Abstract
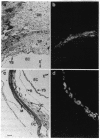
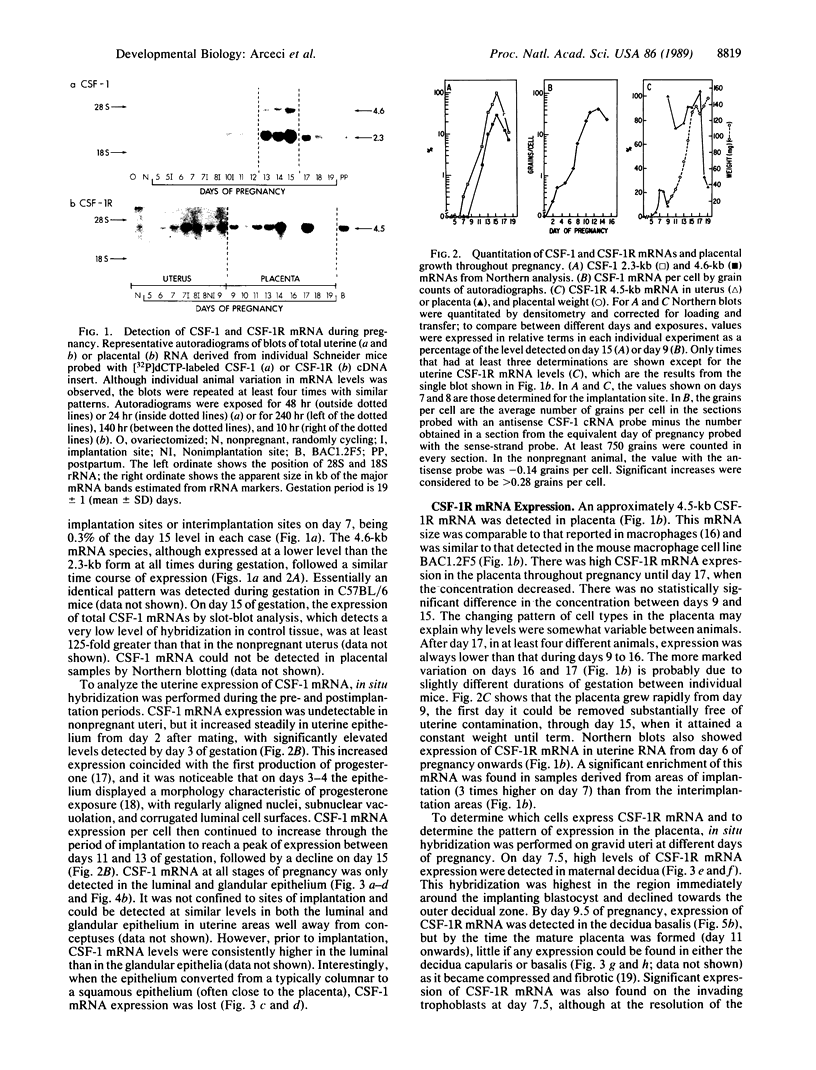
During pregnancy the mouse uterine epithelial synthesis of the mononuclear phagocyte growth factor designated colony-stimulating factor 1 (CSF-1) is regulated by female sex steroids. To study the role of CSF-1 in the pregnant female reproductive tract, the temporal expression and cellular sites of synthesis of CSF-1 and CSF-1 receptor (CSF-1R) mRNA were determined. CSF-1 mRNA, predominantly the 2.3-kilobase (kb) form, was first detected by in situ hybridization in uterine epithelium prior to implantation on day 3 and subsequently increased, reaching a peak at days 14-15. Its expression was restricted to the uterine epithelium at all stages of gestation and was not localized to areas of implantation. CSF-1R mRNA was first detected in maternal decidua at day 6. It was expressed in the decidua basalis during placentation, after which its expression declined. At day 7.5, trophectodermal cells also expressed CSF-1R mRNA; during placentation, it was found also in the diploid trophoblasts. The high level of CSF-1R mRNA expression by trophoblast giant cells was independent of their location around the conceptus. There was a differential distribution of CSF-1R mRNA expression in the mature placenta, with expression in the giant trophoblastic layer greater than spongiotrophoblastic layer greater than labyrinthine layer until term. Yolk sac cells also expressed low levels of CSF-1R mRNA. The coincidence of uterine CSF-1 mRNA expression and CSF-1 synthesis with both placental growth and CSF-1R mRNA expression in decidual cells and trophoblasts strongly implicates CSF-1 in the regulation of placental growth and differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., Croop J. M., Horwitz S. B., Housman D. The gene encoding multidrug resistance is induced and expressed at high levels during pregnancy in the secretory epithelium of the uterus. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4350–4354. doi: 10.1073/pnas.85.12.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanassakis I., Bleackley R. C., Paetkau V., Guilbert L., Barr P. J., Wegmann T. G. The immunostimulatory effect of T cells and T cell lymphokines on murine fetally derived placental cells. J Immunol. 1987 Jan 1;138(1):37–44. [PubMed] [Google Scholar]
- Azoulay M., Webb C. G., Sachs L. Control of hematopoietic cell growth regulators during mouse fetal development. Mol Cell Biol. 1987 Sep;7(9):3361–3364. doi: 10.1128/mcb.7.9.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P. W., Sherman M. I. The biochemistry of differentiation of mouse trophoblast: studies on polyploidy. J Embryol Exp Morphol. 1972 Apr;27(2):447–465. [PubMed] [Google Scholar]
- Bartocci A., Mastrogiannis D. S., Migliorati G., Stockert R. J., Wolkoff A. W., Stanley E. R. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6179–6183. doi: 10.1073/pnas.84.17.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci A., Pollard J. W., Stanley E. R. Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med. 1986 Sep 1;164(3):956–961. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock D. R., Heap R. B., Brown K. D. Polypeptide growth factors in uterine tissues and secretions. J Reprod Fertil. 1989 Mar;85(2):747–758. doi: 10.1530/jrf.0.0850747. [DOI] [PubMed] [Google Scholar]
- Das R. M., Martin L. Uterine DNA synthesis and cell proliferation during early decidualization induced by oil in mice. J Reprod Fertil. 1978 May;53(1):125–128. doi: 10.1530/jrf.0.0530125. [DOI] [PubMed] [Google Scholar]
- Davies J., Glasser S. R. Histological and fine structural observations on the placenta of the rat. Acta Anat (Basel) 1968;69(4):542–608. doi: 10.1159/000143100. [DOI] [PubMed] [Google Scholar]
