Abstract
Drug addiction is a chronic relapsing disorder characterized by compulsive drug seeking and use. Environmental conditioning factors are among the major determinants of relapse in abstinent cocaine users. Here we describe a role of the neuropeptide S (NPS) system in regulating relapse. In rats with a history of cocaine self-administration, presentation of stimuli predictive of drug availability reinstates drug seeking, triggering relapse. Intracerebroventricular (ICV) injection of NPS increased conditioned reinstatement of cocaine seeking, whereas peripheral administration of the NPS receptor antagonist SHA 68 reduced it. Manipulation of the NPS receptor system did not modify cocaine self-administration. We also found that ICV NPS administration activates c-Fos expression in hypocretin-1/orexin-A (Hcrt-1/Ox-A) immunoreactive neurons in the lateral hypothalamus (LH) and in the perifornical area (PeF). Of note, intra-LH and intra-PeF administration of NPS increased conditioned reinstatement of cocaine responding, an effect that was selectively blocked with the Hcrt-1/Ox-A receptor selective antagonist SB334867. Finally, results showed that intra-LH injection of the NPS antagonist [D-Cys(tBu) (5)]NPS blocked cue-induced cocaine seeking, indicating a role for this system in the pathophysiology of drug relapse.
In 2004, Xu and colleagues reported the deorphanization of a novel neuropeptide system named neuropeptide S (NPS). NPS binds to Gs and Gq protein-coupled receptors, previously identified as GPR154 and now referred to as NPSR (1, 2). NPS peptide transcript is expressed predominantly in a small group of neurons located between the locus ceruleus (LC), the Barrington nucleus, and the parabrachial nuclei. NPSR mRNA is expressed throughout the central nervous system with the highest concentration in olfactory structures, the amygdaloid complex, the paraventricular thalamic nucleus, the subiculum, and the lateral (LH), dorsomedial (DMH), and ventromedial hypothalamus (VMH) (1, 2).
Activation of NPSR by intracerebroventricular (ICV) injection of NPS stimulates locomotor activity, increases arousal, and suppresses all stages of sleep (1). ICV injection of NPS has also been shown to stimulate hypothalamic–pituitary–adrenal axis (HPA) activity, enhancing plasma adrenocorticotropic hormone (ACTH) and corticosterone levels (3). Our group has recently found that intra-LH injections of NPS facilitate conditioned reinstatement of alcohol seeking (4). In addition, Pañeda and coworkers reported that ICV NPS itself increased relapse-like behavior in mice previously trained to self-administer cocaine. This latter effect was blocked by CRF1R antagonism and was absent in CRF1 receptor KO mice, suggesting a stress-like effect of NPS under the experimental conditions used (5).
In the present study, to examine further the significance of the NPS system in the pathophysiology of cocaine craving and relapse, we studied the effect of NPS and of the selective NPSR antagonists SHA 68 (3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide) and [D-Cys(tBu) (5)]NPS on cocaine self-administration and on cue-induced reinstatement of cocaine seeking. In addition, guided by c-Fos expression and immunocolocalization mapping studies, we evaluated the effect of NPS on cue-induced cocaine relapse following microinjections into the LH, the perifornical area (PeF), the DMH, and, for control, in the central amygdala (CeA). Finally, we investigated at a functional level whether these NPS effects depend upon interactions with the hypothalamic hypocretin-1/orexin-A (Hcrt-1/Ox-A) system.
Results
NPS Receptor Activation or Blockade Did Not Modify Cocaine Self-Administration in the Rat.
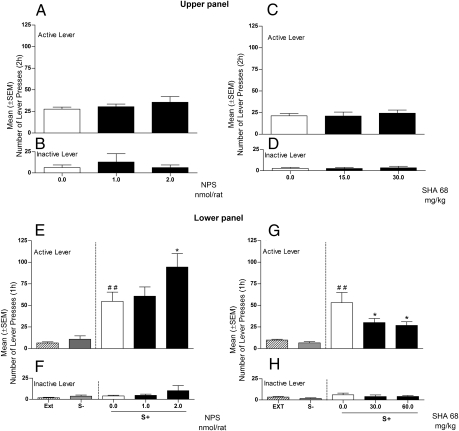
Rats were trained to self-administer cocaine under a fixed ratio (FR)-5 schedule of reinforcement (one cocaine dose: 0.25 mg/0.1 mL, i.v., administered over 10 s). In a Latin square within-subjects design, rats (n = 8) were ICV injected with NPS (0.0, 1.0, and 2.0 nmol). Rats rapidly reached a stable baseline of cocaine self-administration that was not affected by treatment with NPS (F2,7 = 1.139; NS) (Fig. 1A). Responses at the inactive lever were not affected by NPS (Fig. 1B). A second group of animals (n = 7) was treated with the selective NPS receptor antagonist SHA 68. Results showed that cocaine self-administration is not affected by SHA 68 treatment (F2,6 = 0.037; NS), confirming that the NPS system does not play a role in cocaine reinforcement (Fig. 1 C and D).
