Abstract
In the title compound, C9H6Cl2N2O, the dihedral angle between the oxadiazole and benzene rings is 1.7 (2)°. In the crystal, the molecules are linked into chains along the b axis by short intermolecular Cl⋯O contacts [3.019 (3) Å].
Related literature
For general background and the biological activity of oxadiazole compounds, see: Andersen et al. (1994 ▶); Clitherow et al. (1996 ▶); Showell et al. (1991 ▶); Swain et al. (1991 ▶); Watjen et al. (1989 ▶). For a related structure, see: Wang et al. (2006 ▶). For the stability of the temperature controller used for the data collection, see: Cosier & Glazer (1986 ▶).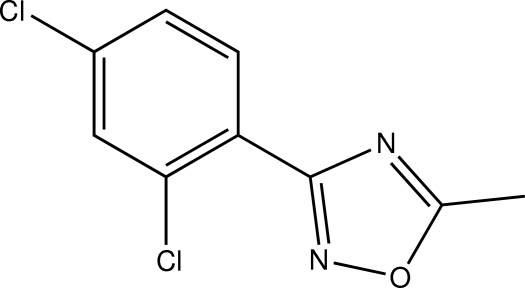
Experimental
Crystal data
C9H6Cl2N2O
M r = 229.06
Monoclinic,

a = 3.8252 (7) Å
b = 21.678 (4) Å
c = 11.0833 (19) Å
β = 92.421 (4)°
V = 918.3 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.67 mm−1
T = 100 K
0.28 × 0.17 × 0.11 mm
Data collection
Bruker SMART APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.833, T max = 0.929
7920 measured reflections
2076 independent reflections
1709 reflections with I > 2σ(I)
R int = 0.048
Refinement
R[F 2 > 2σ(F 2)] = 0.056
wR(F 2) = 0.155
S = 1.20
2076 reflections
128 parameters
H-atom parameters constrained
Δρmax = 0.72 e Å−3
Δρmin = −0.56 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810007932/ci5045sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810007932/ci5045Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
HKF and MMR thank the Malaysian Government and Universiti Sains Malaysia (USM) for the Research University Golden Goose Grant (1001/PFIZIK/811012). AMI is grateful to Professor Sandeep Sancheti, Director, National Institute of Technology-Karnataka, India, for providing research facilities and encouragement.
supplementary crystallographic information
Comment
Heterocyclic compounds are important in recent years due to pharmacological activities. Nitrogen, oxygen containing five- and six-membered heterocyclic compounds have enormous significance in the field of medicinal chemistry. Oxadiazoles play a very vital role in the preparation of various biologically active drugs with anti-inflammatory (Andersen et al., 1994), anti-cancer (Showell et al., 1991), anti-HIV (Watjen et al., 1989), anti-diabetic and anti-microbial (Swain et al., 1991) properties. The results of biological studies showed that oxadiazole derivatives are molecules with maximum anti-inflammatory, analgesic and minimum ulcerogenic and lipid per-oxidation (Clitherow et al., 1996) properties.
Bond lengths and angles are normal (Wang et al., 2006). The mean plane of the oxadiazole ring (C1/C2/N1/N2/O1) is almost coplanar with the mean plane of the C3–C8 benzene ring (Fig. 1), with a dihedral angle of 1.7 (2)°.
The molecules are linked by Cl2···O1(-x, 1/2+y, 3/2-z) short contacts [3.019 (3) Å;] to form chains along the b axis (Fig.2).
Experimental
The title compound was prepared by heating a solution of 2,4-dichloro-N'-hydroxy-benzamidine (1 g, 0.0042 mol) and acetyl chloride (0.38 g, 0.004 mol) in pyridine (30 ml) at 387 K for 1.5 h and the contents were concentrated under vacuum. Further purification was done by column chromatography. The solid obtained was recrystallized using dichloromethane (yield: 1.0 g (76%); m.p. 371-372 K).
