Abstract
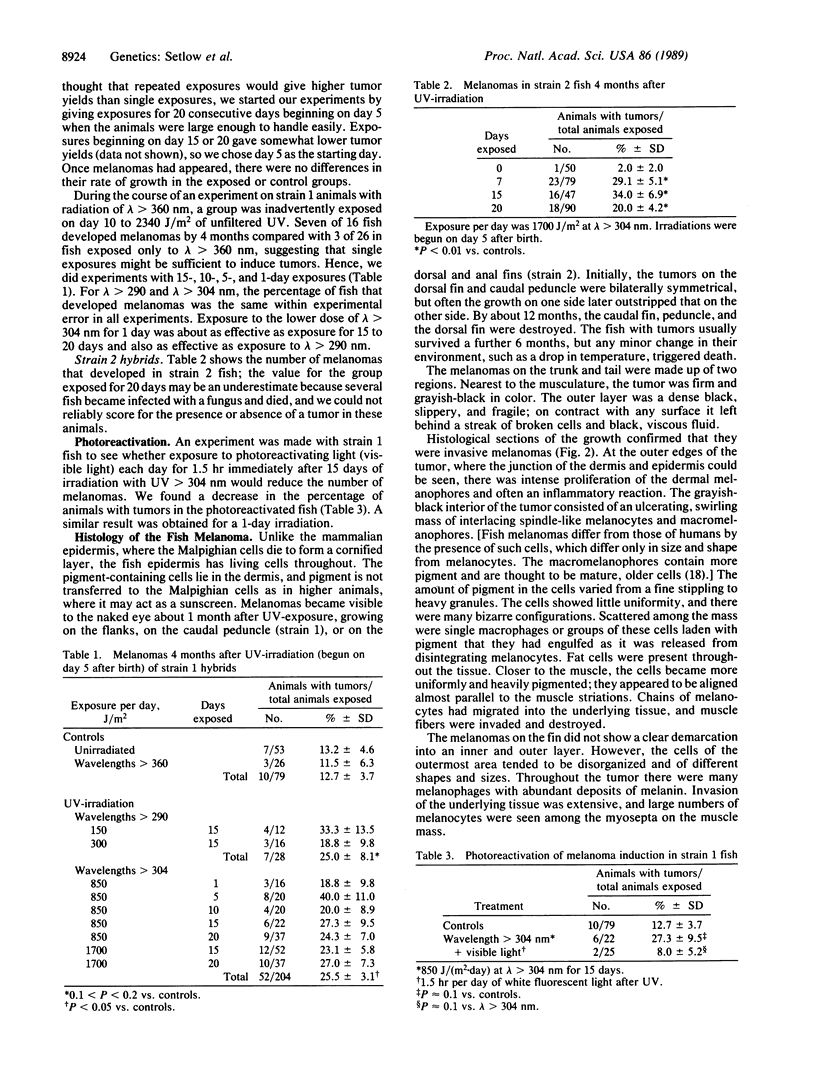
Sunlight exposure is strongly indicated as one of the important etiologic agents in human cutaneous malignant melanoma. However, because of the absence of good animal models, it has not been possible to estimate the wavelengths or wavelength regions involved. We have developed a useful animal model from crosses and backcrosses of platyfish (Xiphophorus maculatus) and swordtails (Xiphophorus helleri). Two strains of these fish are susceptible to invasive melanoma induction by exposure to filtered radiation from sunlamps in the wavelength ranges lambda greater than 290 nm and lambda greater than 304 nm. Multiple exposures on 5-20 consecutive days beginning on day 5 after birth or a single exposure of approximately 200 J/(m2.day) of lambda greater than 304 nm result in a tumor prevalence of 20% to 40% at 4 months of age compared with a background rate of 12% in one strain and 2% in another. Exposure of the fish to visible light after UV exposure reduces the prevalence to background. The melanomas are similar in many respects to mammalian melanomas, as judged by light and electron microscopy. The genetics of the crosses determined by others and the high sensitivity of the hybrids to melanoma induction indicate that the UV radiation probably inactivates the one tumor repressor gene (or a small number of tumor repressor genes) in the hybrid fish. The small size of the animals and their high susceptibility to melanoma induction make them ideal for action spectroscopy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders A., Anders F. Etiology of cancer as studied in the platyfish-swordtail system. Biochim Biophys Acta. 1978 Sep 18;516(1):61–95. doi: 10.1016/0304-419x(78)90004-5. [DOI] [PubMed] [Google Scholar]
- Anders F., Schartl M., Barnekow A., Anders A. Xiphophorus as an in vivo model for studies on normal and defective control of oncogenes. Adv Cancer Res. 1984;42:191–275. doi: 10.1016/s0065-230x(08)60459-5. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B., Anders A., Dippold W. G., Stallcup W. B., Wiegandt H. Melanoma-associated gangliosides in the fish genus Xiphophorus. Cancer Res. 1988 Jun 15;48(12):3454–3460. [PubMed] [Google Scholar]
- GORDON M. The variable expressivity of a pigment cell gene from zero effect to melanotic tumor induction. Cancer Res. 1951 Sep;11(9):676–686. [PubMed] [Google Scholar]