Fig. 1.
(Upper) Effect of ICV treatment with NPS (0.0, 1.0, and 2.0 nmol/rat) or with the NPS antagonist SHA 68 (0.0, 30, and 60 mg/kg) on cocaine self-administration under an FR5 schedule of reinforcement. One group of rats (n = 8) was treated with NPS and another group (n = 7) with SHA 68. (A and C) Active lever; (B and D) inactive lever. (Lower) Effects of ICV injection of NPS (0.0, 1.0, and 2.0 nmol/μL/rat) or IP injection of SHA 68 on cue-induced reinstatement of cocaine seeking (n = 8/group). Reinstatement responses in rats exposed to S+ and S− condition (in the absence of reward delivery) compared with the mean number of lever presses of the last 3 d of extinction (EXT). (E and G) Mean (±SEM) number of responses at the cocaine active lever. (F and H) Mean (±SEM) number of responses at the cocaine inactive lever. Different from extinction, ##P < 0.01. Different from vehicle-treated rats (controls), *P < 0.05.
NPS Increased and SHA 68 Decreased Conditioned Reinstatement of Cocaine Seeking.
The second series of experiments evaluated the effects of targeting the NPS receptor on conditioned reinstatement of cocaine-seeking behavior in a well-established animal model of relapse (6). Briefly, during an initial conditioning phase, rats (n = 16) were trained to self-administer cocaine or saline under an FR1 schedule of reinforcement, and to associate the availability of cocaine or saline with discrete contextual stimuli. Cocaine availability was signaled by an intermittent tone (7 kHz, 70 dB), present throughout the session, which served as a discriminative stimulus (S+). Saline availability was signaled by continuous illumination of the chamber's houselight (S−). To prevent accidental overdosing, drug infusions were followed by a 20-s time-out period (TO), signaled by illumination of a white cue light above the active lever during which time responses at this lever did not result in cocaine delivery. Saline infusions produced by lever presses during S− sessions were similarly followed by a 20-s TO period, signaled by a white noise. Self-administration sessions under these contingencies were conducted in three daily 1-h sessions for 10–12 d. Each training day included one saline and two cocaine sessions conducted in random sequence. Sessions lasted 1 h each. During this phase, a significant overall effect of cocaine vs. saline availability on responding was observed with reliable responding during cocaine sessions and cessation of responding during saline sessions (F1,15 = 193.68; P < 0.001). Following this self-administration/conditioning phase, rats were subjected to repeated extinction sessions (1 h/d) during which cocaine, saline, and associated cues were absent. Responding during these extinction sessions progressively decreased from 21 ± 4.9 on the first day to 5 ± 1.6 on the last extinction day. Reinstatement tests (1 h) began 1 d after the last extinction session by reintroducing the cocaine or saline-associated stimuli. For the reinstatement test, one group of rats (n = 8) was injected ICV with NPS (0.0, 1.0, and 2.0 nmol), while the second group (n = 8) was treated IP with SHA 68 (0.0, 30.0, and 60 mg/kg). To confirm that the reinstatement of extinguished responding was controlled selectively by the cocaine S+, rats were tested with the S− on day 1 and the S+ on day 2, preceded on both testing occasions by injections of respective drug vehicles. ANOVA revealed that reintroduction of cues had a significant overall effect on responding (F2,7 = 13.43; P < 0.001 and F2,7 = 14.32; P < 0.001, for the NPS and the SHA 68 groups, respectively) (Fig. 1 E and G). In both groups of rats, post hoc analysis (Newman-Keuls test) revealed robust reinstatement of responding under the S+ (P < 0.001), but not the S− condition.
