Abstract
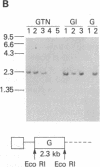
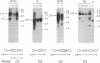
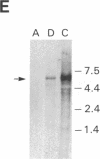
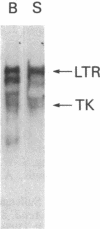
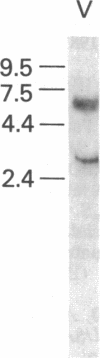
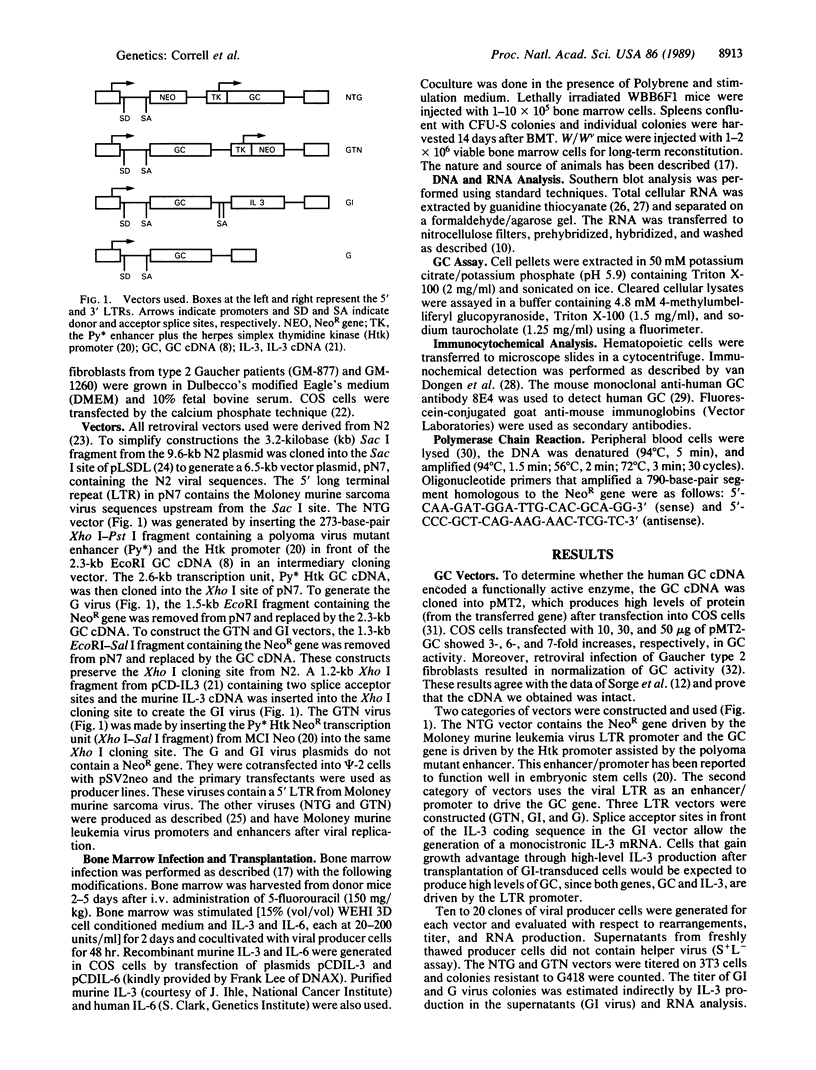
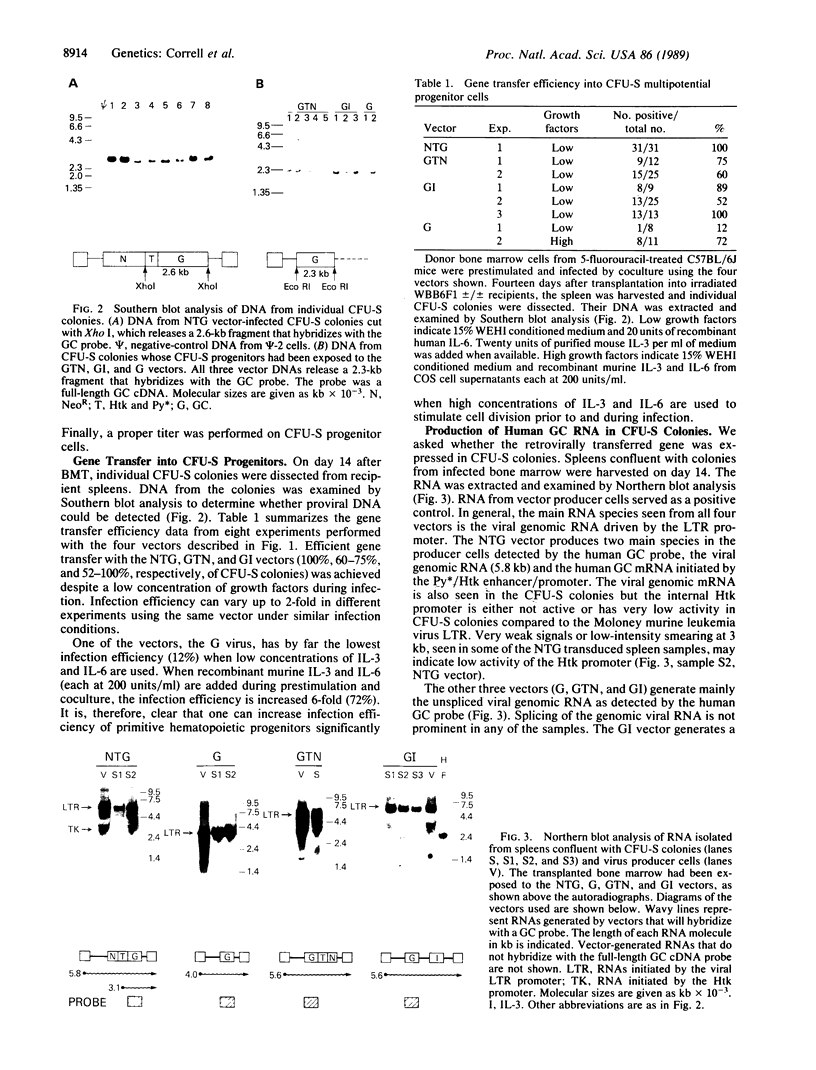
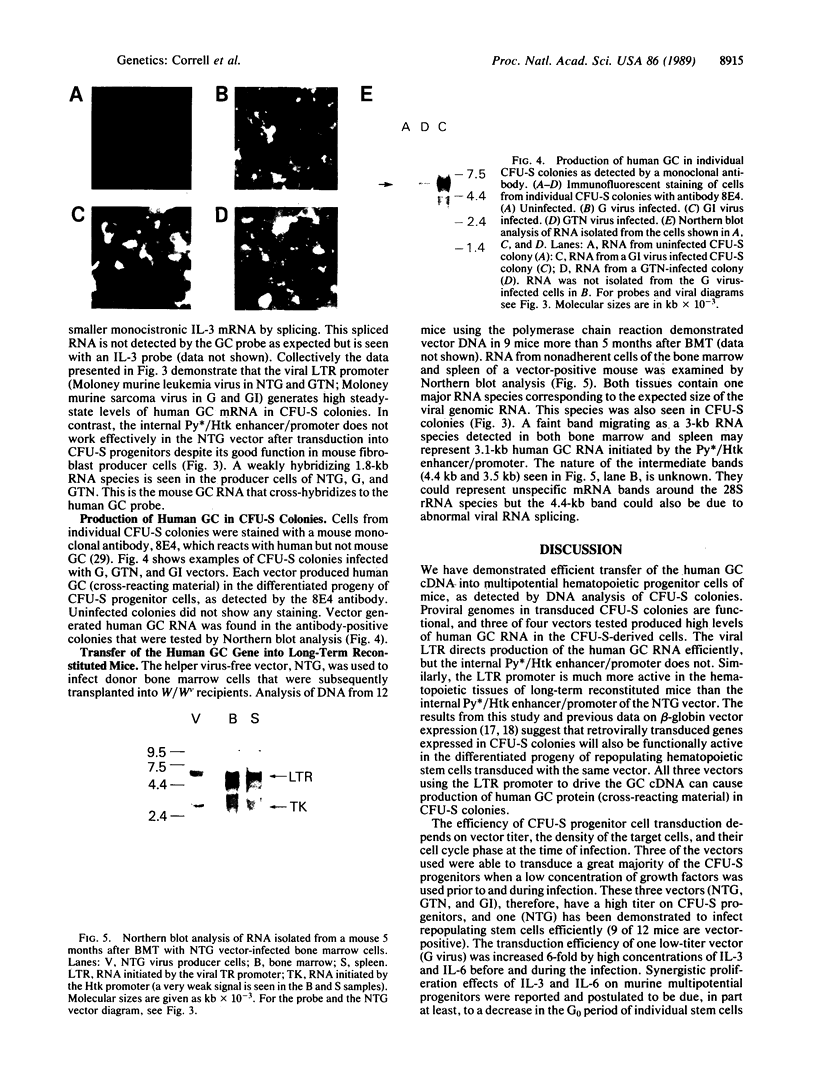
The human glucocerebrosidase (GC) gene has been transferred efficiently into spleen colony-forming unit (CFU-S) multipotential hematopoietic progenitor cells, and production of human GC RNA and protein has been achieved in transduced CFU-S colonies. High-titer retroviral vectors containing the human GC cDNA were constructed. Mouse bone marrow cells were stimulated with hematopoietic growth factors, infected by coculture with producer cells, and injected into lethally irradiated animals. Four vectors were compared with respect to gene-transfer efficiency into CFU-S progenitors. One vector (G vector) required high concentrations of interleukins 3 and 6 during stimulation and coculture for efficient transduction of CFU-S progenitors. The remaining three vectors (NTG, GTN, and GI vectors) transduced these progenitors at infection frequencies approaching 100% using low concentrations of hematopoietic growth factors to stimulate cell division prior to and during the infection. Vectors using the viral long terminal repeat enhancer/promoter to drive the human GC cDNA produced high levels of human GC RNA in the progeny of CFU-S progenitors after gene transfer. When an internal herpes simplex thymidine kinase promoter assisted by a mutant polyoma enhancer was used to drive the human GC cDNA (NTG vector), little or no human GC RNA was detected in transduced CFU-S colonies. All three vectors producing human GC RNA in CFU-S colonies can generate human GC as detected by immunochemical analysis of CFU-S colonies. NTG vector-infected bone marrow cells were transplanted into W/Wv recipients to generate long-term reconstituted mice. The capacity of the viral long terminal repeat and the internal