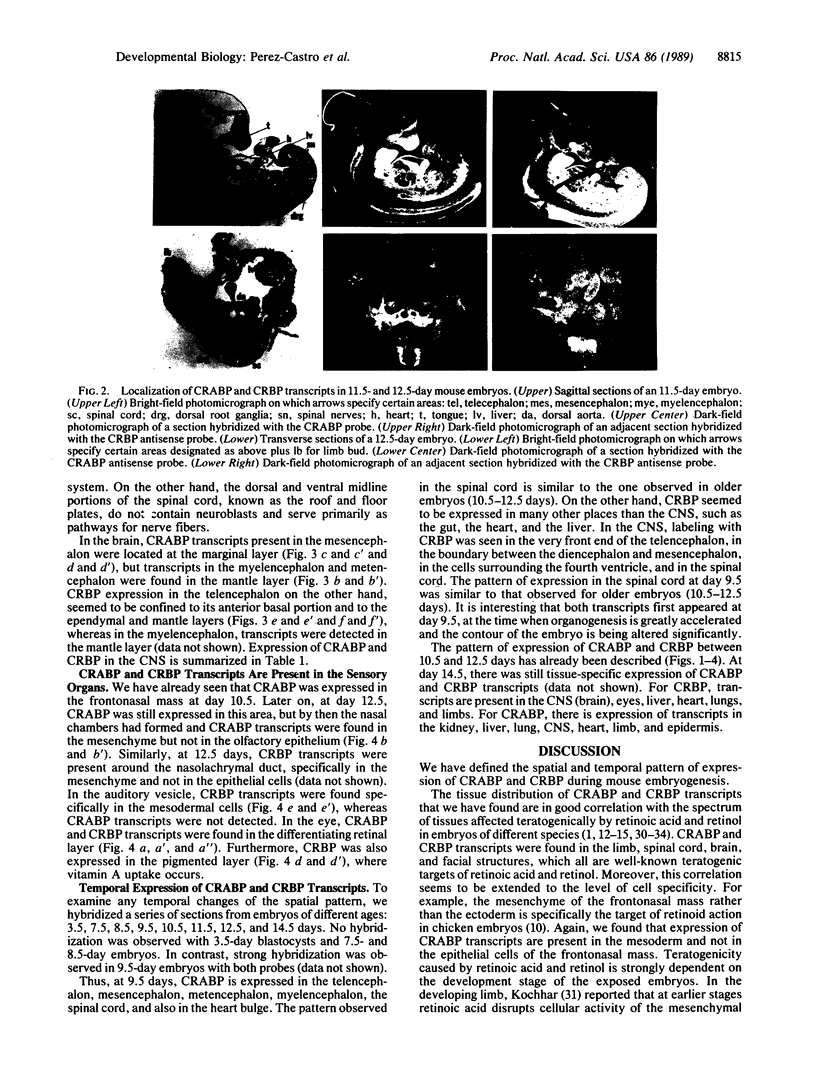
Abstract
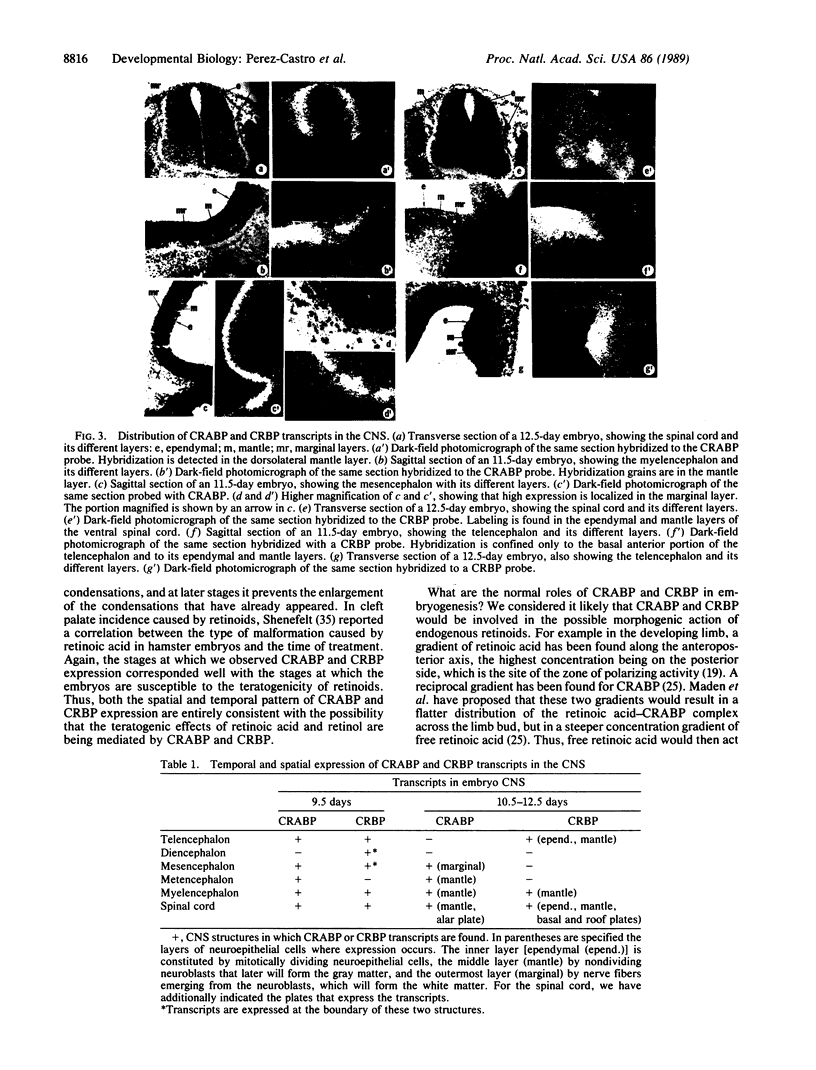
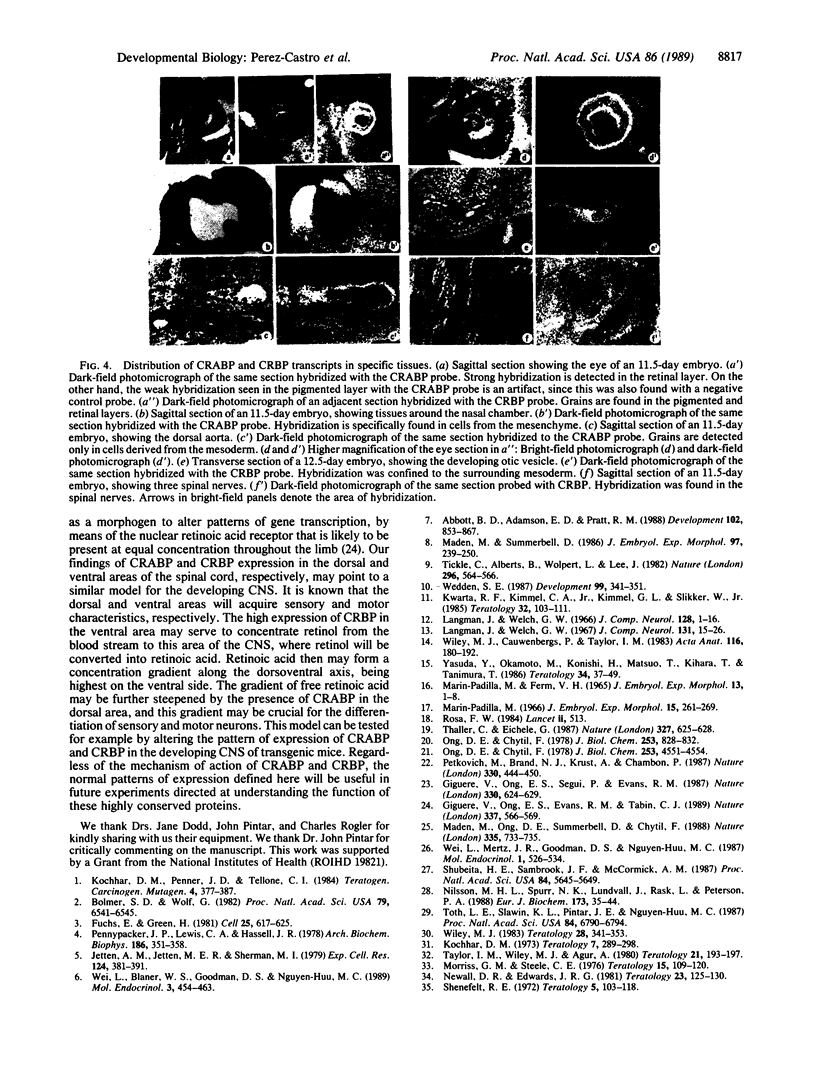
Retinol (vitamin A) and retinoic acid are potent teratogens and also represent good candidates for normal morphogens during development. Their actions may be mediated by the cellular retinoic acid-binding protein (CRABP) and the cellular retinol-binding protein (CRBP). As a step towards understanding the possible function for CRABP and CRBP in morphogenesis, we have used in situ hybridization to analyze their expression during mouse development. Both CRABP and CRBP transcripts were detected at embryonic days 9.5-14.5. (i) In the nervous system, CRABP transcripts were found in the mantle layer of the dorsal spinal cord and hindbrain and in the marginal layer of the midbrain, whereas CRBP transcripts were found in the ependymal and mantle layer of the ventral spinal cord and of the forebrain as well as in the spinal nerves and the roof plate of the spinal cord. (ii) In the eye, CRABP is expressed in the retinal layer, and CRBP is expressed in both retinal and pigmented layers. (iii) In the craniofacial region, CRABP transcripts were found in the mesenchyme of the frontonasal mass and mandible, while CRBP transcripts were found in the mesenchyme of the nasolachrymal duct and surrounding the auditory vesicle. Two general conclusions can be made. First, all of the tissues that are known to be teratogenic targets of retinoic acid and retinol also express CRABP and CRBP transcripts. Second, the specific expression of CRABP and CRBP in numerous developing tissues indicates that these proteins may perform specific functions during morphogenesis of a broad variety of embryonic structures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. D., Adamson E. D., Pratt R. M. Retinoic acid alters EGF receptor expression during palatogenesis. Development. 1988 Apr;102(4):853–867. doi: 10.1242/dev.102.4.853. [DOI] [PubMed] [Google Scholar]
- Bolmer S. D., Wolf G. Retinoids and phorbol esters alter release of fibronectin from enucleated cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6541–6545. doi: 10.1073/pnas.79.21.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Giguère V., Ong E. S., Evans R. M., Tabin C. J. Spatial and temporal expression of the retinoic acid receptor in the regenerating amphibian limb. Nature. 1989 Feb 9;337(6207):566–569. doi: 10.1038/337566a0. [DOI] [PubMed] [Google Scholar]