When in a counterbalanced order (Latin square design), rats were treated with NPS, results showed a significant increase in cocaine seeking (F2,7 = 6.254; P < 0.05). Post hoc comparisons confirmed a significant effect of NPS at the 2.0 nmol dose (P < 0.05) (Fig. 1E). In contrast to NPS, administration of SHA 68 significantly decreased cocaine seeking (F2,7 = 5.90; P < 0.01). Post hoc comparisons confirmed a significant effect of SHA 68 at both doses used (P < 0.05) (Fig. 1G). Inactive lever responding was not altered by treatments (Fig. 1 F and H).
NPS Does Not Affect Responding for Cues Predictive of Saline Availability.
To determine whether the response-reinstating effect associated with NPS system activation is selective, an additional group of rats (n = 8) was treated with NPS before presentation of cues (S−) predictive of the nonreward saline. Also this group of rats responded at significantly higher rates for cocaine compared with saline (F1,7 = 23.411; P < 0.001). During extinction, responding progressively decreased from 13 ± 2.03 on the first day to 5 ± 1.4 on the last extinction day. For reinstatement, a significant overall effect of cues was observed (F2,7 = 2.666; P < 0.001) (Fig. S1A). Post hoc tests confirmed significant reinstatement of responding under S+ (P < 0.001) but not under S− conditions. NPS, given before S− contingency, did not modify lever responding (F2,7 = 0.53; P = NS). Responses at the inactive lever were minimal and not affected by experimental manipulations (Fig. S1B).
NPS-Activated Neurocircuitry as a Candidate to Mediated Effects of NPS on Cocaine Seeking.
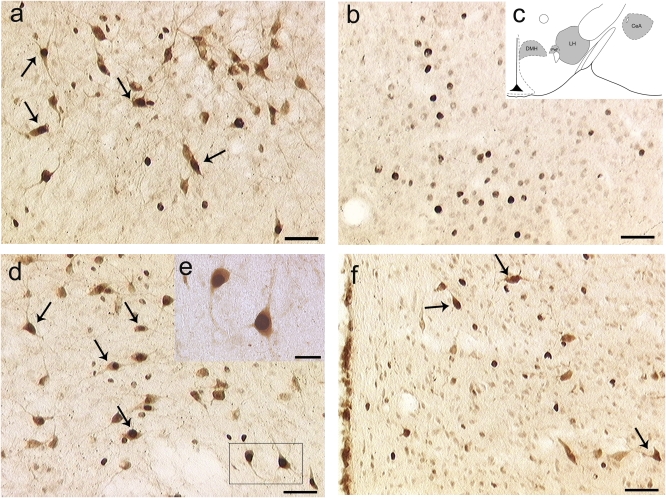
To identify the brain site of action for the NPS effects on reinstatement of cocaine seeking, two groups of rats (n = 4/group) were injected ICV with NPS (2 nmol/rat) or its vehicle, respectively. One hour after injection, animals were killed and brains were removed for c-Fos immunohistochemistry. On the basis of pilot data showing that NPS induces specific c-Fos activation in several midbrain areas, the present experiment used coronal brain sections from AP-1.8 mm to AP-3.6 mm from Bregma (7). Results revealed a significantly higher number of c-Fos immunopositive cells in several hypothalamic structures and in the amygdala of NPS-treated rats compared with controls (Table S1 and Fig. S2). No differences were observed in the caudate putamen or in the ventral portion of the thalamus (Table S1). Notably, following NPS injection, c-Fos immunoreactive neurons were identified in all brain areas that are the primary sources for Hcrt-1/Ox-A projection neurons in the brain, namely the LH, PeF, and DMH. Considering the important role of Hcrt-1/Ox-A in the regulation of drug-seeking behavior (8–10), and knowing that NPS–hypocretin interactions have been described in regulation of alcohol-associated behaviors (4), we investigated whether NPS-induced c-Fos activation occurred in Hcrt-1/Ox-A neurons. For this purpose, additional brain sections from the same group of rats were double stained for c-Fos and Hcrt-1/Ox-A immunoreactivity. Results revealed that hypocretin-positive cells represent 45% of all c-Fos–positive neurons in the LH and PeF but only 8% in the DMH. Moreover, in the LH and in the PeF about 40% of hypocretin-immunoreactive cells are c-Fos positive. In the DMH, colocalization was found in ≈20% of the cells analyzed (Fig. 2 A, D, and F and Table S2). In the CeA, we found extensive c-Fos expression following ICV NPS injection. However, no evidence for colocalization or for Hcrt-1/Ox-A immunoreactivity was found (Fig. 2B). Lastly, we conducted in situ hybridization of NPSR mRNA, combined with immunohistochemistry for Hcrt-1/Ox-A. Confirming previously published data (2), we found that NPSR transcript is expressed in the anterior, dorsomedial, and lateral hypothalamic regions. Interestingly, combined immunocytochemistry with Hcrt-1/Ox-A antibody revealed a fraction (5.8%) of Hcrt-1/Ox-A neurons expressing NPSR mRNA (Fig. S3A), indicating the theoretical possibility that, at least a portion of Hcrt-1/Ox-A neurons, are directly activated by NPS. Of note, cells expressing NPSR transcript but negative to Hcrt-1/Ox-A were also identified (Fig. S3B). These cells were found adjacent to Hcrt-1/Ox-A immunopositive neurons, suggesting that there may be local circuitry through which NPS can indirectly modulate Hcrt-1/Ox-A neurotransmission.
