Abstract
IL-27, consisting of the subunits IL-27p28 and Epstein–Barr virus-induced gene 3 (EBI3), is a heterodimeric cytokine belonging to the IL-6/IL-12 family of cytokines. IL-27p28 is a four-helical cytokine requiring association with the soluble receptor EBI3 to be efficiently secreted and functionally active. Computational and biological analyses of the IL-27 binding site 1 to its receptor revealed important structural proximities with the ciliary neurotrophic factor group of cytokines and highlighted the contribution of p28 Trp97, as well as of EBI3 Phe97, Asp210, and Glu159, as key residues in the interactions between both cytokine subunits. WSX-1 (IL-27R) and gp130 compose the IL-27 receptor-signaling complex, recruiting the STAT-1 and STAT-3 pathways. A study of IL-27 binding site 3 showed that Trp197 was crucial for the cytokine's interaction with gp130, but that the mutated cytokine still recognized IL-27R on the cell surface. IL-27 exerts both pro- and anti-inflammatory functions, promoting proliferation and differentiation of Th1 and inhibiting Th17 differentiation. Our results led us to develop mutated forms of human and mouse IL-27 with antagonistic activities. Using an in vivo mouse model of concanavalin A-induced Th1-cell–mediated hepatitis, we showed that the murine IL-27 antagonist W195A decreased liver inflammation by downregulating the synthesis of CXCR3 ligands and several acute phase proteins. Together, these data suggest that IL-27 antagonism could be of interest in down-modulating acute IL-27–driven Th1-cell–mediated immune response.
Keywords: Th1, cytokine, EBI3, structure, receptor
IL-27 belongs to the IL-6/IL-12 family of cytokines (1). This family of mediators also includes leukemia inhibitory factor, oncostatin M, IL-11, IL-23, ciliary neurotrophic factor (CNTF), cardiotrophin-like cytokine (CLC), neuropoietin (NP), cardiotrophin-1, and IL-31 (2–9).
IL-27 exerts both pro- and anti-inflammatory functions (10–12). This cytokine promotes Th1 differentiation by inducing the expression of T-bet through both signal transducer and activator of transcription 1 (STAT-1)-dependent and -independent mechanisms in the early stages of the immune response (13–15). IL-27 also displays an in vivo suppressive dominant role by down-modulating differentiation of the proinflammatory Th17-cell subset and inducing IL-10 secretion (16–18). Similarly, IL-27 can control parasitemia by suppressing Th2 responses through the STAT-1–mediated down-regulation of GATA-3 expression (14). Recently, IL-27 has been associated with T cell-mediated hepatitis in an acute liver injury model (19).
IL-27 is a heterodimer composed of a cytokine subunit, IL-27p28, and a soluble receptor, Epstein–Barr virus (EBV)-induced gene 3 (EBI3) (1, 20). Similar associations are also observed for the IL-12, IL-23, CNTF/soluble CNTF receptor α, and CLC/cytokine-like factor (CLF) composite cytokines (1, 4, 8, 20–22). IL-27p28 is a “long-chain” cytokine with a four-helix bundle fold; these four helices are named A–D from the N terminus to the C terminus (23). The EBI3 structure consists of a tandem pair of modified fibronectin type III (FnIII) domains named the cytokine-binding domain (CBD). This domain typically contains two pairs of cysteine residues involved in disulfide bridge formation and a characteristic WSXWS signature motif (24). The IL-27 receptor complex is composed of the gp130 and IL-27R transducing chains (25, 26). Binding of IL-27 to its heterodimeric receptor complex leads to the intracellular recruitment and activation of the Janus kinase (JAK)/STAT-1 and STAT-3 pathways (26).
The IL-6/IL-12 family of cytokines bind to their receptors through three independent contact sites numbered 1–3 by analogy with growth hormone and IL-6 (27, 28). Site 1 is composed of residues from the C-terminal parts of the AB loop and the αD helix (29). The counterpart of this binding site on the receptor α-chain involves loops located at the boundary of the two FnIII domains composing the CBD (22, 30). Cytokine binding site 2 is typically composed of solvent-exposed residues of the αA and αC helices and forms a contact site with a first signaling receptor chain. Site 3 is located at the N terminus of the αD helix and engages an Ig domain of a second signaling receptor, identified as gp130 in the case of IL-27.
Using a combination of computational structural analysis, site-directed mutagenesis, biochemical analyses, and functional biology approaches, we identified the IL-27p28 binding sites involved in the recruitment of its different receptor subunits. On the basis of cytokine structural analysis, we designed a mutated form of IL-27 with antagonistic and protective properties in a mouse model of T cell-mediated hepatitis.
Results
Sequence Analysis of IL-27.