Refinement
H atoms were placed in calculated positions [C–H = 0.93–0.96 Å] and refined as riding with Uiso(H) = 1.2eq(C) or 1.5Ueq(methyl C). A rotating group model was used for the methyl group.
Figures
Fig. 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids and the atom-numbering scheme.
Fig. 2.

The crystal structure of the title compound, showing chains along the b axis.
Crystal data
| C9H6Cl2N2O | F(000) = 464 |
| Mr = 229.06 | Dx = 1.657 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3719 reflections |
| a = 3.8252 (7) Å | θ = 2.6–31.1° |
| b = 21.678 (4) Å | µ = 0.67 mm−1 |
| c = 11.0833 (19) Å | T = 100 K |
| β = 92.421 (4)° | Block, colourless |
| V = 918.3 (3) Å3 | 0.28 × 0.17 × 0.11 mm |
| Z = 4 |
Data collection
| Bruker SMART APEXII CCD area-detector diffractometer | 2076 independent reflections |
| Radiation source: fine-focus sealed tube | 1709 reflections with I > 2σ(I) |
| graphite | Rint = 0.048 |
| φ and ω scans | θmax = 27.5°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | h = −4→4 |
| Tmin = 0.833, Tmax = 0.929 | k = −26→28 |
| 7920 measured reflections | l = −13→14 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.056 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.155 | H-atom parameters constrained |
| S = 1.20 | w = 1/[σ2(Fo2) + (0.0428P)2 + 4.0517P] where P = (Fo2 + 2Fc2)/3 |
| 2076 reflections | (Δ/σ)max = 0.001 |
| 128 parameters | Δρmax = 0.72 e Å−3 |
| 0 restraints | Δρmin = −0.56 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cyrosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) K. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.2530 (2) | 0.40084 (4) | 0.92173 (8) | 0.0163 (2) | |
| Cl2 | 0.1238 (3) | 0.63730 (4) | 0.78985 (9) | 0.0185 (3) | |
| O1 | −0.2054 (8) | 0.27327 (13) | 0.6574 (3) | 0.0220 (7) | |
| N1 | −0.3467 (8) | 0.35961 (15) | 0.5636 (3) | 0.0158 (7) | |
| N2 | −0.0709 (10) | 0.32171 (16) | 0.7322 (3) | 0.0194 (7) | |
| C1 | −0.3637 (11) | 0.30045 (18) | 0.5605 (4) | 0.0173 (8) | |
| C2 | −0.1651 (10) | 0.37119 (18) | 0.6715 (3) | 0.0140 (8) | |
| C3 | −0.0869 (9) | 0.43519 (17) | 0.7088 (3) | 0.0124 (7) | |
| C4 | 0.0928 (10) | 0.45392 (18) | 0.8165 (3) | 0.0137 (8) | |
| C5 | 0.1523 (9) | 0.51553 (18) | 0.8418 (4) | 0.0136 (7) | |
| H5A | 0.2684 | 0.5272 | 0.9136 | 0.016* | |
| C6 | 0.0369 (10) | 0.56000 (17) | 0.7588 (3) | 0.0127 (7) | |
| C7 | −0.1429 (10) | 0.54398 (18) | 0.6533 (4) | 0.0151 (8) | |
