Abstract
Stimulation of terrestrial plant production by rising CO2 concentration is projected to reduce the airborne fraction of anthropogenic CO2 emissions. Coupled climate–carbon cycle models are sensitive to this negative feedback on atmospheric CO2, but model projections are uncertain because of the expectation that feedbacks through the nitrogen (N) cycle will reduce this so-called CO2 fertilization effect. We assessed whether N limitation caused a reduced stimulation of net primary productivity (NPP) by elevated atmospheric CO2 concentration over 11 y in a free-air CO2 enrichment (FACE) experiment in a deciduous Liquidambar styraciflua (sweetgum) forest stand in Tennessee. During the first 6 y of the experiment, NPP was significantly enhanced in forest plots exposed to 550 ppm CO2 compared with NPP in plots in current ambient CO2, and this was a consistent and sustained response. However, the enhancement of NPP under elevated CO2 declined from 24% in 2001–2003 to 9% in 2008. Global analyses that assume a sustained CO2 fertilization effect are no longer supported by this FACE experiment. N budget analysis supports the premise that N availability was limiting to tree growth and declining over time —an expected consequence of stand development, which was exacerbated by elevated CO2. Leaf- and stand-level observations provide mechanistic evidence that declining N availability constrained the tree response to elevated CO2; these observations are consistent with stand-level model projections. This FACE experiment provides strong rationale and process understanding for incorporating N limitation and N feedback effects in ecosystem and global models used in climate change assessments.
Keywords: CO2 fertilization, free air CO2 enrichment, global carbon cycle, sweetgum, coupled climate-carbon cycle models
Policy decisions to mitigate climate change require dependable predictions of the forcings and feedbacks between the terrestrial biosphere and the climate (1). Currently, climate models that are coupled to terrestrial and oceanic carbon (C)-cycle models simulate a positive feedback to climate change such that the airborne fraction of anthropogenic CO2 emissions increases with, and amplifies, climatic warming (1). However, the uncertainty in these projections is high, largely because of uncertainty in the offsetting negative feedback that may occur if stimulation of terrestrial plant production by rising CO2 concentration increases land C storage and thereby reduces the airborne fraction of anthropogenic CO2 emissions. Coupled climate–C-cycle models, including those used in the Intergovernmental Panel on Climate Change Fourth Assessment Report (AR4) (2), are sensitive to this negative feedback on atmospheric CO2 (3). For example, dynamic global vegetation models (4) simulate an increased terrestrial C sink resulting from the physiological responses of plants to elevated atmospheric CO2 concentration (eCO2), and when coupled to climate models, inclusion of the CO2 fertilization effect slows the increase in atmospheric CO2 and the trajectory of climatic warming (5). The representation of the so-called CO2 fertilization effect in the 11 models used in AR4 and subsequent models (2, 5, 6) was broadly consistent with experimental evidence available at that time from four free-air CO2 enrichment (FACE) experiments, which indicated that net primary productivity (NPP) of forests was increased by 23 ± 2% in response to atmospheric CO2 enrichment to 550 ppm (7). Substantial uncertainty remains, however, because of the expectation that feedbacks through the nitrogen (N) cycle will reduce the CO2 stimulation of NPP (8–10). These feedbacks were not included in the AR4 models and heretofore have not been confirmed by CO2-enrichment experiments in forests (11). However, N feedbacks have been observed to diminish the CO2 effect on biomass accumulation in nutrient-poor grasslands (12), and severely N-limited forests show little response to eCO2 (13).
To reduce the large uncertainties in climate–C-cycle projections, C-cycle models must be constrained by observational data (3). Forests, which have the capacity to sequester C from the atmosphere in long-lived biomass and soil pools, are an especially important terrestrial C sink (1). However, few forest experiments have been of long enough duration to determine whether an observed stimulation of NPP, which can lead to increased C sequestration, would be sustained through time. The Oak Ridge FACE experiment (14, 15) exposed replicate plots in an established sweetgum (Liquidambar styraciflua) plantation forest to an atmosphere with ∼550 ppm CO2 continuously during the 1998–2009 growing seasons; here we discuss the results of 11 y of CO2 enrichment. Results from the first 6 y of the experiment indicated that NPP was significantly enhanced by eCO2 (Fig. 1) and that this was a consistent and sustained response (7). There was little enhancement of aboveground wood production (Fig. 2); the bulk of the NPP increase occurred as increased fine-root production, especially in the deeper soil profile (16). Increased NPP was associated with greater C input to soil (17) and a gain in soil C (18), and it was this 6-y dataset that was used in a synthesis of NPP responses in FACE experiments (7). Now, with 11 y of data, our analysis must be revised.