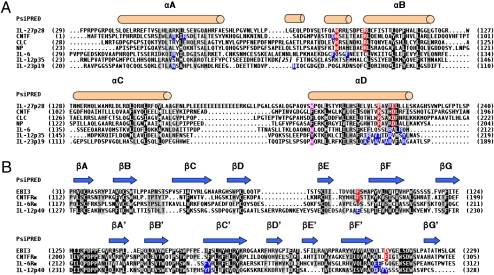
To identify residues involved in the contact sites between both IL-27 subunits as well as with its receptor components, we first performed structure-based multiple sequence alignments. IL-27p28 and EBI3 were aligned to orthologs and paralogs of cytokines and receptors belonging to the IL-6/IL-12 family (Fig. 1).
Fig. 1.
Structure-based multiple sequence alignments of IL-27p28 and EBI3 with paralogs belonging to the IL-6 family of cytokines. (A) Multiple sequence alignments of IL-27p28 with cytokines of the IL-6/IL-12 family. (B) Multiple sequence alignments of EBI3 with receptors of the IL-6/IL-12 family. Black-background letters indicate conserved or type-conserved amino acids according to the BLOSUM45 matrix; 80%-conserved residues are shaded in gray. The NP sequence is the mouse sequence, and the other sequences are human. The assignment of the secondary structure of IL-27p28 and EBI3 as predicted by Psipred is shown. Red-shaded letters indicate residues implicated in the IL-27-p28, CNTF, CLC, and NP binding site 1 and its counterparts in EBI3 and CNTFRα. Pink-shaded letters highlight residues implicated in IL-27p28 and IL-6 binding site 3. Blue-shaded letters indicate residues whose substitution by alanine affected the cytokine and receptor binding site 1 according to the literature.
We first analyzed the C-terminal part of the AB loop and the αD helix of IL-27p28 to determine a potential binding site 1. We searched for conserved amino acid motifs identified within the binding site 1 reported for IL-6, IL-11, CNTF, CLC, and NP (30–35). Interestingly, the Trp97 located in the AB loop of IL-27p28 is conserved with Trp64, Trp85, and Trp94 in CNTF, NP, and CLC, respectively. In previous studies, we demonstrated the key contribution of these residues for binding site 1 of this cytokine subgroup (Fig. 1A) (33, 34). Additional residues contributing to CNTF, NP, and CLC binding site 1 have been identified. They are part of an RXXXD motif located in the C-terminal part of their αD helix (Arg171 and Asp175 in CNTF, Arg190 and Asp194 in NP, and Arg197 and Asp201 in CLC) (33, 34). Interestingly, an equivalent RXXXE motif is conserved in the αD helix of human IL-27p28 (Arg216 and Glu220). In addition, the RXXXD motif is strictly conserved across all of the other IL-27p28 orthologs that we studied. This analysis clearly shows that IL-27p28, CNTF, CLC, and NP share conserved residues within their binding site 1.
Next, we studied EBI3 to identify a putative counterpart to the IL-27p28 site 1. Mutational analyses of interface residues previously carried out on IL-6Rα, IL-12p40, and CNTFRα allowed the identification of several hot-spot residues in the loops connecting the E and F, B′ and C′, and F′ and G′ β-strands (22, 30, 32, 34). These amino acids were highlighted in the multiple sequence alignment (Fig. 1B), and we analyzed residue conservation between EBI3 and these three related α-chains. The EF and B′C′ loops of EBI3 are conserved with those of CNTFRα (Fig. 1B). Interestingly, EBI3 Phe97, located in the EF loop, is conserved with CNTFRα Phe172, a residue that we previously identified as the binding hot spot for CNTFRα cognate ligands (34).
Site 2 identifies a region of interaction between IL-27p28 and IL-27R (28). This motif is less prominent and consists of exposed surfaces of αA and αC helices with multiple discrete and weak interaction points for IL-27R. A number of candidate residues in IL-27p28 were identified but not further analyzed (Fig. S1).
For IL-27p28, we also identified a residue potentially involved in its binding site 3 by homology with IL-6. Site 3 engages the Ig-like domain of gp130 in IL-6 and IL-27 receptor interactions. Crystallographic and mutagenesis studies have identified a tryptophan (IL-6 Trp185) as the key residue mediating IL-6 site 3 interaction with gp130 (26, 28, 30, 36). This tryptophan residue is conserved in IL-27p28 (IL-27p28 Trp197) (Fig. 1A).
In addition, the CD loop of IL-27p28 contained a stretch of 13 consecutive glutamic amino acid residues, which is unique and may be involved in the functional activity of the cytokine.
Molecular Modeling and Docking of IL-27p28 and EBI3.