| H7A | −0.2240 | 0.5740 | 0.5990 | 0.018* | |
| C8 | −0.1993 (10) | 0.48214 (18) | 0.6303 (4) | 0.0153 (8) | |
| H8A | −0.3186 | 0.4712 | 0.5586 | 0.018* | |
| C9 | −0.5226 (12) | 0.2592 (2) | 0.4666 (4) | 0.0237 (9) | |
| H9A | −0.6428 | 0.2835 | 0.4055 | 0.036* | |
| H9B | −0.6856 | 0.2319 | 0.5028 | 0.036* | |
| H9C | −0.3422 | 0.2354 | 0.4308 | 0.036* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0168 (5) | 0.0182 (5) | 0.0138 (5) | 0.0028 (3) | −0.0011 (3) | 0.0023 (4) |
| Cl2 | 0.0197 (5) | 0.0140 (4) | 0.0219 (5) | −0.0024 (3) | 0.0025 (4) | −0.0017 (4) |
| O1 | 0.0309 (17) | 0.0139 (14) | 0.0213 (15) | −0.0017 (12) | 0.0017 (12) | −0.0018 (12) |
| N1 | 0.0130 (15) | 0.0175 (16) | 0.0172 (17) | 0.0000 (12) | 0.0018 (12) | −0.0022 (13) |
| N2 | 0.0257 (19) | 0.0147 (16) | 0.0178 (18) | −0.0002 (14) | 0.0018 (14) | −0.0032 (13) |
| C1 | 0.0148 (19) | 0.0194 (19) | 0.018 (2) | 0.0000 (15) | 0.0051 (15) | −0.0007 (15) |
| C2 | 0.0103 (17) | 0.0185 (19) | 0.0138 (18) | 0.0022 (14) | 0.0081 (14) | 0.0000 (14) |
| C3 | 0.0078 (17) | 0.0152 (18) | 0.0150 (18) | 0.0009 (13) | 0.0080 (14) | −0.0011 (14) |
| C4 | 0.0126 (18) | 0.0171 (19) | 0.0119 (19) | 0.0043 (13) | 0.0054 (14) | 0.0016 (14) |
| C5 | 0.0070 (16) | 0.0208 (19) | 0.0132 (18) | 0.0017 (14) | 0.0022 (13) | −0.0021 (15) |
| C6 | 0.0106 (17) | 0.0134 (17) | 0.0144 (18) | −0.0008 (13) | 0.0033 (13) | −0.0027 (14) |
| C7 | 0.0106 (18) | 0.0174 (19) | 0.0177 (19) | 0.0033 (14) | 0.0056 (14) | 0.0033 (15) |
| C8 | 0.0114 (17) | 0.0181 (19) | 0.017 (2) | 0.0005 (14) | 0.0055 (14) | 0.0004 (15) |
| C9 | 0.025 (2) | 0.018 (2) | 0.029 (2) | −0.0030 (16) | 0.0041 (18) | −0.0072 (17) |
Geometric parameters (Å, °)
| Cl1—C4 | 1.732 (4) | C4—C5 | 1.382 (5) |
| Cl2—C6 | 1.740 (4) | C5—C6 | 1.391 (5) |
| O1—C1 | 1.346 (5) | C5—H5A | 0.93 |
| O1—N2 | 1.421 (4) | C6—C7 | 1.376 (5) |
| N1—C1 | 1.285 (5) | C7—C8 | 1.380 (6) |
| N1—C2 | 1.381 (5) | C7—H7A | 0.93 |
| N2—C2 | 1.308 (5) | C8—H8A | 0.93 |
| C1—C9 | 1.483 (6) | C9—H9A | 0.96 |
| C2—C3 | 1.475 (5) | C9—H9B | 0.96 |
| C3—C8 | 1.396 (5) | C9—H9C | 0.96 |
| C3—C4 | 1.411 (5) | ||
| C1—O1—N2 | 106.4 (3) | C6—C5—H5A | 120.3 |
| C1—N1—C2 | 103.2 (3) | C7—C6—C5 | 121.3 (4) |
| C2—N2—O1 | 102.8 (3) | C7—C6—Cl2 | 119.7 (3) |
| N1—C1—O1 | 113.3 (4) | C5—C6—Cl2 | 119.0 (3) |
| N1—C1—C9 | 129.8 (4) | C6—C7—C8 | 118.1 (4) |
| O1—C1—C9 | 116.9 (4) | C6—C7—H7A | 121.0 |
| N2—C2—N1 | 114.4 (4) | C8—C7—H7A | 121.0 |
