Abstract
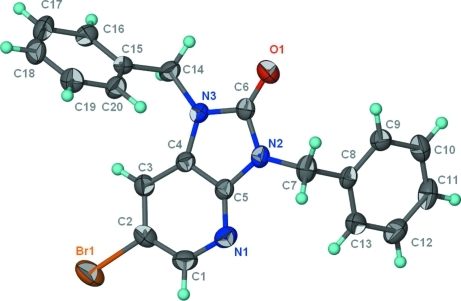
The imidazopyridine fused-ring in the title compound, C20H16BrN3O, is planar (r.m.s. deviation = 0.011 Å). The phenyl rings of the benzyl substitutents twist away from the central five-membered ring in opposite directions; the rings are aligned at 61.3 (1) and 71.2 (1)° with respect to this ring.
Related literature
For the medicinal applications of 1,3-dihydro-imidazo[4,5-b]pyridin-2-ones, see: Barraclough et al. (1990 ▶); Cundy et al. (1997 ▶); Desarro et al. (1994 ▶); Liu et al. (2008 ▶); Mader et al. (2008 ▶); Zaki & Proença (2007 ▶). For the product of the reaction of propargyl bromide with 6-bromo-1,3-dihydro-imidazo[4,5-b]pyridin-2-one in DMF at room and high temperatures, see: Dahmani et al. (2010a
▶,b
▶).
Experimental
Crystal data
C20H16BrN3O
M r = 394.27
Monoclinic,
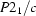
a = 9.1627 (1) Å
b = 25.5071 (3) Å
c = 8.0629 (1) Å
β = 115.571 (1)°
V = 1699.84 (3) Å3
Z = 4
Mo Kα radiation
μ = 2.43 mm−1
T = 293 K
0.42 × 0.18 × 0.13 mm
Data collection
Bruker X8 APEX2 diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.428, T max = 0.743
38010 measured reflections
3903 independent reflections
2967 reflections with I > 2σ(I)
R int = 0.036
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.138
S = 1.07
3903 reflections
226 parameters
H-atom parameters constrained
Δρmax = 0.84 e Å−3
Δρmin = −0.90 e Å−3
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810007713/hg2651sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810007713/hg2651Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Université Sidi Mohammed Ben Abdallah, Université Mohammed V-Agdal and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
Imidazo[4,5-b]pyridines are precursors for the synthesis of a variety of medicinal agents as compounds having the imidazo[4,5-b]pyridine fused-ring system possess a broad range of pharmacological activities (Barraclough et al., 1990; Cundy et al., 1997; Desarro et al., 1994; Liu et al., 2008; Mader et al., 2008; Zaki & Proença, 2007).
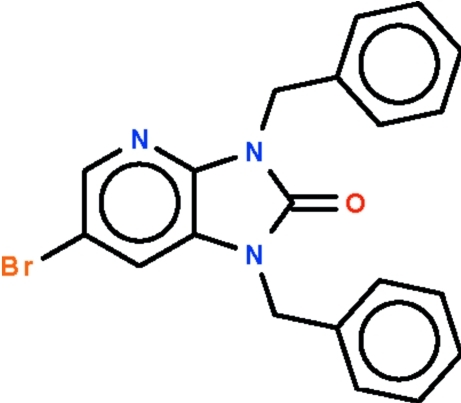
The present study represents the synthesis of the substituted an imidazo[4,5-b]pyridin-2-one derivative by the direct action of benzyl chloride on 6-bromo-1,3-dihydro-imidazo[4,5-b]pyridin-2-one in boiling DMF.
The imidazopyridine fused-ring in C20H16BrN3O (Scheme I, Fig. 1) is planar (r.m.s. deviation 0.011 Å). The phenyl rings of the benzyl substitutents twist away from the central five-membered ring in opposite directions; the rings are aligned at 61.3 (1) and 71.2 (1) ° with respect to this ring.
The temperature of the reaction, in the case of propargyl bromide, governs the nature of the product (Dahmani et al., 2010a, 2010b).