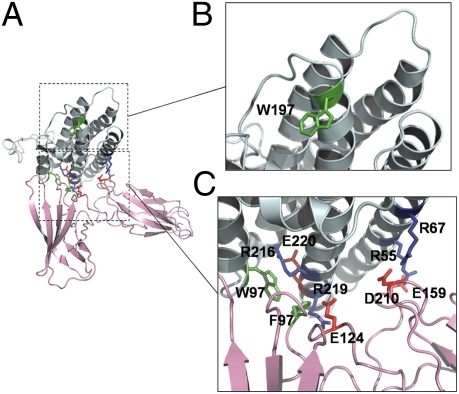
We subsequently built a model of the human p28/EBI3 composite cytokine by homology molecular modeling based on the multiple sequence alignments shown in Fig. 1. Due to the low sequence similarity throughout the IL-6/IL-12 cytokine family, HHpred software was used to determine the nearest template to use for IL-27p28, with the CNTF structure being retained. To improve the refinement of IL-27p28 site 3, the IL-6 structure was also punctually used. Due to its unique amino acid composition, the IL-27p28 CD loop containing the 13-glutamic-amino-acid stretch was de novo-modeled and integrated into the helical core of the protein.
Interactions were predicted using a rigid body docking algorithm, and constraints were based on surface complementarities of both proteins (Fig. S1). A ribbon representation of an IL-27 energy-minimized complex is presented in Fig. 2A. The IL-27p28 structure model adopts a four-helix bundle fold, with two additional short α-helices located in the AB loop of the cytokine subunit. The short α-helix located in the C-terminal end of the AB loop is involved in IL-27p28 site 1 and allows for the correct orientation of the Trp97 side chain. The Arg216 and Glu220 residues are involved in hydrogen bonds with Trp97 and stabilize its side chain in a solvent-exposed conformation (Fig. 2C).
Fig. 2.
Molecular modeling of IL-27. (A) Ribbon representation of IL-27: IL-27p28 and EBI3 are in cyan and pink, respectively. (B) Close-up view of the putative binding site 3 showing predicted hot-spot residue of IL-27p28. (C) Close-up view of the predicted binding site 1 showing the interacting residues at the interface of IL-27p28 and EBI3. Green residues are aromatic amino acids, blue residues are positively charged, and red residues are negatively charged.
The predicted IL-27p28/EBI3 interface is divided into two areas with distinct hydrophobic and polar properties. An aromatic cluster containing EBI3 Phe97 and IL-27p28 Trp97 dominates the hydrophobic area. Charged residues involved in a salt bridge network characterize the polar area. The positively charged surface of IL-27p28 is composed of arginine residues (Arg55, Arg67, and Arg219); these amino acids are located on the A and D helices. EBI3 Glu124, Glu159, and Asp210 form a negatively charged surface, which is the counterpart of the IL-27p28 positively charged area (Fig. S1). Interestingly, the interface between IL-27p28 and EBI3 mimics the interaction of CNTFRα with its different ligands (34). Moreover, the conserved phenylalanine in the EF loop of CNTFRα interacts with the conserved tryptophans located in the AB loop of its cognate ligands and is also surrounded by polar interactions between negatively and positively charged amino acids (34).
Importantly, IL-27p28 Trp197, located in binding site 3 at the tip of the predicted cytokine structure, is solvent-exposed and can potentially recruit the gp130-signaling receptor (Fig. 2B and below).
Site-Directed Mutagenesis of IL-27 Site 1.
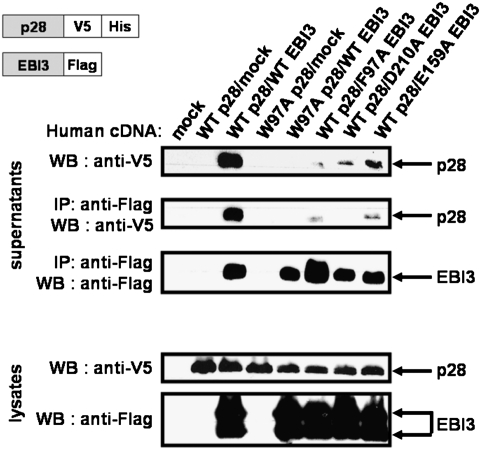
On the basis of the above observations, residues potentially involved in the IL-27p28/EBI3 interaction were selected, and the following mutations were introduced: IL-27p28 W97A, EBI3 F97A, EBI3 E159A, and EBI3 D210A. For protein identification, wild-type (WT) and mutated forms of IL-27p28 were tagged with a V5 epitope tag followed by a His tag, and EBI3 WT and mutant forms were tagged with a Flag epitope tag.
To be secreted and functionally active, human IL-27p28 requires association with EBI3 (1). We therefore tested the capacity for interaction between the IL-27 subunits by coexpressing their WT and mutant forms in mammalian cells. Corresponding cell lysates and culture supernatants were then analyzed for the presence of each protein by Western blot analyses. Fig. 3 shows that the IL-27p28 W97A mutation disrupts the formation and secretion of the heterodimeric cytokine, confirming the predicted contribution of Trp97 to the IL-27p28/EBI3 interaction. Similarly, mutant forms of EBI3 revealed the importance of the Phe97 and Asp210 residues for the stability of the IL-27 heterodimer. Contribution of the Glu159 residue to the site 1 interaction appeared less critical. These results are in accordance with our predictions and underline the key role of site 1 residues in heterodimer formation and secretion.