- Harinck H. I., Bijvoet O. L., van der Meer J. W., Jones B., Onvlee G. J. Regression of bone lesions in Gaucher's disease during treatment with aminohydroxypropylidene bisphosphonate. Lancet. 1984 Sep 1;2(8401):513–513. doi: 10.1016/s0140-6736(84)92579-0. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Sherman M. I. Stimulation of differentiation of several murine embryonal carcinoma cell lines by retinoic acid. Exp Cell Res. 1979 Dec;124(2):381–391. doi: 10.1016/0014-4827(79)90213-1. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M. Limb development in mouse embryos. I. Analysis of teratogenic effects of retinoic acid. Teratology. 1973 Jun;7(3):289–298. doi: 10.1002/tera.1420070310. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M., Penner J. D., Tellone C. I. Comparative teratogenic activities of two retinoids: effects on palate and limb development. Teratog Carcinog Mutagen. 1984;4(4):377–387. doi: 10.1002/tcm.1770040407. [DOI] [PubMed] [Google Scholar]
- Kwarta R. F., Jr, Kimmel C. A., Kimmel G. L., Slikker W., Jr Identification of the cellular retinoic acid binding protein (cRABP) within the embryonic mouse (CD-1) limb bud. Teratology. 1985 Aug;32(1):103–111. doi: 10.1002/tera.1420320114. [DOI] [PubMed] [Google Scholar]
- Langman J., Welch G. W. Effect of vitamin a on development of the central nervous system. J Comp Neurol. 1966 Sep;128(1):1–16. doi: 10.1002/cne.901280102. [DOI] [PubMed] [Google Scholar]
- Langman J., Welch G. W. Excess vitamin A and development of the cerebral cortex. J Comp Neurol. 1967 Sep;131(1):15–26. doi: 10.1002/cne.901310103. [DOI] [PubMed] [Google Scholar]
- MARIN-PADILLA M., FERM V. H. SOMITE NECROSIS AND DEVELOPMENTAL MALFORMATIONS INDUCED BY VITAMIN A IN THE GOLDEN HAMSTER. J Embryol Exp Morphol. 1965 Feb;13:1–8. [PubMed] [Google Scholar]
- Maden M., Ong D. E., Summerbell D., Chytil F. Spatial distribution of cellular protein binding to retinoic acid in the chick limb bud. Nature. 1988 Oct 20;335(6192):733–735. doi: 10.1038/335733a0. [DOI] [PubMed] [Google Scholar]
