 0.0444 (9) | −0.0007 (7) | 0.0147 (7) | −0.0056 (7) |
| C3 | 0.0431 (8) | 0.0349 (7) | 0.0402 (8) | −0.0015 (6) | 0.0088 (6) | −0.0046 (6) |
| C4 | 0.0390 (8) | 0.0345 (7) | 0.0366 (7) | −0.0020 (6) | 0.0061 (6) | −0.0035 (6) |
| C5 | 0.0485 (9) | 0.0430 (8) | 0.0403 (8) | −0.0019 (7) | 0.0094 (7) | 0.0036 (7) |
| C6 | 0.0407 (8) | 0.0345 (7) | 0.0385 (7) | −0.0028 (6) | 0.0068 (6) | −0.0023 (6) |
| C7 | 0.0420 (8) | 0.0353 (7) | 0.0395 (7) | −0.0036 (6) | 0.0064 (6) | −0.0004 (6) |
| C8 | 0.0471 (9) | 0.0360 (8) | 0.0553 (10) | −0.0020 (7) | 0.0105 (8) | −0.0007 (7) |
| C9 | 0.0567 (11) | 0.0391 (9) | 0.0678 (12) | −0.0031 (8) | 0.0092 (9) | 0.0118 (8) |
| C10 | 0.0647 (12) | 0.0560 (11) | 0.0530 (10) | −0.0070 (9) | 0.0141 (9) | 0.0153 (9) |
| C11 | 0.0851 (15) | 0.0567 (12) | 0.0516 (10) | 0.0037 (11) | 0.0298 (10) | 0.0062 (9) |
| C12 | 0.0782 (13) | 0.0393 (9) | 0.0486 (10) | 0.0062 (9) | 0.0240 (9) | 0.0040 (7) |
| C13 | 0.0430 (9) | 0.0371 (8) | 0.0483 (9) | 0.0064 (7) | 0.0091 (7) | 0.0015 (7) |
| C14 | 0.0419 (8) | 0.0331 (7) | 0.0416 (8) | 0.0029 (6) | 0.0147 (6) | −0.0002 (6) |
| C15 | 0.0608 (11) | 0.0390 (8) | 0.0446 (9) | −0.0013 (8) | 0.0092 (8) | 0.0021 (7) |
| C16 | 0.0660 (12) | 0.0574 (11) | 0.0462 (10) | −0.0037 (9) | 0.0015 (9) | −0.0030 (8) |
| C17 | 0.0603 (12) | 0.0584 (12) | 0.0587 (11) | −0.0140 (9) | 0.0152 (9) | −0.0156 (9) |
| C18 | 0.0683 (13) | 0.0396 (9) | 0.0754 (14) | −0.0103 (9) | 0.0233 (11) | −0.0022 (9) |
| C19 | 0.0583 (11) | 0.0388 (9) | 0.0593 (11) | 0.0013 (8) | 0.0139 (9) | 0.0088 (8) |
Geometric parameters (Å, °)
| Br1—C1 | 1.8882 (18) | C9—H9 | 0.9300 |
| N1—C4 | 1.355 (2) | C10—C11 | 1.376 (3) |
| N1—C5 | 1.356 (2) | C10—H10 | 0.9300 |
| N1—C13 | 1.483 (2) | C11—C12 | 1.385 (3) |
| N2—C6 | 1.344 (2) | C11—H11 | 0.9300 |
| N2—C3 | 1.372 (2) | C12—H12 | 0.9300 |
| N3—C4 | 1.329 (2) | C13—C14 | 1.508 (2) |
| N3—C6 | 1.374 (2) | C13—H13A | 0.9700 |
| C1—C5 | 1.375 (3) | C13—H13B | 0.9700 |
| C1—C2 | 1.396 (3) | C14—C19 | 1.385 (2) |
| C2—C3 | 1.373 (2) | C14—C15 | 1.390 (2) |
| C2—H2 | 0.9300 | C15—C16 | 1.382 (3) |
| C3—C4 | 1.438 (2) | C15—H15 | 0.9300 |
| C5—H5 | 0.9300 | C16—C17 | 1.379 (3) |
| C6—C7 | 1.468 (2) | C16—H16 | 0.9300 |