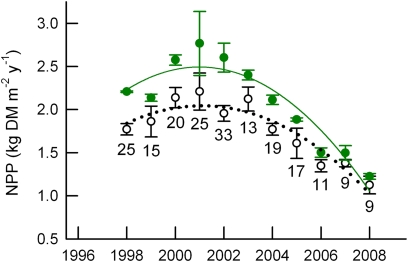
Fig. 1.
Tree growth responses to elevated CO2. NPP [kilograms dry matter (DM) per square meter land area per year] data are the means of three aCO2 plots (open symbols) and two eCO2 plots (solid symbols) ± SEM. The number at each point is the percentage increase under eCO2. Statistical information is given in SI Materials and Methods.
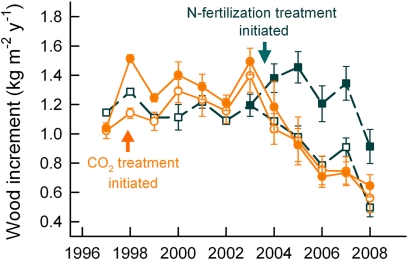
Fig. 2.
Growth response to nitrogen addition. Responses in the N fertilizer experiment (dashed lines) are compared with responses in the FACE experiment (solid lines). Elevated CO2 (solid circles) caused a significant increase in wood increment in the first year after treatment initiation (1998), but the response diminished in subsequent years and in later years was not statistically different from FACE controls (open circles). N fertilization (shaded squares) caused an immediate and sustained increase in wood increment compared with unfertilized plots (open squares) (P < 0.001).
Results
The enhancement of NPP under eCO2 relative to current ambient CO2 (aCO2) declined from 24% in 2001–2003 to 9% in 2008, and there was no significant enhancement (P > 0.18) in any year after 2004 (Fig. 1). During this period, NPP in aCO2 was diminished by 47%, from 2.1 kg dry matter/m2 in 2002 to 1.1 kg/m2 in 2008. The loss in NPP response to eCO2 was entirely accounted for by changes in fine-root production.
Given the speculation that feedbacks through the N cycle could result in the loss of response to eCO2, we tested whether our sweetgum forest was N limited by adding N fertilizer to the sweetgum plantation adjacent to the FACE experiment. N additions resulted in an immediate increase in NPP; much of the increase in NPP was due to greater aboveground production, resulting in a decrease in relative allocation to fine roots (19). This fertilizer response was sustained even as tree growth in control plots (and in the adjacent FACE plots) was declining (Fig. 2).
Canopy-averaged foliar [N] in the FACE experiment declined over time in both aCO2 and eCO2 (Fig. 3A). Foliar [N] did not differ between plots before the onset of the CO2 exposure, but was consistently lower in eCO2 after the treatments were initiated in 1998. There was a linear relationship between NPP and foliar [N] beginning in the third year of treatment (Fig. 3B). The slope of the NPP–[N] relationship was significantly steeper in eCO2 than in aCO2. From this regression analysis, NPP would be equal in the two treatments when foliar [N] declined to 8.6 mg·g−1, which was approximately the [N] of leaf litter in aCO2 from 1998 to 2004 (20).
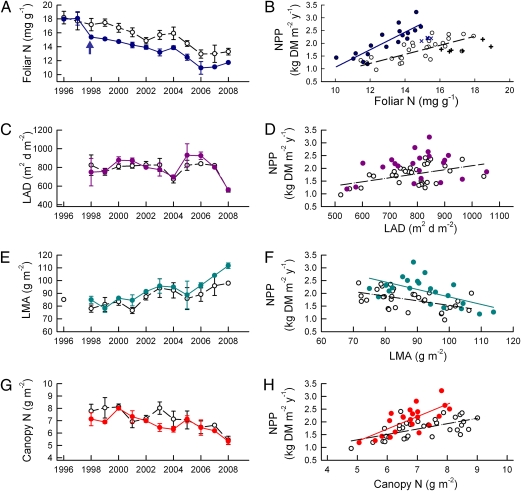
Fig. 3.