Experimental
To a mixture of 6-bromo-1,3-dihydro-imidazo[4,5-b]pyridin-2-one (1 mmol), potassium carbonate (4 mmol) and benzyltributylammonium chloride (0.1 mmol) in DMF was added benzyl chloride (2.5 mmol). The mixture was stirred for 48 hours. After completion of reaction (monitored by TLC), the inorganic salt was filtered and the solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel with ethyl acetate/hexane (1/1) as eluent. Colorless crystals were isolated when the solvent was allowed to evaporate.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.93-0.97 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2U(C).
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of C20H16BrN3O at the 50% probability level; hydrogen atoms are drawn as arbitrary radius.
Crystal data
| C20H16BrN3O | F(000) = 800 |
| Mr = 394.27 | Dx = 1.541 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 9890 reflections |
| a = 9.1627 (1) Å | θ = 2.5–25.0° |
| b = 25.5071 (3) Å | µ = 2.43 mm−1 |
| c = 8.0629 (1) Å | T = 293 K |
| β = 115.571 (1)° | Prism, colorless |
| V = 1699.84 (3) Å3 | 0.42 × 0.18 × 0.13 mm |
| Z = 4 |
Data collection
| Bruker X8 APEX2 diffractometer | 3903 independent reflections |
| Radiation source: fine-focus sealed tube | 2967 reflections with I > 2σ(I) |
| graphite | Rint = 0.036 |
| φ and ω scans | θmax = 27.5°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −11→11 |
| Tmin = 0.428, Tmax = 0.743 | k = −33→33 |
| 38010 measured reflections | l = −9→10 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.041 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.138 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0688P)2 + 1.1691P] where P = (Fo2 + 2Fc2)/3 |
| 3903 reflections | (Δ/σ)max = 0.001 |
| 226 parameters | Δρmax = 0.84 e Å−3 |
| 0 restraints | Δρmin = −0.90 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.75465 (4) | 0.225888 (14) | 0.48173 (6) | 0.07328 (18) | |
| O1 | 0.2072 (3) | 0.45942 (8) | 0.3332 (3) | 0.0570 (5) | |
| N1 | 0.3210 (3) | 0.28134 (9) | 0.4493 (3) | 0.0480 (6) | |
| N2 | 0.2208 (3) | 0.36984 (9) | 0.3920 (3) | 0.0399 (5) | |
| N3 | 0.4318 (3) | 0.40833 (8) | 0.3767 (3) | 0.0398 (5) | |
| C1 | 0.4498 (4) | 0.25184 (12) | 0.4685 (4) | 0.0515 (7) | |
| H1 | 0.4465 | 0.2160 | 0.4879 | 0.062* | |
| C2 | 0.5858 (4) | 0.27231 (11) | 0.4606 (4) | 0.0473 (7) | |
| C3 | 0.6008 (3) | 0.32553 (11) | 0.4321 (4) | 0.0428 (6) | |
| H3 | 0.6926 | 0.3396 | 0.4275 | 0.051* | |
| C4 | 0.4695 (3) | 0.35553 (10) | 0.4115 (3) | 0.0366 (5) | |