Fig. 3.
IL-27/EBI3 binding site 1 study. Cell supernatants were harvested 48 h after transfection, and immunoprecipitations were performed using an anti-Flag mAb. Western blots were performed either directly on culture supernatant and lysates or after an immunoprecipitation step using an appropriate antibody.
Site-Directed Mutagenesis of IL-27 Site 3.
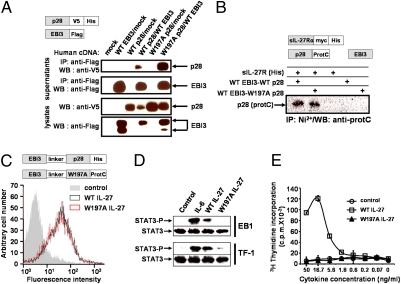
Subsequently, we studied the predicted IL-27 binding site 3. For this purpose, a W197A mutation was introduced into IL-27p28, and this mutant form was tested using the approach described above. Significantly, the W197A IL-27p28 mutant was still able to interact with EBI3 as a stable secreted hetero-complex, as detected by Western blot in Fig. 4A. This observation confirmed that Trp197 was not involved in the cytokine binding site 1 and that the W197A mutation did not significantly alter IL-27p28 folding and structure.
Fig. 4.
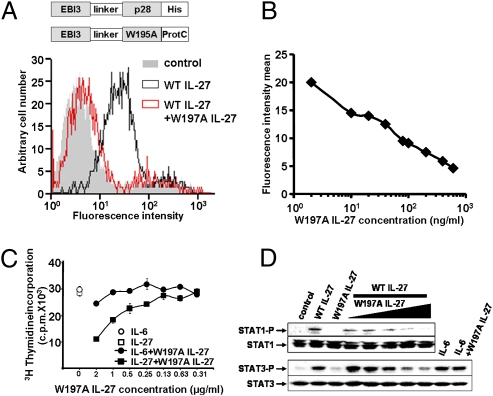
Effects of the W197A mutation on IL-27 secretion, interaction properties, and biological activities. (A) Secretion and interaction of W197A IL-27p28 with WT EBI3. Cos-7 cells were transfected with the WT or mutated form of IL-27p28 and with WT EBI3. Western blot was performed after an immunoprecipitation step using an anti-Flag mAb or on cell lysates. (B) Interaction of WT and W197A EBI3-p28-protC with soluble IL-27R. A soluble form of IL-27R-His was incubated with WT or mutant EBI3-p28protC. After an IL-27R immunoprecipitation step, IL-27p28 association was detected using the anti-ProtC mAb. (C) Binding of hyper WT (black histogram) or hyper W197A IL-27 (red histogram) on EB1 cells was analyzed by flow cytometry. The control histogram is in gray. (D) STAT-3 phosphorylation analysis induced by 50 ng/mL of WT or W197A IL-27 and 5 ng/mL of IL-6 in EB1 and TF-1 cell lines. (E) Proliferation analysis of TF-1 cell line in response to serial dilutions of WT (□) or W197A (▲) IL-27.
We next determined the ability of the W197A IL-27p28/EBI3 composite cytokine to bind IL-27R through its site 2. For this purpose, a histidine-tagged soluble form of IL-27R was incubated with the W197A mutant. Interactions between the modified composite cytokine and IL-27R were then monitored by coimmunoprecipitation. The W197A mutation did not abrogate IL-27 interaction with IL-27R, suggesting that the mutation did not affect the cytokine binding site 2 (Fig. 4B).
To further investigate the biological activities of the IL-27 heterodimer mutant, we engineered tagged fusion proteins (1, 37) (hyper IL-27) by linking the EBI3 chain to the IL-27p28 W197A mutant or to WT IL-27p28. WT and mutant hyper IL-27s were incubated with EB1, an EBV-transformed B-cell line that spontaneously expresses the IL-27 bipartite receptor. Cytokine binding to the cell surface was then monitored by flow cytometry, and similar levels of fluorescence intensity were detected for both WT and mutant variants, indicating that our modified form of IL-27 was still able to bind to the surface of the target cell (Fig. 4C).
We wanted to determine whether the W197A mutant could recruit the STAT-3 signaling pathway. Experiments were carried out using EB1 cells and the erythroleukemia cell line, TF1, also expressing both IL-27 receptor components. A clear induction of STAT-3 Tyr phosphorylation was observed when the cells were incubated in the presence of the WT cytokine, but no STAT-3 phosphorylation could be detected in response to W197A IL-27 (Fig. 4D). Identical results were obtained with STAT1.
In a similar vein, we studied the proliferation of the TF-1 cells in response to both WT and mutant IL-27. As expected, TF1 cells showed no proliferation in the presence of the mutated form of the composite cytokine whereas a clear signal was observed with the WT form (Fig. 4E).