thymidine kinase promoter to direct synthesis of RNA in transduced bone marrow and spleen cells 5 months after bone marrow transplantation reflected the performance of these promoters in NTG-transduced CFU-S colonies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY R. O., KANFER J. N., SHAPIRO D. METABOLISM OF GLUCOCEREBROSIDES. II. EVIDENCE OF AN ENZYMATIC DEFICIENCY IN GAUCHER'S DISEASE. Biochem Biophys Res Commun. 1965 Jan 18;18:221–225. doi: 10.1016/0006-291x(65)90743-6. [DOI] [PubMed] [Google Scholar]
- Barneveld R. A., Tegelaers F. P., Ginns E. I., Visser P., Laanen E. A., Brady R. O., Galjaard H., Barranger J. A., Reuser A. J., Tager J. M. Monoclonal antibodies against human beta-glucocerebrosidase. Eur J Biochem. 1983 Aug 15;134(3):585–589. doi: 10.1111/j.1432-1033.1983.tb07606.x. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Choudary P. V., Tsuji S., Martin B. M., Guild B. C., Mulligan R. C., Murray G. J., Barranger J. A., Ginns E. I. The molecular biology of Gaucher disease and the potential for gene therapy. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):1047–1052. doi: 10.1101/sqb.1986.051.01.121. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Hantzopoulos P. A., Sullenger B. A., Ungers G., Gilboa E. Improved gene expression upon transfer of the adenosine deaminase minigene outside the transcriptional unit of a retroviral vector. Proc Natl Acad Sci U S A. 1989 May;86(10):3519–3523. doi: 10.1073/pnas.86.10.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T., Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986 Jul;6(7):2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Bodine D. M., Perry L., Papayannopoulou T., Nienhuis A. W. Expression of the human beta-globin gene following retroviral-mediated transfer into multipotential hematopoietic progenitors of mice. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6062–6066. doi: 10.1073/pnas.85.16.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Humphries R. K., Gluzman Y., Nienhuis A. W. Transfer of genes into hematopoietic cells using recombinant DNA viruses. Proc Natl Acad Sci U S A. 1985 Jan;82(1):158–162. doi: 10.1073/pnas.82.