| C5 | 0.3351 (3) | 0.33129 (10) | 0.4207 (3) | 0.0366 (5) | |
| C6 | 0.2777 (3) | 0.41774 (10) | 0.3635 (4) | 0.0401 (6) | |
| C7 | 0.0593 (3) | 0.36191 (13) | 0.3822 (4) | 0.0482 (7) | |
| H7A | 0.0226 | 0.3269 | 0.3356 | 0.058* | |
| H7B | −0.0145 | 0.3867 | 0.2952 | 0.058* | |
| C8 | 0.0513 (3) | 0.36830 (10) | 0.5637 (4) | 0.0376 (5) | |
| C9 | 0.0052 (3) | 0.41597 (11) | 0.6102 (4) | 0.0448 (6) | |
| H9 | −0.0162 | 0.4444 | 0.5310 | 0.054* | |
| C10 | −0.0090 (4) | 0.42129 (13) | 0.7723 (4) | 0.0530 (7) | |
| H10 | −0.0399 | 0.4533 | 0.8023 | 0.064* | |
| C11 | 0.0223 (4) | 0.37940 (15) | 0.8905 (4) | 0.0572 (8) | |
| H11 | 0.0105 | 0.3829 | 0.9989 | 0.069* | |
| C12 | 0.0711 (4) | 0.33231 (14) | 0.8480 (5) | 0.0581 (8) | |
| H12 | 0.0946 | 0.3042 | 0.9291 | 0.070* | |
| C13 | 0.0854 (3) | 0.32658 (12) | 0.6849 (4) | 0.0490 (7) | |
| H13 | 0.1180 | 0.2946 | 0.6565 | 0.059* | |
| C14 | 0.5321 (4) | 0.44829 (11) | 0.3487 (4) | 0.0431 (6) | |
| H14A | 0.6349 | 0.4498 | 0.4570 | 0.052* | |
| H14B | 0.4797 | 0.4821 | 0.3362 | 0.052* | |
| C15 | 0.5641 (3) | 0.43916 (9) | 0.1821 (3) | 0.0349 (5) | |
| C16 | 0.6900 (3) | 0.46603 (11) | 0.1695 (4) | 0.0437 (6) | |
| H16 | 0.7526 | 0.4889 | 0.2630 | 0.052* | |
| C17 | 0.7240 (4) | 0.45935 (14) | 0.0203 (4) | 0.0549 (8) | |
| H17 | 0.8092 | 0.4777 | 0.0137 | 0.066* | |
| C18 | 0.6319 (4) | 0.42548 (13) | −0.1197 (4) | 0.0533 (7) | |
| H18 | 0.6559 | 0.4204 | −0.2194 | 0.064* | |
| C19 | 0.5046 (4) | 0.39937 (12) | −0.1105 (4) | 0.0498 (7) | |
| H19 | 0.4411 | 0.3770 | −0.2055 | 0.060* | |
| C20 | 0.4702 (3) | 0.40613 (11) | 0.0401 (4) | 0.0445 (6) | |
| H20 | 0.3836 | 0.3884 | 0.0453 | 0.053* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.0600 (2) | 0.0554 (2) | 0.0961 (3) | 0.01808 (15) | 0.0259 (2) | 0.00089 (17) |
| O1 | 0.0630 (13) | 0.0486 (12) | 0.0681 (14) | 0.0147 (10) | 0.0364 (11) | 0.0062 (10) |
| N1 | 0.0528 (14) | 0.0437 (13) | 0.0526 (14) | −0.0072 (10) | 0.0274 (12) | 0.0003 (10) |
| N2 | 0.0410 (11) | 0.0455 (12) | 0.0394 (12) | −0.0017 (9) | 0.0233 (10) | −0.0020 (9) |
| N3 | 0.0454 (12) | 0.0371 (11) | 0.0455 (12) | −0.0015 (9) | 0.0277 (10) | 0.0009 (9) |
| C1 | 0.0595 (18) | 0.0372 (15) | 0.0584 (18) | −0.0029 (13) | 0.0261 (15) | 0.0018 (13) |
| C2 | 0.0459 (15) | 0.0424 (15) | 0.0494 (16) | 0.0071 (12) | 0.0166 (13) | −0.0007 (12) |
| C3 | 0.0398 (13) | 0.0436 (14) | 0.0478 (15) | 0.0001 (11) | 0.0214 (12) | −0.0026 (12) |