| C7—C12 | 1.388 (3) | C17—C18 | 1.366 (3) |
| C7—C8 | 1.394 (2) | C17—H17 | 0.9300 |
| C8—C9 | 1.385 (3) | C18—C19 | 1.390 (3) |
| C8—H8 | 0.9300 | C18—H18 | 0.9300 |
| C9—C10 | 1.376 (3) | C19—H19 | 0.9300 |
| C4—N1—C5 | 119.22 (14) | C11—C10—H10 | 120.0 |
| C4—N1—C13 | 120.17 (14) | C9—C10—H10 | 120.0 |
| C5—N1—C13 | 120.61 (14) | C10—C11—C12 | 119.8 (2) |
| C6—N2—C3 | 102.99 (13) | C10—C11—H11 | 120.1 |
| C4—N3—C6 | 101.13 (13) | C12—C11—H11 | 120.1 |
| C5—C1—C2 | 122.44 (16) | C11—C12—C7 | 120.86 (18) |
| C5—C1—Br1 | 117.06 (13) | C11—C12—H12 | 119.6 |
| C2—C1—Br1 | 120.49 (14) | C7—C12—H12 | 119.6 |
| C3—C2—C1 | 116.66 (16) | N1—C13—C14 | 112.30 (13) |
| C3—C2—H2 | 121.7 | N1—C13—H13A | 109.1 |
| C1—C2—H2 | 121.7 | C14—C13—H13A | 109.1 |
| N2—C3—C2 | 133.11 (16) | N1—C13—H13B | 109.1 |
| N2—C3—C4 | 106.70 (14) | C14—C13—H13B | 109.1 |
| C2—C3—C4 | 120.18 (16) | H13A—C13—H13B | 107.9 |
| N3—C4—N1 | 127.72 (15) | C19—C14—C15 | 118.70 (17) |
| N3—C4—C3 | 111.64 (14) | C19—C14—C13 | 120.47 (16) |
| N1—C4—C3 | 120.64 (15) | C15—C14—C13 | 120.82 (15) |
| N1—C5—C1 | 120.85 (16) | C16—C15—C14 | 120.56 (17) |
| N1—C5—H5 | 119.6 | C16—C15—H15 | 119.7 |
| C1—C5—H5 | 119.6 | C14—C15—H15 | 119.7 |
| N2—C6—N3 | 117.54 (14) | C17—C16—C15 | 120.1 (2) |
| N2—C6—C7 | 122.76 (14) | C17—C16—H16 | 119.9 |
| N3—C6—C7 | 119.70 (14) | C15—C16—H16 | 119.9 |
| C12—C7—C8 | 118.69 (17) | C18—C17—C16 | 119.88 (19) |
| C12—C7—C6 | 120.15 (15) | C18—C17—H17 | 120.1 |
| C8—C7—C6 | 121.17 (16) | C16—C17—H17 | 120.1 |
| C9—C8—C7 | 120.11 (18) | C17—C18—C19 | 120.47 (18) |
| C9—C8—H8 | 119.9 | C17—C18—H18 | 119.8 |
| C7—C8—H8 | 119.9 | C19—C18—H18 | 119.8 |
| C10—C9—C8 | 120.42 (18) | C14—C19—C18 | 120.25 (18) |
| C10—C9—H9 | 119.8 | C14—C19—H19 | 119.9 |
| C8—C9—H9 | 119.8 | C18—C19—H19 | 119.9 |
| C11—C10—C9 | 120.08 (18) | ||
| C5—C1—C2—C3 | 0.3 (3) | N2—C6—C7—C12 | 177.72 (18) |
| Br1—C1—C2—C3 | 179.25 (13) | N3—C6—C7—C12 | −2.4 (2) |
| C6—N2—C3—C2 | 179.20 (19) | N2—C6—C7—C8 | −2.2 (3) |
| C6—N2—C3—C4 | −0.16 (17) | N3—C6—C7—C8 | 177.69 (16) |
| C1—C2—C3—N2 | −179.77 (18) | C12—C7—C8—C9 | 0.4 (3) |
| C1—C2—C3—C4 | −0.5 (3) | C6—C7—C8—C9 | −179.61 (16) |
| C6—N3—C4—N1 | −179.97 (16) | C7—C8—C9—C10 | 0.2 (3) |