- Maden M., Summerbell D. Retinoic acid-binding protein in the chick limb bud: identification at developmental stages and binding affinities of various retinoids. J Embryol Exp Morphol. 1986 Sep;97:239–250. [PubMed] [Google Scholar]
- Marin-Padilla M. Mesodermal alterations induced by hypervitaminosis A. J Embryol Exp Morphol. 1966 Jun;15(3):261–269. [PubMed] [Google Scholar]
- Morriss G. M., Steele C. E. Comparison of the effects of retinol and retinoic acid on postimplantation rat embryos in vitro. Teratology. 1977 Feb;15(1):109–119. doi: 10.1002/tera.1420150115. [DOI] [PubMed] [Google Scholar]
- Newall D. R., Edwards J. R. The effect of vitamin A on fusion of mouse palates. II. retinyl palmitate, retinol, and retinoic acid in vitro. Teratology. 1981 Feb;23(1):125–130. doi: 10.1002/tera.1420230115. [DOI] [PubMed] [Google Scholar]
- Nilsson M. H., Spurr N. K., Lundvall J., Rask L., Peterson P. A. Human cellular retinol-binding protein gene organization and chromosomal location. Eur J Biochem. 1988 Apr 5;173(1):35–44. doi: 10.1111/j.1432-1033.1988.tb13963.x. [DOI] [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Cellular retinoic acid-binding protein from rat testis. Purification and characterization. J Biol Chem. 1978 Jul 10;253(13):4551–4554. [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Cellular retinol-binding protein from rat liver. Purification and characterization. J Biol Chem. 1978 Feb 10;253(3):828–832. [PubMed] [Google Scholar]
- Pennypacker J. P., Lewis C. A., Hassell J. R. Altered proteoglycan metabolism in mouse limb mesenchyme cell cultures treated with vitamin A. Arch Biochem Biophys. 1978 Mar;186(2):351–358. doi: 10.1016/0003-9861(78)90445-9. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Shenefelt R. E. Morphogenesis of malformations in hamsters caused by retinoic acid: relation to dose and stage at treatment. Teratology. 1972 Feb;5(1):103–118. doi: 10.1002/tera.1420050115. [DOI] [PubMed] [Google Scholar]