| C4 | 0.0424 (13) | 0.0371 (12) | 0.0336 (12) | −0.0029 (10) | 0.0194 (11) | −0.0028 (10) |
| C5 | 0.0392 (13) | 0.0400 (13) | 0.0336 (12) | −0.0026 (10) | 0.0186 (10) | −0.0031 (10) |
| C6 | 0.0482 (15) | 0.0438 (14) | 0.0346 (13) | 0.0014 (12) | 0.0239 (11) | −0.0020 (11) |
| C7 | 0.0359 (13) | 0.0663 (19) | 0.0426 (15) | −0.0056 (12) | 0.0172 (12) | −0.0081 (13) |
| C8 | 0.0291 (11) | 0.0463 (14) | 0.0404 (13) | −0.0053 (10) | 0.0177 (10) | −0.0025 (11) |
| C9 | 0.0402 (14) | 0.0445 (15) | 0.0506 (16) | 0.0020 (11) | 0.0204 (12) | 0.0050 (12) |
| C10 | 0.0482 (16) | 0.0594 (18) | 0.0570 (18) | 0.0009 (14) | 0.0279 (14) | −0.0135 (15) |
| C11 | 0.0468 (16) | 0.090 (2) | 0.0415 (16) | −0.0001 (16) | 0.0251 (13) | −0.0038 (16) |
| C12 | 0.0531 (18) | 0.069 (2) | 0.0566 (19) | 0.0009 (15) | 0.0276 (15) | 0.0177 (16) |
| C13 | 0.0460 (15) | 0.0441 (15) | 0.0637 (18) | 0.0007 (12) | 0.0302 (14) | 0.0014 (13) |
| C14 | 0.0544 (16) | 0.0379 (13) | 0.0447 (15) | −0.0103 (12) | 0.0287 (13) | −0.0057 (11) |
| C15 | 0.0382 (12) | 0.0309 (12) | 0.0383 (13) | 0.0023 (10) | 0.0190 (11) | 0.0031 (10) |
| C16 | 0.0412 (14) | 0.0442 (15) | 0.0437 (15) | −0.0059 (11) | 0.0166 (12) | 0.0001 (11) |
| C17 | 0.0486 (17) | 0.066 (2) | 0.0605 (19) | −0.0051 (14) | 0.0338 (16) | 0.0068 (15) |
| C18 | 0.0600 (18) | 0.0642 (19) | 0.0467 (16) | 0.0081 (15) | 0.0333 (15) | 0.0053 (14) |
| C19 | 0.0571 (17) | 0.0519 (16) | 0.0401 (15) | −0.0015 (13) | 0.0206 (13) | −0.0074 (12) |
| C20 | 0.0460 (15) | 0.0461 (15) | 0.0463 (15) | −0.0085 (12) | 0.0245 (13) | −0.0048 (12) |
Geometric parameters (Å, °)
| Br1—C2 | 1.897 (3) | C9—H9 | 0.9300 |
| O1—C6 | 1.213 (3) | C10—C11 | 1.377 (5) |
| N1—C5 | 1.311 (3) | C10—H10 | 0.9300 |
| N1—C1 | 1.351 (4) | C11—C12 | 1.376 (5) |
| N2—C5 | 1.382 (3) | C11—H11 | 0.9300 |
| N2—C6 | 1.386 (3) | C12—C13 | 1.384 (5) |
| N2—C7 | 1.462 (3) | C12—H12 | 0.9300 |
| N3—C4 | 1.389 (3) | C13—H13 | 0.9300 |
| N3—C6 | 1.391 (3) | C14—C15 | 1.512 (4) |
| N3—C14 | 1.453 (3) | C14—H14A | 0.9700 |
| C1—C2 | 1.377 (4) | C14—H14B | 0.9700 |
| C1—H1 | 0.9300 | C15—C20 | 1.382 (4) |
| C2—C3 | 1.393 (4) | C15—C16 | 1.383 (4) |
| C3—C4 | 1.374 (4) | C16—C17 | 1.377 (4) |
| C3—H3 | 0.9300 | C16—H16 | 0.9300 |
| C4—C5 | 1.408 (4) | C17—C18 | 1.382 (5) |
| C7—C8 | 1.505 (4) | C17—H17 | 0.9300 |
| C7—H7A | 0.9700 | C18—C19 | 1.372 (5) |
| C7—H7B | 0.9700 | C18—H18 | 0.9300 |
| C8—C13 | 1.386 (4) | C19—C20 | 1.391 (4) |
| C8—C9 | 1.390 (4) | C19—H19 | 0.9300 |
| C9—C10 | 1.376 (4) | C20—H20 | 0.9300 |
