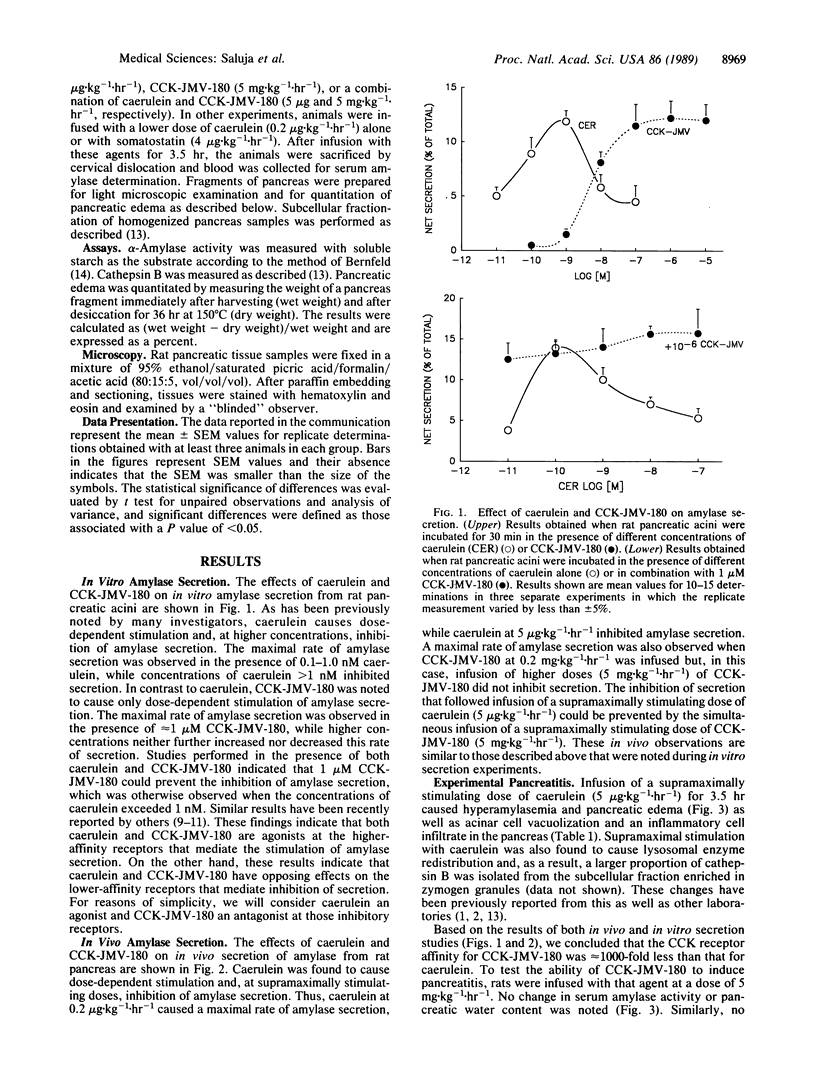
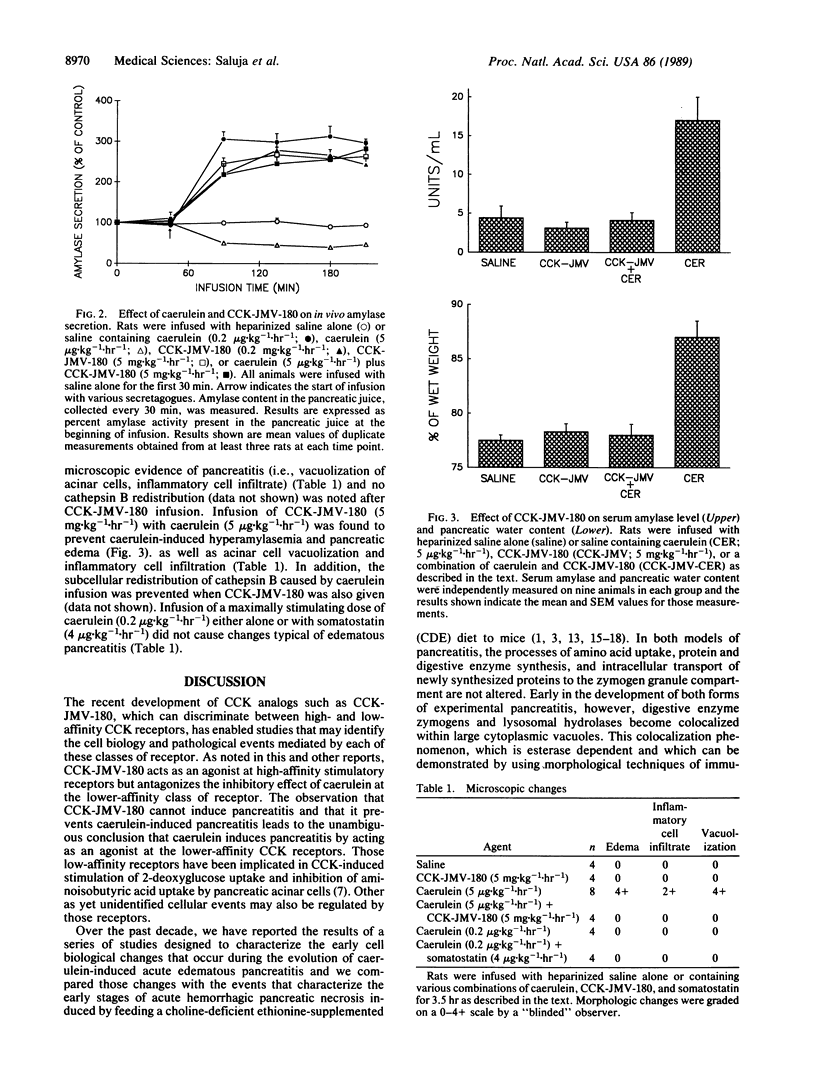
Abstract
Rats infused with a supramaximally stimulating dose of the cholecystokinin (CCK) analog caerulein develop acute edematous pancreatitis. Using CCK-JMV-180, a recently developed CCK analog that acts as an agonist at high-affinity CCK receptors but antagonizes the effect of CCK at low-affinity receptors, we have determined that caerulein induces pancreatitis by interacting with low-affinity CCK receptors. Those low-affinity receptors mediate CCK-induced inhibition of digestive enzyme secretion from the pancreas. Our observations, therefore, suggest that this form of experimental pancreatitis results from the inhibition of pancreatic digestive enzyme secretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Field B. E., Hepner G. W., Shabot M. M., Schwartz A. A., State D., Worthen N., Wilson R. Nasogastric suction in alcoholic pancreatitis. Dig Dis Sci. 1979 May;24(5):339–344. doi: 10.1007/BF01297118. [DOI] [PubMed] [Google Scholar]
- Gaisano H. Y., Klueppelberg U. G., Pinon D. I., Pfenning M. A., Powers S. P., Miller L. J. Novel tool for the study of cholecystokinin-stimulated pancreatic enzyme secretion. J Clin Invest. 1989 Jan;83(1):321–325. doi: 10.1172/JCI113877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas M. C., Lignon M. F., Rodriguez M., Mendre C., Fulcrand P., Laur J., Martinez J. Structure-activity relationship studies on cholecystokinin: analogues with partial agonist activity. Am J Physiol. 1988 Feb;254(2 Pt 1):G176–G182. doi: 10.1152/ajpgi.1988.254.2.G176. [DOI] [PubMed] [Google Scholar]
- Gilliland L., Steer M. L. Effects of ethionine on digestive enzyme synthesis and discharge by mouse pancreas. Am J Physiol. 1980 Nov;239(5):G418–G426. doi: 10.1152/ajpgi.1980.239.5.G418. [DOI] [PubMed] [Google Scholar]