Taken together, these results indicate that, despite its binding to IL-27R, the W197A mutant form of IL-27 fails to activate the bipartite IL-27 receptor and initiate subsequent signaling events.
Finally, to evaluate a putative functional role for the 13-glutamic-amino-acid repeat, this sequence was deleted from the IL-27p28 CD loop. The ΔE13p28 mutant was coexpressed either with EBI3 using an IRES vector or as a fusion protein linking to the EBI3 moiety. The results obtained showed an almost total extinction of secretion of the composite cytokine when both subunits were expressed together, indicating that the E13 stretch contributed to the folding and/or to the recognition of p28 by EBI3 (Fig. S2 A–C). Interestingly, the ΔE13 hyper IL-27 was well secreted as a fusion protein but failed to bind to its receptor complex in contrast to the parental hyper IL-27, reinforcing the idea of a p28 partial denaturation when the motif is deleted (Fig. S2).
Antagonistic Activities of Human W197A IL-27.
Previous studies described the development of nonsignaling interleukin antagonist molecules (38, 39). For cytokines related to IL-27, it has been well documented that selective mutation of key residues could abrogate binding to site 3 while maintaining binding to sites 1 and 2 (40). We therefore tested the W197A mutant's ability to compete for receptor binding with the WT form of the cytokine. For this purpose, EB1 cells were incubated with WT IL-27 alone or with both IL-27 and varying amounts of W197A protein (Fig. 5 A and B). Cell-surface binding of WT IL-27, tagged with a histidine epitope, was monitored by flow cytometry. The presence of W197A IL-27 led to a near-complete inhibition of WT IL-27 binding at the highest concentration of mutant tested (representing a 400-fold molar excess over WT).
Fig. 5.
Antagonistic activities of W197A IL-27. (A) Inhibition of WT IL-27-His binding on cells by W197A IL-27. After a preincubation step with 600 ng of W197A IL-27 for 30 min at 4 °C, 2 ng of WT IL-27 was added to EB1 cells for 30 min more. IL-27 binding was detected by flow cytometry using an anti-His mAb. (B) The same experiment was repeated by preincubating EB1 cells with increasing concentrations of W197A IL-27, and IL-27 binding was measured by flow cytometry. Fluorescence intensity means were then plotted against W197A IL-27 concentrations to determine its dose-dependent antagonistic effect. (C) TF1 cell line proliferation analysis. Cells were grown in the presence of 10 ng/mL of IL-27 or 0.5 ng/mL of IL-6 and W197A IL-27 serial dilutions. (D) Inhibition of STAT-1 and STAT-3 phosphorylation by W197A IL-27. TF-1 cells were preincubated with increasing concentrations of W197A IL-27 (0.25–4 μg/mL) and were activated for 10 min with 10 ng/mL of WT IL-27. As control, cells were preincubated with 4 μg/mL of W197A IL-27 and activated with 5 ng/mL of IL-6.
Additional experiments were carried out to characterize the properties of the W197A IL-27 antagonist using TF-1 cells. Similar to the observations above, increasing concentrations of W197A IL-27 progressively decreased the cell proliferation and STAT-1/STAT-3 tyrosine phosphorylation observed in response to IL-27 (Fig. 5 C and D). These data demonstrate that W197A IL-27 behaves as an antagonist to WT IL-27 in vitro.
Antagonist Activities of Mouse W195A IL-27.
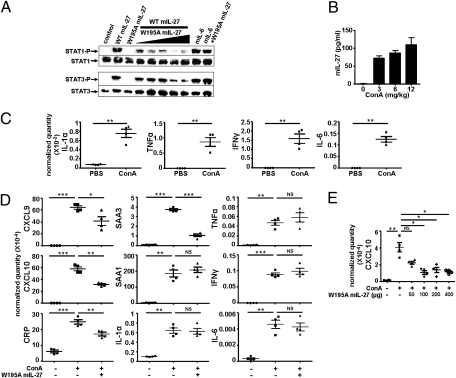
We subsequently produced a mouse equivalent of human hyper W197A IL-27 and studied its neutralizing effects in vivo. For this purpose Trp195, corresponding to the Trp197 residue in humans, was substituted by alanine in the mouse sequence. First, we determined the antagonistic capacity of the W195A murine hyper construct to neutralize IL-27–induced signal transduction using mouse splenocytes. We observed that increasing mutant concentrations inhibited the induction of STAT-1 and STAT-3 phosphorylation (Fig. 6A).
Fig. 6.