Relationship of canopy parameters to NPP. (A) N concentration in leaves throughout the canopy of each plot after canopy expansion was complete in July or August of each year. The arrow indicates onset of the CO2 treatments. (B) The linear relationship between NPP and foliar [N], excluding data from 1998 to 1999 (x and + symbols). (C) Leaf area duration (m2 leaf × d·m−2 ground). (D) NPP increased with increasing LAD in aCO2 but there was no significant relationship between NPP and LAD in eCO2. (E) LMA, leaf mass per unit area (g DM/m2 leaf area) averaged over the canopy. (F) Linear relationships between NPP and LMA. (G) Total N content of canopy at seasonal peak leaf area (g N/m2 ground area). (H) Linear relationship between NPP and canopy N. A, C, E, and G data are the means of three aCO2 plots (open symbols, dashed black lines) and two eCO2 plots (solid symbols, solid colored lines) ± SEM. B, D, F, and H data are values for each plot–year combination in aCO2 (open symbols) and eCO2 (solid symbols) from 1998 to 2008 with regression lines as indicated. Statistical information is given in SI Materials and Methods.
During the first 2 y of the experiment, NPP was not closely related to foliar [N], and other year-to-year fluctuations in NPP did not always correspond to differences in [N], indicating that foliar [N] was not the only regulator of NPP. NPP also depends on interrelated factors describing canopy structure, including leaf area duration (LAD) (the integration of leaf area index over the year), leaf mass per unit area (LMA), and total canopy N content. LAD varied year to year without any clear trend over time and with no effect of [CO2] (Fig. 3C). NPP increased with LAD in aCO2, but there was no significant relationship between NPP and LAD in eCO2 (Fig. 3D). LMA increased during the experiment (Fig. 3E), and NPP was inversely correlated with LMA (Fig. 3F). Canopy N content did not exhibit any clear trend through time except for a decline over the last 3 y (Fig. 3G), but NPP was nevertheless correlated with canopy N (Fig. 3H). The influences of LAD, LMA, and canopy N on NPP were small compared with that of foliar [N]. In a multivariate regression framework, foliar [N] was the only significant predictor of NPP in eCO2, accounting for 73% of the variation. In aCO2 foliar [N] explained 57% of the variation in NPP, and LAD explained an additional 15%.
Light-saturated photosynthetic rates were significantly greater (P < 0.05) at eCO2 than at aCO2 in 1999 (means ± SEM were 14.5 ± 1.8 μmol·m−2·s−1 vs. 10.4 ± 1.0 μmol·m−2·s−1) (21). However, photosynthesis was lower in both treatments in 2008, and there was no longer a significant stimulation by eCO2 (7.6 ± 0.7 μmol·m−2·s−1 vs. 6.4 ± 0.7 μmol·m−2·s−1, P > 0.26). Reductions in leaf photosynthesis through time and with CO2 treatment reflect differences in the parameters of photosynthetic biochemistry, Vcmax (the maximum rate of carboxylation) and Jmax (maximum rate of electron transport at saturating irradiance.) Foliar N per unit leaf area, Narea, in the upper 2 m of the canopy decreased from 1999 to 2008 and was less in eCO2 than in aCO2; hence, Vcmax and Jmax were reduced concomitantly. There was no change in the relationships between Vcmax or Jmax and Narea with time or with CO2 enrichment.
Discussion
Global analyses that assume a sustained CO2 fertilization effect are no longer supported by this FACE experiment. Previous reports from this experiment that were based on observations over 6 y of treatment and combined with results from three other forest FACE experiments indicated that the CO2 fertilization effect on NPP was sustained and consistent across a broad range of productivity (7). Observations over a longer period were necessary to reveal the loss in response reported here, which challenges the use of the previous report as a benchmark for models.
The diminishing response of forest NPP to eCO2 must be interpreted in relation to the coinciding decline of NPP in aCO2, although we note that low productivity by itself does not necessarily imply lower relative responsiveness to eCO2 (7). A decline in forest production with age is a pattern normally observed during forest stand development as a consequence of various environmental or internal factors (22, 23). Although the decline can be large and is a near universal phenomenon, the physiological mechanisms responsible for decline are not completely understood; possible explanations include increased respiration-to-photosynthesis balance, increased hydraulic resistance, decreased nutrient supply, or various changes in stand structure (22, 23). Generally, more productive stands (including plantations established for short-rotation forestry) reach their peak productivity earlier and productivity declines more steeply (22). Consistent with model projections (24), we attribute the observed decline in NPP in this ecosystem to a constraint imposed by limited and declining N availability. In N-limited forests, the N that is sequestered into perennial tissue during stand development or immobilized into decomposing plant litter and soil organic pools must be replaced with additional N inputs, or tree growth will decline (25). The response to N fertilization in the adjacent sweetgum stand provides direct evidence that the forest stand was N limited.