| C6—N3—C4—C3 | −0.15 (18) | C8—C9—C10—C11 | −0.6 (3) |
| C5—N1—C4—N3 | 179.35 (16) | C9—C10—C11—C12 | 0.2 (4) |
| C13—N1—C4—N3 | −0.7 (3) | C10—C11—C12—C7 | 0.5 (4) |
| C5—N1—C4—C3 | −0.5 (2) | C8—C7—C12—C11 | −0.8 (3) |
| C13—N1—C4—C3 | 179.47 (15) | C6—C7—C12—C11 | 179.25 (19) |
| N2—C3—C4—N3 | 0.21 (19) | C4—N1—C13—C14 | −94.67 (18) |
| C2—C3—C4—N3 | −179.25 (16) | C5—N1—C13—C14 | 85.26 (19) |
| N2—C3—C4—N1 | −179.95 (15) | N1—C13—C14—C19 | −119.47 (17) |
| C2—C3—C4—N1 | 0.6 (2) | N1—C13—C14—C15 | 60.9 (2) |
| C4—N1—C5—C1 | 0.3 (2) | C19—C14—C15—C16 | 0.5 (3) |
| C13—N1—C5—C1 | −179.68 (16) | C13—C14—C15—C16 | −179.83 (17) |
| C2—C1—C5—N1 | −0.2 (3) | C14—C15—C16—C17 | −1.8 (3) |
| Br1—C1—C5—N1 | −179.17 (13) | C15—C16—C17—C18 | 1.4 (3) |
| C3—N2—C6—N3 | 0.1 (2) | C16—C17—C18—C19 | 0.2 (3) |
| C3—N2—C6—C7 | 180.00 (15) | C15—C14—C19—C18 | 1.1 (3) |
| C4—N3—C6—N2 | 0.05 (19) | C13—C14—C19—C18 | −178.56 (17) |
| C4—N3—C6—C7 | −179.88 (14) | C17—C18—C19—C14 | −1.5 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: PK2232).
References
- Aridoss, G., Balasubramanian, S., Parthiban, P. & Kabilan, S. (2006). Eur. J. Med. Chem. 41, 268–275. [DOI] [PubMed]
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Benham, C. D., Blackburn, T. P., Johns, A., Kotecha, N. R., Martin, R. T., Thomas, D. R., Thompson, M. & Ward, R. W. (1995). Bioorg. Med. Chem. Lett.5, 2455–2460.
- Bruker (2008). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cundy, D. J., Holan, G., Otaegui, M. & Simpson, G. W. (1997). Bioorg. Med. Chem. Lett.7, 669–674.
- Kale, R. P., Shaikh, M. U., Jadhav, G. R. & Gill, C. H. (2009). Tetrahedron Lett.50, 1780–1782.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Walsh, T. F., Fitch, K. J., MacCoss, M., Chang, R. S. L., Kivlighn, S. D., Lotti, V. J., Siegl, P. K. S., Patchett, A. & Greenlee, W. J. (1994). Bioorg. Med. Chem. Lett. 4, 219–222.
- Westrip, S. P. (2010). publCIF In preparation.
- Zaki, M. E. A. & Proença, M. F. (2007). Tetrahedron, 63, 3745–3753.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053681001038X/pk2232sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681001038X/pk2232Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report