Fig. 2.
Dual labeling of c-Fos and Hcrt-1/Ox-A immunoreactivity in: (A) the lateral hypothalamus (LH); (B) the central amygdala (CeA); (D) the perifornical area (PeF); (F) the dorsomedial hypothalamic nucleus (DMH) of rats (n = 4) injected with NPS (2 nmol) ICV. Coronal sections were taken at AP coordinates identical to those used for intra-LH, -CeA, -PeF, and -DMH NPS injections. c-Fos and Hcrt-1/Ox-A were visualized with biotinylated antibodies leading, respectively, to black and brown immunoreactive products. Arrows indicate colocalization of c-Fos (dark nuclei) with Hcrt-1/Ox-A (brown). (C) Depiction of coronal sections used for analysis of the LH, CeA, PeF, and DMH; (E) magnified detail of D, showing that in the LH and PeF, c-Fos and Hcrt-1/OX-A immunoreactivity are coexpressed in about 40% of the counted cells. In the DMH, coexpression was found in about 20% of the counted neurons. In the CeA, a detectable expression of c-Fos was also found but in the absence of Hcrt-1/OX-A immunoreactivity. [Scale bars, 50 μm (A, B, D, and F) and 20 μm (E).]
NPS and Hcrt-1/Ox-A Systems Interact to Promote Cocaine Seeking: Functional Evidence.
Guided by the immunohistochemical results, we evaluated the effects of site-specific microinjection of NPS into brain areas highly activated by ICV NPS. For this purpose, separate groups of rats (n = 7–8/group), subjected to the same cocaine self-administration and conditioning procedure as above, were microinjected with NPS (0.0, 0.1, and 0.5 nmol/rat) into the LH, PeF, DMH, and CeA. The LH, PeF, and DMH were chosen because combined immunocytochemistry suggested NPS can activate Hcrt-1/Ox-A neurons in these areas. The CeA was selected because it also shows substantial c-Fos activation after ICV NPS injection (Table S1); it is rich in NPSR transcript (2), but does not colocalize with Hcrt-1/Ox-A.
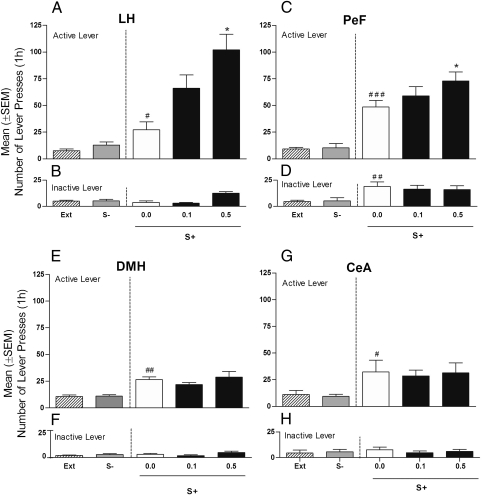
All groups of cannulated rats responded at a higher rate for cocaine than for saline [LH, (F1,6 = 89.58; P < 0.01); PeF, (F1,7 = 38.83; P < 0.001); DMH, (F1,7 = 10.63; P < 0.05); and CeA, (F1,6 = 6.15; P < 0.05)]. During the extinction phase, responding progressively decreased in all animals. Reexposure to the cocaine vs. nonreward cues produced a significant overall difference on responding in all four groups of rats difference from extinction: LH, (F2,6 = 4.06; P < 0.05); PeF, (F2,7 = 52.66; P < 0.001); DMH, (F2,7 = 28.28; P < 0.001); and CeA, (F2,6 = 6.151; P < 0.05) (Fig. 3 A, C, E, and G). Post hoc tests confirmed that responding was significantly increased during S+ but not S− presentation. NPS treatment significantly increased conditioned reinstatement in rats that received intra-LH (F2,6 = 9.41; P < 0.01) and intra-PeF administration (F2,7 = 3.83; P < 0.05) of the peptide, but not in DMH (F2,7 = 0.87; P > 0.05) or CeA microinjected animals (F2,6 = 0.069; P > 0.05). Post hoc comparisons revealed a significant effect of NPS at 0.1 (P < 0.05) and 0.5 nmol (P < 0.01) in the LH (i.e., at doses 10 times lower than those needed to reinstate cocaine seeking after ICV administration). A significant but less robust increase of