Although N limitation in this stand is established, the question whether the CO2 response was N limited requires further analysis. Our analysis of the relationship between NPP and foliar [N] is guided by the predictions of a simple model of the sweetgum stand's C–N–water economy (9, 24). Consistent with our analysis that both leaf- and canopy-level characteristics are important determinants of NPP, the model predicts an optimal balance with respect to foliar [N], stomatal conductance, and stand leaf area index (LAI) at which NPP is maximized; the optimum point, which is determined independently for each year, varies as resources change over time. With water and N availability prescribed at levels determined for 1998–2002, the model predicts maximum NPP to occur at a foliar [N] of 16.6 mg·g−1 in aCO2 and 14.4 mg·g−1 in eCO2 (24), which compares well with regression analysis of our data (Fig. 3B). NPP is maximized at a lower value of [N] in eCO2 because of greater photosynthetic N-use efficiency, which permits a reduction in the costs associated with foliar N (24). Also consistent with the optimization model, the slope of the NPP–[N] relationship was steeper in eCO2 than in aCO2 (Fig. 3B), which explains the gradual loss of NPP response to CO2 enrichment. At the somewhat higher levels of foliar [N] observed at the beginning of the experiment, the relationship between foliar [N] and NPP was weaker and other canopy or environmental factors were likely to have been important.
These stand-level observations and models have mechanistic support from measurements of leaf-level photosynthesis. The simple optimization model can explain the N constraint on CO2 enhancement from plant physiological considerations (9, 24), specifically the reduced stimulation of photosynthesis by eCO2 as foliar [N] declines. The loss of photosynthetic response to eCO2 was a proximate cause of the decline in NPP response. In contrast, there was no decline in photosynthetic response in Populus tremuloides trees after 11 y in a FACE experiment (26).
The long-term relationship among NPP, eCO2, and N cycling was predicted by the progressive nitrogen limitation (PNL) hypothesis (27), whereby increased sequestration of N in long-lived biomass or soil pools under eCO2 causes N availability to decline and induces a negative feedback on further productivity increases in eCO2. PNL, however, has long been recognized as a consequence of normal forest development even without the influence of eCO2 (25), and evidence from N cycling studies on forest plantations suggests that prospects for long-term growth responses are poor unless additional N is supplied by either N fixation or increased atmospheric deposition (10, 25). Similar mechanisms of N sequestration leading to acute N deficiency also pertain to older and unmanaged forests (28), but they may be slower to develop and more difficult to detect than in a faster growing plantation.
In our sweetgum forest, annual sequestration of N into wood (20) and soil organic matter (18) exceeded the input of exogenous N into this ecosystem, implying a drawing down of the soil N capital (25). Mechanisms have been proposed whereby eCO2 could increase N availability and compensate for increased N demand, such as stimulation of N mineralization by increased labile C inputs (29) and increased allocation to mycorrhizal fungi (11). However, occasional measurements of N mineralization in the top 10–15 cm of the soil (30–32) have not been sufficient to document a change in N availability through time or with eCO2. A more integrative (spatially and temporally) indicator of N availability is provided by the ratio of 15N to 14N in leaf litter. The isotopic signature of inorganic N in soil that is available for plant uptake depends on the balance between immobilization and nitrification of the NH4 pool. As immobilization increases relative to nitrification, indicating less available N, the available N pool becomes isotopically lighter (33), and this signal is reflected in the 15N/14N ratio (expressed as δ15N) in foliage (34). Although other factors (e.g., mycorrhizae and nitrate leaching) can also affect the isotopic signature, these factors could be discounted in the sweetgum stand (35). Hence, δ15N in leaves and freshly fallen leaf litter is an indicator of N availability in the ecosystem. In this sweetgum stand, δ15N of leaf litter declined linearly from 1998 to 2005 (litter from later years could not be analyzed), and it declined more steeply in eCO2 (Fig. 4A), providing evidence for declining N availability during stand development, which was exacerbated by eCO2 and consistent with the progressive N limitation hypothesis.