Antagonistic activities of mouse W195A IL-27. (A) Inhibition of STAT-1 and STAT-3 phosphorylation by W195A mIL-27. Mouse splenocytes were preincubated with increasing concentrations of W195A mIL-27 (0.25–4 μg/mL) and were activated for 10 min with WT mIL-27 (10 ng/mL). As control, cells were preincubated with 4 μg/mL W197A mIL-27 and then activated with 5 ng/mL mIL-6. (B) Mouse ConA treatment. After 4 h, blood was collected, and mIL-27 was ELISA-quantified in the serum. (C) Mice were treated with a 3-mg/kg ConA injection. After 4 h, the liver was removed, the RNAs were extracted, and expression of IL-1α, TNFα, IFNγ, and IL-6 was analyzed by qPCR. Results are expressed in relative quantity normalized with housekeeping genes. (D) Mice received a first injection of 200 μg W195A mIL-27 or PBS in the tail vein before a ConA injection 15 min later. RNA extraction was carried out at 4 h, as described in B and C. Expression of chemokines, cytokines, and acute-phase proteins was determined by qPCR. (E) Dose–response effect of W195A IL-27. Increasing concentrations of W195A IL-27 were injected to mice. The samples were handled and analyzed as in C and D.
As IL-27 was recently shown to play a central pathogenic role in the concanavalin A (ConA) model of Th1-cell–mediated liver injury, we tested W195A IL-27 as an antagonist in this model. A significant increase in circulating levels of IL-27 was detected upon ConA administration, in agreement with the Siebler et al. study (Fig. 6B) (19), as well as a marked induction of liver proinflammatory cytokines (Fig. 6C). From a high-throughput screening assay, we determined that liver transcripts of the Th1 cell chemokines CXCL9 and CXCL10 were extremely sensitive to ConA treatment (Fig. 6D). Four hours after injection of ConA, or of W195A IL-27 plus ConA, liver RNAs were extracted and expression of both chemokines was determined. The results obtained showed a clear reduction in CXCL9 and CXCL10 transcription levels in mice treated with W195A IL-27 (Fig. 6 D and E). In addition, the induction of expression of the acute phase proteins (serum amyloid A) SAA3 and C-reactive protein were significantly reduced after treatment, whereas expression of other acute-phase reactants, such as SAA1, which are mainly driven by IL-1, IL-6, and TNFα (41), remained insensitive to the injection of W195 IL-27 (Fig. 6D).
In conclusion, the W195A mutated form of IL-27 was able to antagonize the endogenous wild-type form of the cytokine in vivo in a model of Th1-cell–mediated liver injury and to subsequently neutralize part of the liver proinflammatory cascade.
Discussion
IL-27 is a key regulator of the immune system and appears to act as both a pro- and an anti-inflammatory cytokine (11). The dual role of this cytokine suggests that it may be an interesting therapeutic target as an immune modulator. Therefore, it is of interest to design specific antagonists of IL-27 activity using small molecules, antibodies, or engineered proteins.
IL-27 is a heterodimeric cytokine composed of the four-helical cytokine IL-27p28 and the soluble receptor EBI3. IL-27p28 has to be associated with EBI3 for efficient secretion and functional activity (1). Computational and site-directed mutagenesis analyses have shown that IL-27p28 Trp97 and EBI3 Phe97 residues form an aromatic–aromatic interaction surrounded by salt bridges at the cytokine–receptor interface. Similar interactions were previously observed at the contact sites for related cytokine–receptor α-chain complexes such as the IL-6/IL-6Rα, IL-11/IL-11Rα, IL-12p35/p40, or IL-23p19/p40 (22, 30, 32, 42, 43). The characterization of IL-27 site 1 showed that the key aromatic residues involved in the IL-27p28–EBI3 interaction are conserved with amino acids contributing to the interaction between CNTFRα and its three different ligands: CNTF, CLC, and NP (31, 33, 34, 44). These structural similarities were unexpected considering the different physiological roles of the IL-27R and CNTFR pathways; however, we failed to detect any interaction between IL-27p28 and CNTFR, in agreement with the distinct tissue expressions of these two genes (1, 45, 46).
A study of IL-27 binding site 3, which engages the Ig-like domain of gp130 (28), showed that Trp197 was crucial for this interaction. By analogy with an IL-4 mutein converting the cytokine to an antagonist by a single amino acid replacement (38, 39), we carried out the substitution of Trp197 with an alanine residue. Site-directed mutagenesis of this residue led to the development of an IL-27 antagonist. Interestingly, the equivalent residue in CNTF has previously been substituted to generate a mutant neutralizing the CNTF receptor pathway (47). The present study indicates that a similar approach can be used to inhibit IL-12–type composite cytokines.
A recent publication reported the association of p28 with the soluble cytokine receptor CLF to form another composite receptor structurally related to IL-27 (48). It would be interesting to identify the nature of the contact site(s) involved in the p28–CLF interaction, as well as the possibility that the present IL-27 antagonist can also neutralize this newly described composite cytokine.