| C5—N1—C1 | 114.4 (2) | C9—C10—C11 | 120.3 (3) |
| C5—N2—C6 | 110.0 (2) | C9—C10—H10 | 119.9 |
| C5—N2—C7 | 126.0 (2) | C11—C10—H10 | 119.9 |
| C6—N2—C7 | 123.9 (2) | C12—C11—C10 | 119.9 (3) |
| C4—N3—C6 | 109.8 (2) | C12—C11—H11 | 120.1 |
| C4—N3—C14 | 126.4 (2) | C10—C11—H11 | 120.1 |
| C6—N3—C14 | 123.8 (2) | C11—C12—C13 | 120.2 (3) |
| N1—C1—C2 | 123.1 (3) | C11—C12—H12 | 119.9 |
| N1—C1—H1 | 118.4 | C13—C12—H12 | 119.9 |
| C2—C1—H1 | 118.4 | C12—C13—C8 | 120.2 (3) |
| C1—C2—C3 | 122.2 (3) | C12—C13—H13 | 119.9 |
| C1—C2—Br1 | 118.6 (2) | C8—C13—H13 | 119.9 |
| C3—C2—Br1 | 119.2 (2) | N3—C14—C15 | 114.1 (2) |
| C4—C3—C2 | 114.8 (3) | N3—C14—H14A | 108.7 |
| C4—C3—H3 | 122.6 | C15—C14—H14A | 108.7 |
| C2—C3—H3 | 122.6 | N3—C14—H14B | 108.7 |
| C3—C4—N3 | 133.9 (2) | C15—C14—H14B | 108.7 |
| C3—C4—C5 | 119.3 (2) | H14A—C14—H14B | 107.6 |
| N3—C4—C5 | 106.9 (2) | C20—C15—C16 | 118.8 (2) |
| N1—C5—N2 | 126.6 (2) | C20—C15—C14 | 122.7 (2) |
| N1—C5—C4 | 126.2 (3) | C16—C15—C14 | 118.5 (2) |
| N2—C5—C4 | 107.2 (2) | C17—C16—C15 | 120.9 (3) |
| O1—C6—N2 | 126.9 (3) | C17—C16—H16 | 119.6 |
| O1—C6—N3 | 127.0 (3) | C15—C16—H16 | 119.6 |
| N2—C6—N3 | 106.1 (2) | C16—C17—C18 | 120.1 (3) |
| N2—C7—C8 | 113.9 (2) | C16—C17—H17 | 120.0 |
| N2—C7—H7A | 108.8 | C18—C17—H17 | 120.0 |
| C8—C7—H7A | 108.8 | C19—C18—C17 | 119.6 (3) |
| N2—C7—H7B | 108.8 | C19—C18—H18 | 120.2 |
| C8—C7—H7B | 108.8 | C17—C18—H18 | 120.2 |
| H7A—C7—H7B | 107.7 | C18—C19—C20 | 120.3 (3) |
| C13—C8—C9 | 118.9 (2) | C18—C19—H19 | 119.8 |
| C13—C8—C7 | 120.7 (3) | C20—C19—H19 | 119.8 |
| C9—C8—C7 | 120.3 (3) | C15—C20—C19 | 120.3 (3) |
| C10—C9—C8 | 120.4 (3) | C15—C20—H20 | 119.9 |
| C10—C9—H9 | 119.8 | C19—C20—H20 | 119.9 |
| C8—C9—H9 | 119.8 | ||
| C5—N1—C1—C2 | 0.6 (4) | C4—N3—C6—N2 | 0.4 (3) |
| N1—C1—C2—C3 | 0.0 (5) | C14—N3—C6—N2 | 177.7 (2) |
| N1—C1—C2—Br1 | −178.0 (2) | C5—N2—C7—C8 | 92.2 (3) |
| C1—C2—C3—C4 | −0.4 (4) | C6—N2—C7—C8 | −91.5 (3) |
| Br1—C2—C3—C4 | 177.6 (2) | N2—C7—C8—C13 | −86.0 (3) |
| C2—C3—C4—N3 | −178.0 (3) | N2—C7—C8—C9 | 95.7 (3) |
| C2—C3—C4—C5 | 0.1 (4) | C13—C8—C9—C10 | −1.1 (4) |
| C6—N3—C4—C3 | 178.2 (3) | C7—C8—C9—C10 | 177.2 (3) |
| C14—N3—C4—C3 | 0.9 (5) | C8—C9—C10—C11 | 0.0 (4) |
| C6—N3—C4—C5 | −0.1 (3) | C9—C10—C11—C12 | 1.3 (5) |
| C14—N3—C4—C5 | −177.4 (2) | C10—C11—C12—C13 | −1.4 (5) |
| C1—N1—C5—N2 | 178.3 (3) | C11—C12—C13—C8 | 0.3 (5) |
| C1—N1—C5—C4 | −1.0 (4) | C9—C8—C13—C12 | 1.0 (4) |
| C6—N2—C5—N1 | −178.9 (3) | C7—C8—C13—C12 | −177.3 (3) |