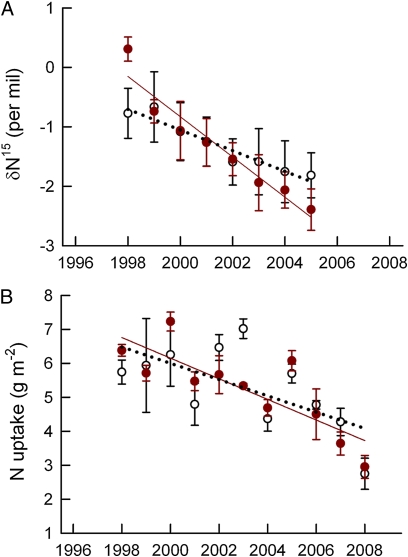
Fig. 4.
Nitrogen availability. (A) The δ15N of freshly fallen leaf litter (35), which is an indicator of N availability, declined significantly through time, and the decline in eCO2 was significantly steeper than that in aCO2. (B) Annual N uptake to aboveground tree parts per unit ground area. N uptake declined through time, but there was no significant difference between treatments. Data shown are the means of three aCO2 plots (open symbols, dotted black regression line) and two eCO2 plots (solid symbols, solid colored regression line) ± SEM. Statistical information is given in SI Materials and Methods.
Annual N uptake into aboveground plant parts (Fig. 4B), which can be considered another measure of the net N availability to trees after the requirement for fine-root production is met, also declined during the experiment. During the course of this experiment, sweetgum trees in eCO2 were able to use the additional C resources for increased fine-root production, especially deeper in the soil profile (17). Deeper roots provided access to more N (32), and total N uptake was greater (11), but after accounting for the additional N required for fine roots, there was no increase in uptake to the aboveground tree. As a result, the decline in aboveground uptake was similar in aCO2 and eCO2 (Fig. 4B). Foliar [N] declined, and with it so did photosynthesis and the C supply for additional fine-root production. Hence, the compensatory mechanism of increased root production, which initially averted an N limitation to CO2 fertilization, could not be sustained in this ecosystem. Responses in other ecosystems would be expected to vary depending on C allocation patterns of the tree species, N fertility of the soil, and the relationship between them.
We considered other possible explanations besides declining N availability for the decline in stand NPP and the loss in capacity to respond to eCO2. The effect of eCO2 on NPP was primarily an enhancement of fine-root production (16, 17), so there has been little change in tree size that could account for differences in growth rate. The average basal area of trees in eCO2 in 2008 was just 5.8% greater than in aCO2, and stand basal area was 1.5% less. There has been no effect of eCO2 on tree height that could have created hydraulic constraints to productivity (22), and sap flow did not decline over time as trees grew taller (15). Average soil moisture (0- to 20-cm depth) during the summer growing season (June through September) varied year to year and tended to be greater in eCO2 than in aCO2 (Fig. S1A), but was only weakly associated with NPP (Fig. S1B). Progressively drier summers from 2004 to 2007 may be partially responsible for declining NPP, but NPP continued to decline in 2008 despite more mesic conditions. However, carryover effects of the 2007 drought (Fig. S1A) on LAD in 2008 (Fig. 3C), and indirect effects of soil moisture on N availability, cannot be dismissed. As already discussed, some of the variation in NPP in aCO2 is explained by annual differences in LAD. Other potential causes of forest growth decline such as reproduction, mortality, or crown abrasion (22) do not apply to this stand. N limitation remains the most likely causative factor.
The NPP response to eCO2 observed in this FACE experiment contrasts with that of a similar experiment in a Pinus taeda forest stand in North Carolina (Duke FACE), where NPP has remained enhanced in eCO2 (36). Greater nutrient demand of the deciduous sweetgum trees compared with the evergreen pine trees may have caused N deficiency and associated growth decline to occur earlier in the sweetgum stand, but a similar response may eventually occur in the pine stand as well. This hypothesis needs to be explored with ecosystem models. The two forest stands differed in their C allocation patterns in that the productivity gains in the sweetgum trees occurred primarily in fast-turnover pools (fine roots), whereas in the pine trees, the gains were in woody tissue (37). It will be important for models to capture the C allocation patterns correctly because they also have implications for N turnover and availability (38). Furthermore, the observations that rooting depth increased in eCO2 in these and other experiments (38), and that deeper rooting provides access to more available N (32), highlight the need for models to incorporate soil depth in their representation of N availability.