- Koike H., Steer M. L., Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol. 1982 Apr;242(4):G297–G307. doi: 10.1152/ajpgi.1982.242.4.G297. [DOI] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Niederau C., Ferrell L. D., Grendell J. H. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology. 1985 May;88(5 Pt 1):1192–1204. doi: 10.1016/s0016-5085(85)80079-2. [DOI] [PubMed] [Google Scholar]
- Ohshio G., Saluja A. K., Leli U., Sengupta A., Steer M. L. Esterase inhibitors prevent lysosomal enzyme redistribution in two noninvasive models of experimental pancreatitis. Gastroenterology. 1989 Mar;96(3):853–859. [PubMed] [Google Scholar]
- Powers R. E., Saluja A. K., Houlihan M. J., Steer M. L. Diminished agonist-stimulated inositol trisphosphate generation blocks stimulus-secretion coupling in mouse pancreatic acini during diet-induced experimental pancreatitis. J Clin Invest. 1986 May;77(5):1668–1674. doi: 10.1172/JCI112484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan P. T., Malagelada J. R., Go V. L., Wolf A. M., DiMagno E. P. A prospective study of the antisecretory and therapeutic effects of cimetidine and glucagon in human acute pancreatitis. Mayo Clin Proc. 1981 Aug;56(8):499–503. [PubMed] [Google Scholar]
- Saito I., Hashimoto S., Saluja A., Steer M. L., Meldolesi J. Intracellular transport of pancreatic zymogens during caerulein supramaximal stimulation. Am J Physiol. 1987 Oct;253(4 Pt 1):G517–G526. doi: 10.1152/ajpgi.1987.253.4.G517. [DOI] [PubMed] [Google Scholar]
- Saluja A., Hashimoto S., Saluja M., Powers R. E., Meldolesi J., Steer M. L. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol. 1987 Oct;253(4 Pt 1):G508–G516. doi: 10.1152/ajpgi.1987.253.4.G508. [DOI] [PubMed] [Google Scholar]