cue-induced responding was also found in intra-PeF treated rats at 0.5 nmol (P < 0.05). For further evaluation of NPS effect in the DMH (an area where some activation of Hcrt-1/Ox-A cells following ICV NPS was observed), the same group of rats previously tested with NPS 0.1 and 0.5 nmol/rat was given a higher dose of the peptide (2.0 nmol/rat) or vehicle. At this dose, which is identical to the one effective after ICV injection, NPS elicited a significant (F2,7 = 22.98; P < 0.001) reinstatement of responding (Fig. S4). Inactive lever responses were very low in all experimental groups but were significantly increased by intra-LH injection of 0.5 nmol of NPS (P < 0.01) (Fig. 3B). This NPS effect was, however, an isolated finding because it was not observed in the other experiments where, as in this one, NPS was given into the LH (Fig. 4). Responding at the inactive lever was not modified following intra-PeF, intra-DMH, or intra-CeA NPS administration (Fig. 3 D, F, and H).
Fig. 3.
Effect of intracranial bilateral injection of NPS (0.1 and 0.5 nmol/0.6 μL/rat) or its vehicle (0.0) on cue-induced reinstatement of cocaine seeking. Reinstatement responses in rats (n = 7–8/group) exposed to S+ and S− conditions (in the absence of cocaine or saline) compared with the mean number of lever responses during the last 3 d of the extinction period (EXT). Mean (± SEM) number of responses at the (A) cocaine active and (B) inactive levers in rats with cannulae aimed at the LH. Mean (± SEM) number of responses at the (C) cocaine active and (D) inactive levers in rats with cannulae aimed at the PeF. Mean (± SEM) number of responses at the (E) cocaine active and (F) inactive levers in rats with cannulae aimed at the DMH. Mean (± SEM) number of responses at the (G) cocaine active and (H) inactive levers in rats with cannulae aimed at the CeA. Different from extinction, ##P < 0.01 and #P < 0.05. Different from vehicle-treated rats (controls), *P < 0.05 and **P < 0.01.
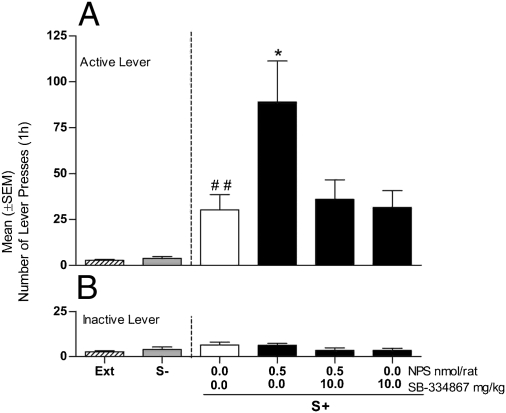
Fig. 4.
Effect of treatment with the Hcrt-1/Ox-A antagonist SB-334867 (10 mg/kg, i.p.) or its vehicle (0.0) on the consequences of intra-LH injection of NPS (0.5 nmol/0.6 μL/rat) or its vehicle (0.0) on cue-induced reinstatement of cocaine seeking in rats (n = 7). Reinstatement responses in rats exposed to the S+ and S− conditions (in the absence of cocaine or saline) compared with the mean number of lever responses during the last 3 d of extinction (EXT). (A) Mean (±SEM) number of responses at the active lever. (B) Mean (±SEM) number of responses at the inactive lever. Different from extinction, ##P < 0.01. Different from vehicle-treated rats (controls), *P < 0.05.
Given that the LH was the area most sensitive to the proreinstating actions of NPS, an additional group of rats (n = 8) was used to study the effect of intra-LH injection of the NPSR antagonist [D-cys(tBu) (5)]NPS (11). Consistent with findings obtained with the peripherally active NPS receptor antagonist SHA 68, results showed that intra-LH infusion of [D-Cys(tBu) (5)]NPS (30 nmol) significantly (P < 0.05) reduced cue-induced cocaine relapse (Fig. S5).