IL-12 family members play essential roles in the regulation of T-helper-cell differentiation. IL-27 is unique in that it induces Th1 differentiation and suppresses Th2 and Th17 immune responses. In IL-27R–deficient mice, the hyper-production of various proinflammatory cytokines was reported, concomitant with severe inflammation in affected organs (16–18, 25, 49–52). Furthermore, the study of Siebler et al. demonstrated that IL-27 acted very early in a ConA model of Th1-cell–mediated liver injury, in agreement with the pro-Th1 activity of IL-27 (1, 19). We have shown that our IL-27 antagonist can moderate liver inflammation by down-regulating the synthesis of CXCR3 ligands, as well as a subset of acute-phase proteins.
In conclusion, we have identified the key residues contributing to IL-27 binding sites 1 and 3 on the basis of computational predictions. The results obtained underline the structural similarities between the CNTF cytokine subgroup and IL-27. Our data allowed us to develop an antagonist for IL-27 as a tool to study models of acute immune diseases and Th1-cell polarization.
Materials and Methods
Sequence Alignments and Molecular Modeling.
Sequence alignments and protein modeling were performed using T-Coffee and Modeler, respectively (34, 44). Models were energy minimized with Profiles-3D and Procheck softwares (SI Materials and Methods). Models will be made available upon request.
Site-Directed Mutagenesis and Quantitative PCR.
Hyper IL-27 constructs were generated by linking EBI3 cDNA with an oligonucleotide encoding a (G4S)2 polypeptide in 3′ and cloning it into a pcDNA3.1 vector (Invitrogen) before an insertion of IL-27p28 cDNA. P28 and EBI3 bicistronic expression was carried out using the pIRES vector (Clontech). For quantitative PCR (qPCR), RNAs were reversed-transcribed using random hexamer primers and SuperScript II reverse transcriptase. The ΔCt method was used for quantification (SI Materials and Methods).
Cells, Reagents and Protein Purification, and Protein Analyses.
Cos-7, HEK-293, TF1, and EB1 cells were grown as described (2, 44). Recombinant proteins, generated by transfecting the Cos-7 or the HEK-293 cell lines, were purified by affinity chromatography followed by an anionic column HPLC step. Protein and cell analyses were carried out as previously described (33, 34, 44) (SI Materials and Methods).
Mouse Model.
BALB/c mice were injected with W195A mIL-27 and 3 mg/kg of ConA as described (19) (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank G. Elson (NovImmune) for his helpful comments and review of the paper. We thank P. Chiron, Y. Danger, J. Gayon, L. Grimaud, C. Guillet, and E. Ravon for helpful technical assistance. We thank O. Devergne (UMR CNRS 8147, Hôpital Necker, Paris) for providing an anti-EBI3 mAb. L.B. was supported by a grant from the Ministère de la Recherche et de l'Enseignement Supérieur. This study was supported by the Ciblage Moléculaire et Applications Thérapeutiques Program from Région Pays de la Loire.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005793107/-/DCSupplemental.
References
- 1.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Derouet D, et al. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proc Natl Acad Sci USA. 2004;101:4827–4832. doi: 10.1073/pnas.0306178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 5.Senaldi G, et al. Novel neurotrophin-1/B cell-stimulating factor-3: A cytokine of the IL-6 family. Proc Natl Acad Sci USA. 1999;96:11458–11463. doi: 10.1073/pnas.96.20.11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 7.Lin LF, et al. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF) Science. 1989;246:1023–1025. doi: 10.1126/science.2587985. [DOI] [PubMed] [Google Scholar]
- 8.Davis S, et al. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science. 1993;259:1736–1739. doi: 10.1126/science.7681218. [DOI] [PubMed] [Google Scholar]
- 9.Dillon SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 10.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med. 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 11.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: Related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: A double-edged sword for offense and defense. J Leukoc Biol. 2009;86:1295–1303. doi: 10.1189/jlb.0609445. [DOI] [PubMed] [Google Scholar]
- 13.Takeda A, et al. Cutting edge: Role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 14.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamano S, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 16.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 17.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 18.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 19.Siebler J, et al. Cutting edge: A key pathogenic role of IL-27 in T cell-mediated hepatitis. J Immunol. 2008;180:30–33. doi: 10.4049/jimmunol.180.1.30. [DOI] [PubMed] [Google Scholar]
- 20.Devergne O, et al. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elson GC, et al. CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat Neurosci. 2000;3:867–872. doi: 10.1038/78765. [DOI] [PubMed] [Google Scholar]
- 22.Yoon C, et al. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. EMBO J. 2000;19:3530–3541. doi: 10.1093/emboj/19.14.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazan JF. Neuropoietic cytokines in the hematopoietic fold. Neuron. 1991;7:197–208. doi: 10.1016/0896-6273(91)90258-2. [DOI] [PubMed] [Google Scholar]