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Papayannopoulou T., Schweiger S. G., Stamatoyannopoulos G., Nienhuis A. W. Retroviral-mediated transfer of genomic globin genes leads to regulated production of RNA and protein. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2411–2415. doi: 10.1073/pnas.84.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Lim B., Williams D. A., Orkin S. H. Retrovirus-mediated gene transfer of human adenosine deaminase: expression of functional enzyme in murine hematopoietic stem cells in vivo. Mol Cell Biol. 1987 Oct;7(10):3459–3465. doi: 10.1128/mcb.7.10.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Sidransky E., Ginns E. I. Gaucher's disease: advances and challenges. Adv Pediatr. 1989;36:277–306. [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Rappeport J. M., Ginns E. I. Bone-marrow transplantation in severe Gaucher's disease. N Engl J Med. 1984 Jul 12;311(2):84–88. doi: 10.1056/NEJM198407123110203. [DOI] [PubMed] [Google Scholar]
- Reiner O., Wilder S., Givol D., Horowitz M. Efficient in vitro and in vivo expression of human glucocerebrosidase cDNA. DNA. 1987 Apr;6(2):101–108. doi: 10.1089/dna.1987.6.101. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Groth C. G., Erikson A., Bäckman L., Granqvist S., Månsson J. E., Svennerholm L. Long-term follow-up of the first successful bone marrow transplantation in Gaucher disease. Transplantation. 1988 Jul;46(1):66–70. doi: 10.1097/00007890-198807000-00011. [DOI] [PubMed] [Google Scholar]
- Sorge J., Kuhl W., West C., Beutler E. Complete correction of the enzymatic defect of type I Gaucher disease fibroblasts by retroviral-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Feb;84(4):906–909. doi: 10.1073/pnas.84.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., West C., Westwood B., Beutler E. Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7289–7293. doi: 10.1073/pnas.82.21.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starer F., Sargent J. D., Hobbs J. R. Regression of the radiological changes of Gaucher's disease following bone marrow transplantation. Br J Radiol. 1987 Dec;60(720):1189–1195. doi: 10.1259/0007-1285-60-720-1189. [DOI] [PubMed] [Google Scholar]