- Saluja A., Saito I., Saluja M., Houlihan M. J., Powers R. E., Meldolesi J., Steer M. In vivo rat pancreatic acinar cell function during supramaximal stimulation with caerulein. Am J Physiol. 1985 Dec;249(6 Pt 1):G702–G710. doi: 10.1152/ajpgi.1985.249.6.G702. [DOI] [PubMed] [Google Scholar]
- Sankaran H., Goldfine I. D., Bailey A., Licko V., Williams J. A. Relationship of cholecystokinin receptor binding to regulation of biological functions in pancreatic acini. Am J Physiol. 1982 Mar;242(3):G250–G257. doi: 10.1152/ajpgi.1982.242.3.G250. [DOI] [PubMed] [Google Scholar]
- Silverman M., Ilardi C., Bank S., Kranz V., Lendvai S. Effects of the cholecystokinin receptor antagonist L-364,718 on experimental pancreatitis in mice. Gastroenterology. 1989 Jan;96(1):186–192. doi: 10.1016/0016-5085(89)90779-8. [DOI] [PubMed] [Google Scholar]
- Stark H. A., Sharp C. M., Sutliff V. E., Martinez J., Jensen R. T., Gardner J. D. CCK-JMV-180: a peptide that distinguishes high-affinity cholecystokinin receptors from low-affinity cholecystokinin receptors. Biochim Biophys Acta. 1989 Feb 9;1010(2):145–150. doi: 10.1016/0167-4889(89)90154-7. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J., Figarella C. Pancreatitis. The role of lysosomes. Dig Dis Sci. 1984 Oct;29(10):934–938. doi: 10.1007/BF01312483. [DOI] [PubMed] [Google Scholar]
- Villanueva M. L., Collins S. M., Jensen R. T., Gardner J. D. Structural requirements for action of cholecystokinin on enzyme secretion from pancreatic acini. Am J Physiol. 1982 Apr;242(4):G416–G422. doi: 10.1152/ajpgi.1982.242.4.G416. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]