- 24.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 26.Pflanz S, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 27.de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: Crystal structure of the complex. Science. 1992;255:306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 28.Skiniotis G, Lupardus PJ, Martick M, Walz T, Garcia KC. Structural organization of a full-length gp130/LIF-R cytokine receptor transmembrane complex. Mol Cell. 2008;31:737–748. doi: 10.1016/j.molcel.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bravo J, Heath JK. Receptor recognition by gp130 cytokines. EMBO J. 2000;19:2399–2411. doi: 10.1093/emboj/19.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 31.Panayotatos N, et al. Localization of functional receptor epitopes on the structure of ciliary neurotrophic factor indicates a conserved, function-related epitope topography among helical cytokines. J Biol Chem. 1995;270:14007–14014. doi: 10.1074/jbc.270.23.14007. [DOI] [PubMed] [Google Scholar]
- 32.Kalai M, et al. Analysis of the human interleukin-6/human interleukin-6 receptor binding interface at the amino acid level: Proposed mechanism of interaction. Blood. 1997;89:1319–1333. [PubMed] [Google Scholar]
- 33.Perret D, et al. Two different contact sites are recruited by cardiotrophin-like cytokine (CLC) to generate the CLC/CLF and CLC/sCNTFRalpha composite cytokines. J Biol Chem. 2004;279:43961–43970. doi: 10.1074/jbc.M407686200. [DOI] [PubMed] [Google Scholar]
- 34.Rousseau F, et al. Ciliary neurotrophic factor, cardiotrophin-like cytokine, and neuropoietin share a conserved binding site on the ciliary neurotrophic factor receptor alpha chain. J Biol Chem. 2008;283:30341–30350. doi: 10.1074/jbc.M803239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tacken I, et al. Definition of receptor binding sites on human interleukin-11 by molecular modeling-guided mutagenesis. Eur J Biochem. 1999;265:645–655. doi: 10.1046/j.1432-1327.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 36.Hammacher A, et al. The immunoglobulin-like module of gp130 is required for signaling by interleukin-6, but not by leukemia inhibitory factor. J Biol Chem. 1998;273:22701–22707. doi: 10.1074/jbc.273.35.22701. [DOI] [PubMed] [Google Scholar]
- 37.Fischer M, et al. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol. 1997;15:142–145. doi: 10.1038/nbt0297-142. [DOI] [PubMed] [Google Scholar]
- 38.Kruse N, Tony HP, Sebald W. Conversion of human interleukin-4 into a high affinity antagonist by a single amino acid replacement. EMBO J. 1992;11:3237–3244. doi: 10.1002/j.1460-2075.1992.tb05401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller T, Sebald W, Oschkinat H. Antagonist design through forced electrostatic mismatch. Nat Struct Biol. 1994;1:674–676. doi: 10.1038/nsb1094-674. [DOI] [PubMed] [Google Scholar]
- 40.Kallen KJ, Grötzinger J, Rose-John S. New perspectives on the design of cytokines and growth factors. Trends Biotechnol. 2000;18:455–461. doi: 10.1016/s0167-7799(00)01492-x. [DOI] [PubMed] [Google Scholar]
- 41.Thorn CF, Whitehead AS. Differential transcription of the mouse acute phase serum amyloid A genes in response to pro-inflammatory cytokines. Amyloid. 2002;9:229–236. doi: 10.3109/13506120209114098. [DOI] [PubMed] [Google Scholar]
- 42.Harmegnies D, et al. Characterization of a potent human interleukin-11 agonist. Biochem J. 2003;375:23–32. doi: 10.1042/BJ20030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lupardus PJ, Garcia KC. The structure of interleukin-23 reveals the molecular basis of p40 subunit sharing with interleukin-12. J Mol Biol. 2008;382:931–941. doi: 10.1016/j.jmb.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rousseau F, et al. Inactivation of cardiotrophin-like cytokine, a second ligand for ciliary neurotrophic factor receptor, leads to cold-induced sweating syndrome in a patient. Proc Natl Acad Sci USA. 2006;103:10068–10073. doi: 10.1073/pnas.0509598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ip NY, et al. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993;10:89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- 46.MacLennan AJ, et al. Immunohistochemical localization of ciliary neurotrophic factor receptor alpha expression in the rat nervous system. J Neurosci. 1996;16:621–630. doi: 10.1523/JNEUROSCI.16-02-00621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Marco A, et al. Identification of ciliary neurotrophic factor (CNTF) residues essential for leukemia inhibitory factor receptor binding and generation of CNTF receptor antagonists. Proc Natl Acad Sci USA. 1996;93:9247–9252. doi: 10.1073/pnas.93.17.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crabé S, et al. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J Immunol. 2009;183:7692–7702. doi: 10.4049/jimmunol.0901464. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida H, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 50.Bancroft AJ, Humphreys NE, Worthington JJ, Yoshida H, Grencis RK. WSX-1: A key role in induction of chronic intestinal nematode infection. J Immunol. 2004;172:7635–7641. doi: 10.4049/jimmunol.172.12.7635. [DOI] [PubMed] [Google Scholar]
- 51.Pearl JE, et al. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 52.Artis D, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.