- Theophilus B., Latham T., Grabowski G. A., Smith F. I. Gaucher disease: molecular heterogeneity and phenotype-genotype correlations. Am J Hum Genet. 1989 Aug;45(2):212–225. [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Stubblefield B. K., Mayor J. A., Barranger J. A., Ginns E. I. A mutation in the human glucocerebrosidase gene in neuronopathic Gaucher's disease. N Engl J Med. 1987 Mar 5;316(10):570–575. doi: 10.1056/NEJM198703053161002. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Winfield S., Barranger J. A., Ginns E. I. Nucleotide sequence of cDNA containing the complete coding sequence for human lysosomal glucocerebrosidase. J Biol Chem. 1986 Jan 5;261(1):50–53. [PubMed] [Google Scholar]
- Van Dongen J. M., Barneveld R. A., Geuze H. J., Galjaard H. Immunocytochemistry of lysosomal hydrolases and their precursor forms in normal and mutant human cells. Histochem J. 1984 Sep;16(9):941–954. doi: 10.1007/BF01003850. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Dunbar C. E., Bodine D. M., Ruscetti S., Nienhuis A. W. Retrovirus-mediated transfer and expression of the interleukin-3 gene in mouse hematopoietic cells result in a myeloproliferative disorder. Mol Cell Biol. 1989 Feb;9(2):798–808. doi: 10.1128/mcb.9.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Nienhuis A. W. Retroviral transfer and expression of the interleukin-3 gene in hemopoietic cells. Genes Dev. 1987 Jun;1(4):358–365. doi: 10.1101/gad.1.4.358. [DOI] [PubMed] [Google Scholar]
- Zimran A., Sorge J., Gross E., Kubitz M., West C., Beutler E. Prediction of severity of Gaucher's disease by identification of mutations at DNA level. Lancet. 1989 Aug 12;2(8659):349–352. doi: 10.1016/s0140-6736(89)90536-9. [DOI] [PubMed] [Google Scholar]