A final experiment was conducted to provide functional evidence for an interaction between the NPS and Hcrt-1/Ox-A systems within the LH. For this purpose, we tested for cue-induced reinstatement of cocaine seeking a group of rats (n = 7) treated with the selective Hcrt-1/OX-A receptor-1 antagonist SB-334867 (10 mg/kg, i.p.) given 30 min before intra-LH NPS (0.5 nmol) administration.
As in the preceding experiments, reexposure to the cocaine cue had a significant overall effect on cocaine seeking (F2,6 = 9.883; P < 0.01) compared with extinction, and intra-LH NPS treatment significantly increased this behavior (F3,6 = 4.75; P < 0.05) (Fig. 4A). Importantly, however, post hoc comparisons revealed a significant increase of cocaine-seeking behavior after NPS injection (P < 0.05) that was completely prevented by treatment with 10 mg/kg of SB-334867. At the dose tested, the Hcr-1/Ox-A receptor antagonist alone did not modify cue-induced reinstatement, which remained at the same level of vehicle-treated rats but was significantly higher compared with extinction responses (Fig. 4A). Inactive lever responses were not affected by NPS or SB-334867 (Fig. 4B).
Discussion
The major finding of this study was that NPS potently increases conditioned reinstatement of cocaine-seeking in rats, whereas selective blockade of NPS receptors significantly reduced it in a dose-dependent manner. Moreover, results indicated that NPS activates Hcrt1/Ox-A immunoreactive neurons in the hypothalamus and that in the LH there is a measurable expression of NPSR transcript in Hcrt1/Ox-A cells. In the LH, cells expressing NPSR mRNA but negative for Hcrt-1/Ox-A were also present. These cells were found in close vicinity to Hcrt-1/Ox-A neurons, suggesting the possibility that NPS may also regulate Hcrt-1/Ox-A function indirectly through local circuitry. An alternative possibility is that NPS activates distal neuronal pathways (i.e., in periventricular areas) that through indirect mechanisms may contribute to stimulate c-Fos expression in the hypothalamus. Of note, intra-LH injection of NPS significantly increased conditioned reinstatement of cocaine seeking. A similar effect was also observed after NPS injection into the PeF. In this structure, however, the effect of NPS required a higher dose, and it can therefore not be excluded that the PeF effect results from diffusion into the nearby LH. A key role for the LH is supported by additional experiments with the selective NPS receptor antagonist [D-Cys(tBu) (5)]NPS, which showed that blockade of NPSR within the LH is sufficient to abolish cue-induced cocaine relapse. Finally, results showed that increase in reinstatement responding following intra-LH NPS injection is abolished by pretreatment with the selective Hcrt1/Ox-A receptor antagonist SB-334867.
Control experiments indicated that central NPS injections do not affect lever responding for cues previously associated with ICV saline delivery. This finding, together with data showing that the peptide did not change operant responding at the inactive lever, demonstrates that NPS effects on reinstatement are selective and not secondary to an increase of locomotor activation or a general increase in arousal (1). Results also showed that neither exogenous NPS nor administration of selective NPSR antagonists affect cocaine self-administration. This suggests that the NPS system does not modulate the primary reinforcing properties of cocaine. Of note, in the present study NPS and NPS antagonists were used at doses that are specific and that do not lead to behavioral effects per se (11–13).
These data provide compelling support for a role of the NPS system in the regulation of cocaine craving. Our data indicate that activation of the NPSR facilitates Hcrt-1/Ox-A system activity in the hypothalamus, and that this activity, in turn, drives the marked increase of cocaine reinstatement observed upon NPS administration. A growing literature suggests that Hcrt-1/Ox-A plays a central role in the modulation of addiction-related behaviors across several classes of drugs of abuse, including cocaine, alcohol, and opiates (8–10). On the basis of these findings, Hcrt-1/Ox-A antagonism has been proposed as a possible mechanism for pharmacotherapy of drug addiction (8–10, 14). The present findings support the significance of the Hcrt-1/Ox-A system in the regulation of cocaine seeking and uncover an important role of the NPS system as an upstream modulator of the Hcrt-1/Ox-A system. Consistent with this view, our data indicate that blockade of NPS receptors like Hcrt-1/Ox-A antagonism prevents the reinstatement of cocaine seeking without blocking its intake (8–10, 15).