| N2—C2—C3 | 125.4 (4) | C7—C8—C3 | 123.5 (4) |
| N1—C2—C3 | 120.2 (3) | C7—C8—H8A | 118.3 |
| C8—C3—C4 | 116.4 (3) | C3—C8—H8A | 118.3 |
| C8—C3—C2 | 117.2 (3) | C1—C9—H9A | 109.5 |
| C4—C3—C2 | 126.4 (3) | C1—C9—H9B | 109.5 |
| C5—C4—C3 | 121.3 (3) | H9A—C9—H9B | 109.5 |
| C5—C4—Cl1 | 117.1 (3) | C1—C9—H9C | 109.5 |
| C3—C4—Cl1 | 121.6 (3) | H9A—C9—H9C | 109.5 |
| C4—C5—C6 | 119.4 (3) | H9B—C9—H9C | 109.5 |
| C4—C5—H5A | 120.3 | ||
| C1—O1—N2—C2 | 0.1 (4) | C8—C3—C4—C5 | 0.0 (5) |
| C2—N1—C1—O1 | −0.4 (5) | C2—C3—C4—C5 | 179.8 (3) |
| C2—N1—C1—C9 | −179.1 (4) | C8—C3—C4—Cl1 | −179.1 (3) |
| N2—O1—C1—N1 | 0.2 (5) | C2—C3—C4—Cl1 | 0.6 (5) |
| N2—O1—C1—C9 | 179.1 (3) | C3—C4—C5—C6 | −0.8 (6) |
| O1—N2—C2—N1 | −0.4 (4) | Cl1—C4—C5—C6 | 178.4 (3) |
| O1—N2—C2—C3 | −179.3 (3) | C4—C5—C6—C7 | 1.6 (6) |
| C1—N1—C2—N2 | 0.5 (5) | C4—C5—C6—Cl2 | −178.3 (3) |
| C1—N1—C2—C3 | 179.5 (3) | C5—C6—C7—C8 | −1.5 (6) |
| N2—C2—C3—C8 | 178.0 (4) | Cl2—C6—C7—C8 | 178.4 (3) |
| N1—C2—C3—C8 | −0.8 (5) | C6—C7—C8—C3 | 0.7 (6) |
| N2—C2—C3—C4 | −1.8 (6) | C4—C3—C8—C7 | 0.1 (6) |
| N1—C2—C3—C4 | 179.4 (4) | C2—C3—C8—C7 | −179.7 (4) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CI5045).
References
- Andersen, K. E., Jørgensen, A. S. & Bræstrup, C. (1994). Eur. J. Med. Chem.29, 393-399.
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Clitherow, J. W., Beswick, P., Irving, W. J., Scopes, D. I. C., Barnes, J. C., Clapham, J., Brown, J. D., Evans, D. J. & Hayes, A. G. (1996). Bioorg. Med. Chem. Lett.6, 833–838.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Showell, G. A., Gibbons, T. L., Kneen, C. O., MacLeod, A. M., Merchant, K., Saunders, J., Freedman, S. B., Patel, S. & Baker, R. (1991). J. Med. Chem.34, 1086–1094. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Swain, C. J., Baker, R., Kneen, C., Moseley, J., Saunders, J., Seward, E. M., Stevenson, G., Beer, M., Stanton, J. & Watling, K. (1991). J. Med. Chem.34, 140–151. [DOI] [PubMed]
- Wang, H.-B., Liu, Z.-Q., Wang, H.-B. & Yan, X.-C. (2006). Acta Cryst. E62, o4715–o4716.
- Watjen, F., Baker, R., Engelstoff, M., Herbert, R., MacLeod, A., Knight, A., Merchant, K., Moseley, J., Saunders, J., Swain, C. J., Wang, E. & Springer, J. P. (1989). J. Med. Chem.32, 282–2291. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810007932/ci5045sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810007932/ci5045Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