- Shubeita H. E., Sambrook J. F., McCormick A. M. Molecular cloning and analysis of functional cDNA and genomic clones encoding bovine cellular retinoic acid-binding protein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5645–5649. doi: 10.1073/pnas.84.16.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. M., Wiley M. J., Agur A. Retinoic acid-induced heart malformations in the hamster. Teratology. 1980 Apr;21(2):193–197. doi: 10.1002/tera.1420210210. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Tickle C., Alberts B., Wolpert L., Lee J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 1982 Apr 8;296(5857):564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Toth L. E., Slawin K. L., Pintar J. E., Nguyen-Huu M. C. Region-specific expression of mouse homeobox genes in the embryonic mesoderm and central nervous system. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6790–6794. doi: 10.1073/pnas.84.19.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedden S. E. Epithelial-mesenchymal interactions in the development of chick facial primordia and the target of retinoid action. Development. 1987 Mar;99(3):341–351. doi: 10.1242/dev.99.3.341. [DOI] [PubMed] [Google Scholar]
- Wei L. N., Blaner W. S., Goodman D. S., Nguyen-Huu M. C. Regulation of the cellular retinoid-binding proteins and their messenger ribonucleic acids during P19 embryonal carcinoma cell differentiation induced by retinoic acid. Mol Endocrinol. 1989 Mar;3(3):454–463. doi: 10.1210/mend-3-3-454. [DOI] [PubMed] [Google Scholar]
- Wei L. N., Mertz J. R., Goodman D. S., Nguyen-Huu M. C. Cellular retinoic acid- and cellular retinol-binding proteins: complementary deoxyribonucleic acid cloning, chromosomal assignment, and tissue specific expression. Mol Endocrinol. 1987 Aug;1(8):526–534. doi: 10.1210/mend-1-8-526. [DOI] [PubMed] [Google Scholar]
- Wiley M. J., Cauwenbergs P., Taylor I. M. Effects of retinoic acid on the development of the facial skeleton in hamsters: early changes involving cranial neural crest cells. Acta Anat (Basel) 1983;116(2):180–192. doi: 10.1159/000145741. [DOI] [PubMed] [Google Scholar]
- Wiley M. J. The pathogenesis of retinoic acid-induced vertebral abnormalities in golden Syrian hamster fetuses. Teratology. 1983 Dec;28(3):341–353. doi: 10.1002/tera.1420280306. [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Okamoto M., Konishi H., Matsuo T., Kihara T., Tanimura T. Developmental anomalies induced by all-trans retinoic acid in fetal mice: I. Macroscopic findings. Teratology. 1986 Aug;34(1):37–49. doi: 10.1002/tera.1420340106. [DOI] [PubMed] [Google Scholar]