- Finn C. A., Martin L. The onset of progesterone secretion during pregnancy in the mouse. J Reprod Fertil. 1971 May;25(2):299–300. doi: 10.1530/jrf.0.0250299. [DOI] [PubMed] [Google Scholar]
- Hoshina M., Nishio A., Bo M., Boime I., Mochizuki M. [The expression of oncogene fms in human chorionic tissue]. Nihon Sanka Fujinka Gakkai Zasshi. 1985 Dec;37(12):2791–2798. [PubMed] [Google Scholar]
- Hunt J. S., Manning L. S., Mitchell D., Selanders J. R., Wood G. W. Localization and characterization of macrophages in murine uterus. J Leukoc Biol. 1985 Aug;38(2):255–265. doi: 10.1002/jlb.38.2.255. [DOI] [PubMed] [Google Scholar]
- Hunt J. S., Manning L. S., Wood G. W. Macrophages in murine uterus are immunosuppressive. Cell Immunol. 1984 May;85(2):499–510. doi: 10.1016/0008-8749(84)90262-4. [DOI] [PubMed] [Google Scholar]
- Johnson G. R., Burgess A. W. Molecular and biological properties of a macrophage colony-stimulating factor from mouse yolk sacs. J Cell Biol. 1978 Apr;77(1):35–47. doi: 10.1083/jcb.77.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner M. B., Martin G. A., Noble J. A., Wittman V. P., Warren M. K., McGrogan M., Stanley E. R. cDNA cloning and expression of murine macrophage colony-stimulating factor from L929 cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6706–6710. doi: 10.1073/pnas.85.18.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L., Finn C. A., Trinder G. DNA synthesis in the endometrium of progesterone-treated mice. J Endocrinol. 1973 Feb;56(2):303–307. doi: 10.1677/joe.0.0560303. [DOI] [PubMed] [Google Scholar]
- Mercola M., Stiles C. D. Growth factor superfamilies and mammalian embryogenesis. Development. 1988 Mar;102(3):451–460. doi: 10.1242/dev.102.3.451. [DOI] [PubMed] [Google Scholar]
- Morgan C., Pollard J. W., Stanley E. R. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC1.2F5. J Cell Physiol. 1987 Mar;130(3):420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Adamson E. D., Tremblay J. M., Müller D., Cline M. J., Verma I. M. Transcription of c-onc genes c-rasKi and c-fms during mouse development. Mol Cell Biol. 1983 Jun;3(6):1062–1069. doi: 10.1128/mcb.3.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Verma I. M., Adamson E. D. Expression of c-onc genes: c-fos transcripts accumulate to high levels during development of mouse placenta, yolk sac and amnion. EMBO J. 1983;2(5):679–684. doi: 10.1002/j.1460-2075.1983.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. W., Bartocci A., Arceci R., Orlofsky A., Ladner M. B., Stanley E. R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987 Dec 3;330(6147):484–486. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- Regenstreif L. J., Rossant J. Expression of the c-fms proto-oncogene and of the cytokine, CSF-1, during mouse embryogenesis. Dev Biol. 1989 May;133(1):284–294. doi: 10.1016/0012-1606(89)90319-9. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Roussel M. F., Ashmun R. A., Ralph P., Price K., Sherr C. J. Synthesis of membrane-bound colony-stimulating factor 1 (CSF-1) and downmodulation of CSF-1 receptors in NIH 3T3 cells transformed by cotransfection of the human CSF-1 and c-fms (CSF-1 receptor) genes. Mol Cell Biol. 1987 Jul;7(7):2378–2387. doi: 10.1128/mcb.7.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell V. M., Rohrschneider L. R. Murine c-fms cDNA: cloning, sequence analysis and retroviral expression. Oncogene Res. 1987 Sep-Oct;1(4):311–324. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Griffin J. D., Rambaldi A., Chen Z. G., Mantovani A. Induction of monocyte migration by recombinant macrophage colony-stimulating factor. J Immunol. 1988 Jul 15;141(2):575–579. [PubMed] [Google Scholar]
- Wegmann T. G. Maternal T cells promote placental growth and prevent spontaneous abortion. Immunol Lett. 1988 Apr;17(4):297–302. doi: 10.1016/0165-2478(88)90001-6. [DOI] [PubMed] [Google Scholar]