- Gordon M. The Genetics of a Viviparous Top-Minnow Platypoecilus; the Inheritance of Two Kinds of Melanophores. Genetics. 1927 May;12(3):253–283. doi: 10.1093/genetics/12.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Tumor suppressors: recessive mutations that lead to cancer. Cell. 1988 Apr 22;53(2):173–174. doi: 10.1016/0092-8674(88)90376-5. [DOI] [PubMed] [Google Scholar]
- Kallman K. D., Schreibman M. P. The origin and possible genetic control of new, stable pigment patterns in the poeciliid fish Xiphophorus maculatus. J Exp Zool. 1971 Feb;176(2):147–168. doi: 10.1002/jez.1401760204. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984 Apr;5(4):511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Fisher M. S. Immunologic aspects of tumor induction by ultraviolet radiation. Natl Cancer Inst Monogr. 1978 Dec;(50):179–183. [PubMed] [Google Scholar]
- Kripke M. L., Fisher M. S. Immunologic parameters of ultraviolet carcinogenesis. J Natl Cancer Inst. 1976 Jul;57(1):211–215. doi: 10.1093/jnci/57.1.211. [DOI] [PubMed] [Google Scholar]
- Ley R. D., Applegate L. A., Padilla R. S., Stuart T. D. Ultraviolet radiation--induced malignant melanoma in Monodelphis domestica. Photochem Photobiol. 1989 Jul;50(1):1–5. doi: 10.1111/j.1751-1097.1989.tb04123.x. [DOI] [PubMed] [Google Scholar]
- Riehl R., Schartl M., Kollinger G. Comparative studies on the ultrastructure of malignant melanoma in fish and human by freeze-etching and transmission electron microscopy. J Cancer Res Clin Oncol. 1984;107(1):21–31. doi: 10.1007/BF00395486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Setlow J. K. Effects of radiation on polynucleotides. Annu Rev Biophys Bioeng. 1972;1:293–346. doi: 10.1146/annurev.bb.01.060172.001453. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Cimino J. S., Delihas N., Shih A. G., Oliver R. P. Ultraviolet light-induced transformation of human cells to anchorage-independent growth. Cancer Res. 1980 Jun;40(6):1934–1939. [PubMed] [Google Scholar]
- Sutherland B. M., Delihas N. C., Oliver R. P., Sutherland J. C. Action spectra for ultraviolet light-induced transformation of human cells to anchorage-independent growth. Cancer Res. 1981 Jun;41(6):2211–2214. [PubMed] [Google Scholar]
- Sutherland B. M. Photoreactivation: evaluation of pyrimidine dimers in ultraviolet radiation-induced cell transformation. Natl Cancer Inst Monogr. 1978 Dec;(50):129–132. [PubMed] [Google Scholar]