Models are essential for projecting the long-term response of diverse and complex forest ecosystems to atmospheric and climatic change because these responses may be beyond the reach of direct experimental manipulation and observation. To gain confidence in model projections, it is important to have benchmarks with which we can challenge the models. We submit that the loss of response to eCO2 observed in this experiment will be captured in models only if there is an adequate representation of the N cycle. The detection of the loss of response and its connection to the developing N limitation was aided by the use of a fast-growing, densely planted, and unfertilized tree plantation in which stand development processes were accelerated compared with a native forest. It is not yet clear whether foliar [N] and CO2 enhancement of NPP in this experimental forest stand would continue to decline or after 11 y reached a new steady state indicative of long-term forest response to eCO2. Growth declines in native, unmanaged forests also have been documented and are sometimes associated with a progressive N limitation (27). However, the evaluation of the persistence of responses to eCO2 and detection of critical feedbacks through the N cycle will be much more difficult in native forests, where the processes observed in a fast-growing plantation will take longer to develop and be obscured by many other influences. The pattern of forest ecosystem N development can vary enormously depending on vegetation and site history (25). Extension of our results to other forests must be made with respect to stand development and include consideration of disturbance effects, species replacements, atmospheric N deposition, and climate change, all of which can alter the N cycle and C–N interactions.
Given the importance of the CO2 fertilization effect in coupled climate–C-cycle models that predict future climate change, there is an urgent need for additional long-term experiments focusing on interactions between C and N cycles in forests. Failure to characterize these interactions and incorporate suitable algorithms into models will lead to unreliable predictions of the response of the terrestrial biosphere to atmospheric and climatic change. It may be fortuitous, but ultimately misleading, that models that ignore the N cycle matched the previously reported FACE synthesis data, which preceded the onset of N limitation reported here. A longer record of experimental data, in combination with more sophisticated modeling, will provide more dependable predictions of future responses.
Materials and Methods
Experimental Design.
The experiment was established in Oak Ridge, Tennessee (35° 54′ N; 84° 20′ W) in 1997 in a fully established 10-y-old plantation forest of the deciduous, broadleaf tree, sweetgum (L. styraciflua L.). The experiment comprised five 25-m-diameter plots, each with ≈90 trees, an initial basal area of 29 m2·ha−1, and peak LAI of 5.7 (14, 15). Beginning in April 1998, two plots were exposed continuously during daylight hours throughout the growing season to an elevated concentration of CO2 (∼550 ppm), using the FACE apparatus (39). No N fertilizer was added to the FACE plots; annual N deposition is 12–15 kg·ha−1 (40). The experiment was terminated in 2009.
Nitrogen Fertilization.
Urea fertilizer (200 kg N·ha−1) was added in early spring, 2004–2008 to replicate plots on part of the sweetgum plantation adjacent to the FACE experiment (19). There were two fertilized and two control plots (12 × 16 m) in each of three blocks. Wood increment was measured as change in stem circumference and converted to dry mass using previously determined allometric relationships (SI Materials and Methods).
Net Primary Productivity.
NPP was calculated as the sum of wood (bole, branch, and coarse root) increment, leaf litter production, and fine-root production (refs. 15 and 17 and SI Materials and Methods). Foliar N concentration was measured in leaves collected from throughout the canopy in August each year (20). Oven-dried and finely ground tissue was analyzed using a C–N analyzer (Carlo Erba or Costech Analytical Technologies) with atropine as a standard and National Institute of Standards and Technology (NIST) apple leaves as internal quality checks. NPP, foliar [N], LAD, LMA, and canopy N content data were analyzed statistically as repeated measures data, using a mixed model with covariance structure chosen to minimize Akaike's information criterion.
N Uptake and Availability.
N uptake was calculated as the annual peak N content of the canopy plus N content of wood increment minus the amount supplied by internal recycling from the previous year's canopy, which was assumed to equal the N content of the canopy minus that of litterfall (11). The 15N/14N ratio of archived samples of freshly fallen leaf litter was determined on an Integra-CN, continuous flow, isotope ratio, mass-spectrometer (PDZ-Europa), using ammonium sulfate (δ15N = −0.4 ‰), traceable to NIST, as an internal standard (38). Because a 15N label was applied to the site in 2006, natural abundance measurements of litter could not be made after 2005.
Photosynthesis.
Light-saturated photosynthetic rate was measured in upper canopy leaves, using a Li-Cor 6400 system (Li-Cor Biosciences) from 1998 to 2000 (21) and again in July 2008. Response curves of assimilation vs. internal leaf CO2 were used to estimate maximum Rubisco activity, Vcmax, and potential electron transport rate, Jmax, using a consistent set of leaf photosynthesis model equations (41).