Although NPS and Hcrt-1/Ox-A appear to be organized in series in regulation of cocaine seeking, important differences exist between these two systems in the regulation of consummatory behaviors. For example, stimulation of hypothalamic Hcrt-1/Ox-A activity increases the motivation for natural reinforcers (i.e., sweet food) and increases their consumption, whereas blockade of Hcrt-1/Ox-A receptors leads to reduction of palatable food intake and lower responses conditioned to its availability (16). Conversely, central administration of NPS markedly inhibits palatable food consumption in sated rats (13). One hypothesis to explain this apparent discrepancy in the pharmacological profile of NPS is that NPS does not mediate a generalized activation of the Hcrt-1/Ox-A system, but rather stimulates a specific subset of Hcrt-1/Ox-A neurons, affecting relapse to cocaine seeking but not reward processing in general. Consistent with this hypothesis, our histochemistry showed that NPS activates about 30–40% of Hcrt-1/Ox-A immunopositive cells in the LH and in the PeF, whereas its effect on DMH Hcrt-1/Ox-A neurons is minimal. In addition, our data indicate that NPSRs are localized only in a small proportion (5.9%) of Hcrt-1/Ox-A neurons. Our data also indicate that in the hypothalamus there is another population of NPSR-positive cells that does not colocalize with Hcrt-1/Ox-A, but that can influence its function indirectly through local circuitry.
Neuroanatomical studies have shown the presence of anterogradely labeled axons from the LH in the ventral tegmental area (VTA). These are located in close proximity to the dendrites and somata of dopamine (DA) neurons, and 20% of these LH cells express hypocretin immunoreactivity (17). In addition, neurochemical data suggest that LH Hcrt-1/OX-A neurons facilitate dopamine neurotransmission in the nucleus accumbens (15, 18), and electrophysiological findings have established that Hcrt-1/Ox-A can facilitate accumbens DA activity via stimulation of DA neurons in the ventral tegmental area (19). All these actions are consistent with a role of the Hcrt-1/Ox-A in modulating drug seeking and reward. One possibility is that stimulation of NPS receptors in the LH and possibly in the PeF might result in the activation of Hcrt-1/Ox-A neurons projecting to the VTA, and possibly other mesolimbic areas, that in turn might be responsible for modulating relapse-like behavior. Our findings also indicate that NPS manipulation did not influence the primary reinforcing properties of cocaine. This is consistent with previous studies showing that blockade of Hcrt-1/Ox-A receptors does not affect cocaine self-administration measured under a fixed ratio contingency similar to that used in our study (20, 21). In addition to the VTA, other areas shown to mediate responses to drug cues and found to be rich in Hcrt-1/Ox-A receptors are the amygdaloid complex, the prefrontal cortex, and the bed nucleus of the stria terminalis (22–24). It is also possible therefore that the NPS effects observed here are mediated by activation of subsets of Hcrt-1/Ox-A fibers that project into these structures.
Finally, neuroanatomical data show that a subset of efferent hypothalamic Hcrt-1/Ox-A neurons project to the LC, where they make synaptic contact with noradrenergic (NE) neurons (25, 26). At the cellular level, hypocretin increases postjunctional neuronal activity and facilitates synchronization of noradrenergic neurotransmission (27). Considering the role of noradrenergic LC neurotransmission in the regulation of conditioned responding, it cannot be excluded that the effects of NPS on cue-induced relapse are mediated by Hcrt-1/OX-A efferents to the LC. We find this less likely, because the LC is predominantly innervated by Hcrt-1/OX-A neurons branching from the DMH, an area that showed little response to NPS injection, whereas our data suggest that NPS effects are likely mediated by Hcrt-1/OX-A cells originating from the LH and in part from the PeF, which only sparsely project to the LC (25, 26, 28). Future studies will have to identify the terminal areas reached by the subpopulation of Hcrt-1/OX-A neurons that are activated by NPS.
In conclusion, our study demonstrates that activation of NPSR in the LH facilitates relapse to cocaine seeking elicited by drug-related conditioned environmental cues. This effect is selective for cues predictive of drug availability as opposed to stimuli predictive of the nonreward saline and is mediated, at least in part, by activation of Hcrt-1/Ox-A neurons in the LH. More importantly, we have shown that administration of two chemically distinct NPS receptor antagonists potently and selectively prevented cue-induced cocaine seeking, suggesting that endogenous NPS may have a role in the pathophysiology of drug relapse. On the basis of these findings, we hypothesize that NPS receptor antagonism may represent a unique pharmacotherapeutic strategy for the treatment of cocaine craving and prevention of relapse in cocaine-addicted individuals.