| C7—N2—C5—N1 | −2.1 (4) | C4—N3—C14—C15 | 62.6 (4) |
| C6—N2—C5—C4 | 0.5 (3) | C6—N3—C14—C15 | −114.3 (3) |
| C7—N2—C5—C4 | 177.3 (2) | N3—C14—C15—C20 | 18.6 (4) |
| C3—C4—C5—N1 | 0.6 (4) | N3—C14—C15—C16 | −163.1 (2) |
| N3—C4—C5—N1 | 179.2 (3) | C20—C15—C16—C17 | −1.3 (4) |
| C3—C4—C5—N2 | −178.8 (2) | C14—C15—C16—C17 | −179.7 (3) |
| N3—C4—C5—N2 | −0.2 (3) | C15—C16—C17—C18 | 0.1 (5) |
| C5—N2—C6—O1 | 179.4 (3) | C16—C17—C18—C19 | 1.2 (5) |
| C7—N2—C6—O1 | 2.6 (4) | C17—C18—C19—C20 | −1.2 (5) |
| C5—N2—C6—N3 | −0.5 (3) | C16—C15—C20—C19 | 1.4 (4) |
| C7—N2—C6—N3 | −177.4 (2) | C14—C15—C20—C19 | 179.7 (3) |
| C4—N3—C6—O1 | −179.6 (3) | C18—C19—C20—C15 | −0.1 (5) |
| C14—N3—C6—O1 | −2.2 (4) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG2651).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Barraclough, P., Black, J. W., Cambridge, D., Collard, D., Firmin, D., Gerskowitch, V. P., Glen, R. C., Giles, H. & Hill, A. P. (1990). J. Med. Chem 33, 2231–2239. [DOI] [PubMed]
- Bruker (2008). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cundy, D. J., Holan, G., Otaegui, M. & Simpson, G. W. (1997). Bioorg. Med. Chem. Lett.7, 669–674.
- Dahmani, S., Haoudi, A., Capet, F., Essassi, E. M. & Ng, S. W. (2010a). Acta Cryst. E66, o755. [DOI] [PMC free article] [PubMed]
- Dahmani, S., Haoudi, A., Capet, F., Essassi, E. M. & Ng, S. W. (2010b). Acta Cryst. E66, o756. [DOI] [PMC free article] [PubMed]
- Desarro, A., Desarro, G., Chimirri, A., Grasso, S., Monforte, A. M. & Zappala, M. (1994). Gen. Pharmacol.25,1027–1031.
- Liu, L., Xu, P., Zhou, L. & Lei, P. S. (2008). Chin. Chem. Lett.19, 1–4.
- Mader, M., de Dios, A., Shih, C., Bonjouklian, R., Li, T. C., White, W., de Uralde, B. L., Sánchez-Martinez, C., del Prado, M., Jaramillo, C., de Diego, E., Cabrejas, L. M. M., Dominguez, C., Montero, C., Shepherd, T., Dally, R., Toth, J. E., Chatterjee, A., Pleite, S., Blanco-Urgoiti, J., Perez, L., Barberis, M., Lorite, M. J., Jambrina, E., Nevill, C. R., Lee, P. A., Schultz, R. C., Wolos, J. A., Li, L. C., Campbell, R. M. & Anderson, B. D. (2008). Bioorg. Med. Chem. Lett.18, 179–183. [DOI] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). publCIF In preparation.
- Zaki, M. E. A. & Proença, M. F. (2007). Tetrahedron, 63, 3745–3753.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810007713/hg2651sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810007713/hg2651Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report