Data Online.
Data from the FACE experiment are publicly available at http://public.ornl.gov/face/ORNL/ornl_home.shtml.
Supplementary Material
Acknowledgments
We thank J. Childs, J. Riggs, and D. Sluss for sustained assistance with operation of the experimental facility; S. Jawdy, C. Sheehan, C. DeVan, K. Sides, E. Felker-Quinn, G. Jimenez, C. Campany, and C. Bruno for assistance with data collection and sample processing; and M. L. Tharp for data management. Funding was provided by the US Department of Energy, Office of Science, Biological and Environmental Research Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006463107/-/DCSupplemental.
References
- 1.Bonan GB. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science. 2008;320:1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- 2.Denman KL, et al. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon SD, et al., editors. Cambridge, UK: Cambridge Univ Press; 2007. Chap 7, pp 499–587. [Google Scholar]
- 3.Friedlingstein P, et al. Climate-carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J Clim. 2006;19:3337–3353. [Google Scholar]
- 4.Cramer W, et al. Global response of terrestrial ecosystem structure and function to CO2 and climate change: Results from six dynamic global vegetation models. Glob Change Biol. 2001;7:357–373. [Google Scholar]
- 5.Matthews HD. Implications of CO2 fertilization for future climate change in a coupled climate-carbon model. Glob Change Biol. 2007;13:1068–1078. [Google Scholar]
- 6.Hickler T, et al. CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Glob Change Biol. 2008;14:1531–1542. [Google Scholar]
- 7.Norby RJ, et al. Forest response to elevated CO2 is conserved across a broad range of productivity. Proc Natl Acad Sci USA. 2005;102:18052–18056. doi: 10.1073/pnas.0509478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornton PE, Lamarque JF, Rosenbloom NA, Mahowald NM. Influence of carbon-nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Glob Biogeochem Cycles. 2007;21:GB4018. [Google Scholar]
- 9.McMurtrie RE, et al. Why is plant-growth response to elevated CO2 amplified when water is limiting, but reduced when nitrogen is limiting? A growth-optimisation hypothesis. Funct Plant Biol. 2008;35:521–534. doi: 10.1071/FP08128. [DOI] [PubMed] [Google Scholar]
- 10.Hungate BA, Dukes JS, Shaw MR, Luo Y, Field CB. Atmospheric science. Nitrogen and climate change. Science. 2003;302:1512–1513. doi: 10.1126/science.1091390. [DOI] [PubMed] [Google Scholar]
- 11.Finzi AC, et al. Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci USA. 2007;104:14014–14019. doi: 10.1073/pnas.0706518104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reich PB, et al. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature. 2006;440:922–925. doi: 10.1038/nature04486. [DOI] [PubMed] [Google Scholar]
- 13.Oren R, et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature. 2001;411:469–472. doi: 10.1038/35078064. [DOI] [PubMed] [Google Scholar]
- 14.Norby RJ, et al. Net primary productivity of a CO2-enriched deciduous forest and the implications for carbon storage. Ecol Appl. 2002;12:1261–1266. [Google Scholar]
- 15.Norby RJ, et al. In: Managed Ecosystems and CO2: Case Studies, Processes, and Perspectives. Nösberger J, et al., editors. Berlin: Springer; 2006. pp. 231–251. [Google Scholar]
- 16.Norby RJ, Ledford J, Reilly CD, Miller NE, O'Neill EG. Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci USA. 2004;101:9689–9693. doi: 10.1073/pnas.0403491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iversen CM, Ledford J, Norby RJ. CO2 enrichment increases carbon and nitrogen input from fine roots in a deciduous forest. New Phytol. 2008;179:837–847. doi: 10.1111/j.1469-8137.2008.02516.x. [DOI] [PubMed] [Google Scholar]
- 18.Jastrow JD, et al. Elevated atmospheric carbon dioxide increases soil carbon. Glob Change Biol. 2005;11:2057–2064. doi: 10.1111/j.1365-2486.2005.01077.x. [DOI] [PubMed] [Google Scholar]
- 19.Iversen CM, Norby RJ. Nitrogen limitation in a sweetgum plantation: Implications for carbon allocation and storage. Can J For Res. 2008;38:1021–1032. [Google Scholar]