Materials and Methods
Experimental Subjects.
Male Long Evans rats (Charles River) were used. The study was conducted in adherence to the European Community Council Directive for Care and Use of Laboratory Animals and the National Institutes of Health Guidelines for Care and Use of Laboratory Animals.
Intracranial and I.V. Surgery.
For intracranial surgery, guide cannulae were implanted with the following coordinates (7) with reference to Bregma: ICV, anterior-posterior (AP), −1.0; lateral (L), −1.8; ventral (V), 2.0; LH, AP-2.8; L ± 2.0; V-7.5; PeF, AP-3.0; L ± 1.1; V-7.5; DMH, AP-3.0; L ± 1.8; V-7.7; angle 10°; CeA, AP-1.8; L ± 3.9 and V-7.0. For the LH, PeF, DMH, and CeA, cannulae were implanted bilaterally. Drugs were administered through an injector protruding beyond the cannula tip: 2.5 mm (ICV); 1.6 mm (LH); 2 mm (PeF and DMH); and 1.5 (CeA). Chronic jugular i.v. catheter implantation was conducted as previously described (6).
Drug Injections and Histological Analysis.
For details, see SI Materials and Methods.
Self-Administration Apparatus.
Self-administration stations consisted of operant conditioning chambers (Med Associates) equipped with an infusion pump for cocaine delivery (6).
Histochemistry.
For c-Fos immunostaining and for c-Fos and Hcrt-1/Ox-A double immunostaining, rats (n = 4 each group) were injected with NPS 2 nmol/ rat or its vehicle and 1 h later were killed to be processed for immunoistochemistry (SI Materials and Methods).
For colocalization of Hcrt-1/Ox-A immunoreactivity and NPSR transcript, two additional rats were used (SI Materials and Methods).
Statistical Analysis.
Data were analyzed using appropriate within- and between-factorial ANOVAs. Analysis of variance was followed by Newman-Keuls post hoc tests when appropriate. Power analysis for minimal sample size estimation was carried out to rule out possible misinterpretation of results.
Supplementary Material
Acknowledgments
We thank Sheila Beatty for linguistic revision of the paper. This work was supported by Grant AA014351 (to F.W.), and by a Compagnia San Paolo Foundation grant (to R.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004100107/-/DCSupplemental.
References
- 1.Xu YL, et al. Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- 3.Smith KL, et al. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147:3510–3518. doi: 10.1210/en.2005-1280. [DOI] [PubMed] [Google Scholar]
- 4.Cannella N, et al. Persistent increase of alcohol-seeking evoked by neuropeptide S: An effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacology. 2009;34:2125–2134. doi: 10.1038/npp.2009.37. [DOI] [PubMed] [Google Scholar]
- 5.Pañeda C, et al. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J Neurosci. 2009;29:4155–4161. doi: 10.1523/JNEUROSCI.5256-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: Reversal by D(1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. San Diego: Academic Press; 1998. [Google Scholar]
- 8.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 9.Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Boutrel B, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camarda V, et al. In vitro and in vivo pharmacological characterization of the neuropeptide s receptor antagonist [D-Cys(tBu)5]neuropeptide S. J Pharmacol Exp Ther. 2009;328:549–555. doi: 10.1124/jpet.108.143867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruzza C, et al. Further studies on the pharmacological profile of the neuropeptide S receptor antagonist SHA 68. Peptides. 2010;31:915–925. doi: 10.1016/j.peptides.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Fedeli A, et al. The paraventricular nucleus of the hypothalamus is a neuroanatomical substrate for the inhibition of palatable food intake by neuropeptide S. Eur J Neurosci. 2009;30:1594–1602. doi: 10.1111/j.1460-9568.2009.06948.x. [DOI] [PubMed] [Google Scholar]
- 14.Aston-Jones G, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.España RA, et al. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cason AM, et al. Role of orexin/hypocretin in reward-seeking and addiction: Implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 18.Narita M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bäckberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: Focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- 23.Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 24.Feltenstein MW, See RE. The neurocircuitry of addiction: An overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horvath TL, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 26.Hagan JJ, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Pol AN, et al. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541:169–185. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gompf HS, Aston-Jones G. Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity. Brain Res. 2008;1224:43–52. doi: 10.1016/j.brainres.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.