- 20.Norby RJ, Iversen CM. Nitrogen uptake, distribution, turnover, and efficiency of use in a CO2-enriched sweetgum forest. Ecology. 2006;87:5–14. doi: 10.1890/04-1950. [DOI] [PubMed] [Google Scholar]
- 21.Sholtis JD, Gunderson CA, Norby RJ, Tissue DT. Persistent stimulation of photosynthesis by elevated CO2 in a sweetgum (Liquidambar styraciflua) forest stand. New Phytol. 2004;162:343–354. [Google Scholar]
- 22.Ryan MG, Binkley D, Fownes JH. Age-related decline in forest productivity: Pattern and process. Adv Ecol Res. 1997;27:213–262. [Google Scholar]
- 23.Murty D, McMurtrie RE. The decline of forest productivity as stands age: A model-based method for analyzing causes for the decline. Ecol Modell. 2000;134:185–205. [Google Scholar]
- 24.Dewar RC, Franklin O, Mäkelä A, McMurtrie RE, Valentine HT. Optimal function explains plant responses to global change. Bioscience. 2009;59:127–139. [Google Scholar]
- 25.Johnson DW. Progressive N limitation in forests: Review and implications for long-term responses to elevated CO2. Ecology. 2006;87:64–75. doi: 10.1890/04-1781. [DOI] [PubMed] [Google Scholar]
- 26.Darbah JNT, et al. Will photosynthetic capacity of aspen trees acclimate after long-term exposure to elevated CO2 and O3? Environ Pollut. 2010;158:983–991. doi: 10.1016/j.envpol.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Luo Y, et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience. 2004;54:731–739. [Google Scholar]
- 28.Richter DD, et al. Legacies of agriculture and forest regrowth in the nitrogen of old-field soils. For Ecol Manage. 2000;138:233–248. [Google Scholar]
- 29.Zak DR, et al. Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant Soil. 1993;151:105–117. [Google Scholar]
- 30.Zak DR, Holmes WE, Finzi AC, Norby RJ, Schlesinger WH. Soil nitrogen cycling under elevated CO2: A synthesis of forest face experiments. Ecol Appl. 2003;13:1508–1514. [Google Scholar]
- 31.Austin EE, Castro HF, Sides KE, Schadt CW, Classen AT. Assessment of 10 years of CO2 fumigation on soil microbial communities and function in a sweetgum plantation. Soil Biol Biochem. 2009;41:514–520. [Google Scholar]
- 32.Iversen CM, Hooker TD, Classen AT, Norby RJ. Net mineralization of N at deeper soil depths as a potential mechanism for sustained forest production under elevated [CO2] Glob Change Biol. 2010 10.1111/j.1365-2486.2010.02240.x. [Google Scholar]
- 33.Garten CT., Jr. Variation in foliar 15N abundance and the availability of soil nitrogen on Walker Branch Watershed. Ecology. 1993;74:2098–2113. [Google Scholar]
- 34.Craine JM, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009;183:980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 35.Garten CT, Iversen CM, Norby RJ. Litterfall 15N abundance indicates declining soil nitrogen availability in a free air CO2-enrichment experiment. Ecology. 2010 doi: 10.1890/10-0293.1. in press. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy HR, et al. Re-assessment of plant carbon dynamics at the Duke free-air CO(2) enrichment site: Interactions of atmospheric [CO(2)] with nitrogen and water availability over stand development. New Phytol. 2010;185:514–528. doi: 10.1111/j.1469-8137.2009.03078.x. [DOI] [PubMed] [Google Scholar]
- 37.DeLucia EH, Moore DJ, Norby RJ. Contrasting responses of forest ecosystems to rising atmospheric CO2: Implications for the global C cycle. Glob Biogeochem Cycles. 2005;19:GB3006. [Google Scholar]
- 38.Iversen CM. Digging deeper: Fine-root responses to rising atmospheric CO concentration in forested ecosystems. New Phytol. 2010;186:346–357. doi: 10.1111/j.1469-8137.2009.03122.x. [DOI] [PubMed] [Google Scholar]
- 39.Hendrey GR, Ellsworth DS, Lewin KF, Nagy J. A free-air enrichment system for exposing tall forest vegetation to elevated atmospheric CO2. Glob Change Biol. 1999;5:293–309. [Google Scholar]
- 40.Johnson DW, et al. Effects of elevated CO2 on nutrient cycling in a sweetgum plantation. Biogeochemistry. 2004;69:379–403. [Google Scholar]
- 41.Medlyn BE, et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 2002;25:1167–1179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.