Abstract
The acute diarrheal disease cholera is caused by the marine bacterium Vibrio cholerae. A type VI secretion system (T6SS), which is structurally similar to the bacteriophage cell-puncturing device, has been recently identified in V. cholerae and is used by this organism to confer virulence toward phagocytic eukaryotes, such as J774 murine macrophages and Dictyostelium discoideum. We tested the interbacterial virulence of V. cholerae strain V52, an O37 serogroup with a constitutively active T6SS. V52 was found to be highly virulent toward multiple Gram-negative bacteria, including Escherichia coli and Salmonella Typhimurium, and caused up to a 100,000-fold reduction in E. coli survival. Because the T6SS-deficient mutants V52ΔvasK and V52ΔvasH showed toxicity defects that could be complemented, virulence displayed by V. cholerae depends on a functional T6SS. V. cholerae V52 and strains of the O1 serogroup were resistant to V52, suggesting that V. cholerae has acquired immunity independently of its serogroup. We hypothesize that the T6SS, in addition to targeting eukaryotic host cells, confers toxicity toward other bacteria, providing a means of interspecies competition to enhance environmental survival. Thus, the V. cholerae T6SS may enhance the survival of V. cholerae in its aquatic ecosystem during the transmission of cholera and between epidemics.
Keywords: bacterial secretion, microbial competition, bacterial killing
Diarrheal diseases including cholera are the leading cause of morbidity and the second-most common cause of death among children under the age of 5 worldwide (1, 2). Cholera alone, caused by the marine bacterium Vibrio cholerae, is responsible for several million cases and an estimated 120,000 deaths annually.* Among the >200 serogroups of V. cholerae, O1 and O139 are responsible for cholera pandemics, whereas non-O1 and non-O139 are often associated with localized outbreaks of cholera-like illnesses.
V. cholerae possesses a type VI secretion system (T6SS) that has been identified in >80 Gram-negative bacterial genomes (3, 4). The Mougous group recently demonstrated that Pseudomonas aeruginosa and Burkholderia thailandensis use their T6SS to compete and interact with other bacteria (5, 6). In V. cholerae, the T6SS confers virulence toward the phagocytic amoeba Dictyostelium discoideum and J774 murine macrophages; however, its role in the diarrheal disease cholera remains unclear (3). Although the structure of the secretion apparatus has not been clearly defined, structural similarities to the bacteriophage cell-puncturing device have been identified (7, 8). In the current model substrates pass through the T6SS conduit and are injected directly into the host cytoplasm. A typical T6SS encodes 15–25 genes in a single locus. The export of hemolysin coregulated protein (Hcp), a small 17.4-kDa protein capable of forming nanotubes in vitro, is the hallmark of T6SSs because Hcp is consistently found in the supernatant of bacteria harboring a functional T6SS (9, 10).
Interestingly, Hcp and the valine–glycine repeat (VgrG) proteins VgrG1–VgrG3 are both secreted and required for the secretion of other T6SS substrates. The VgrG proteins are homologous to the cell-puncturing needle of T4 bacteriophage and are presumed to form a complex at the tip of the T6SS apparatus (3). Hcp is structurally similar to the lambda phage major tail protein gpV, which serves as a conduit for bacteriophage genome delivery (7, 8, 11). Another essential T6SS component, VasK, is predicted to be a component of the inner membrane complex. V. cholerae cells lacking vasK are unable to export either Hcp or VgrG proteins; as a result these cells do not engage in T6SS-mediated toxicity (3).
Regulation of the T6SS in V. cholerae is poorly understood. We recently identified the T6SS regulator VasH, predicted to encode a transcriptional regulator and activator of the alternate σ-factor σ54, essential for Hcp production (3). In pandemic strains, expression of T6SS genes remains undetected under laboratory conditions. In contrast, the O37 serogroup strain V52 constitutively expresses the T6SS and serves as our experimental model system for studying T6SS-mediated virulence (3).
V. cholerae’s primary reservoir is an aquatic environment, where it exists in a free-living state associated with phytoplankton, zooplankton, algae, or copepods (12, 13). Transmission into a host's gastrointestinal system occurs by ingestion of contaminated food or water. Bacteria surviving the acidity of the stomach colonize the small intestine and cause increased intestinal secretions, leading to large volumes of watery diarrhea (14). V. cholerae exits the human body during such diarrheal purges, allowing disease transmission or seeding an environmental reservoir.
It has long been speculated that V. cholerae has evolved mechanisms to kill bacterial competitors. Chakrabarty et al. identified 11 diffusible bacteriocins with toxicity toward the enteric bacteria Escherichia coli, Shigella sonnei, and Shigella flexneri (15). However, expression of such bacteriocins and mechanisms underlying their antimicrobial properties are not well understood. Thus, we investigated whether the T6SS contributes to the antimicrobial properties of V. cholerae. Interbacterial virulence was determined by exposing bacterial prey (the organism that is attacked) to a bacterial predator (a V. cholerae strain that is attacking) and measuring the survival of bacterial prey. Here, we present experimental evidence to demonstrate the antimicrobial properties of the V. cholerae T6SS. We propose that interbacterial toxicity promotes V. cholerae survival in the environment and/or during colonization of a host.
Results
V. cholerae Is Highly Virulent Toward Selected Gram-Negative Bacteria.
Various Gram-negative and Gram-positive bacteria were selected as prey to represent the microbiota encountered by V. cholerae in the environment or during host colonization. A V52 strain lacking the accessory toxins hlyA, rtxA, and hapA was found to be highly virulent toward various Gram-negative bacteria including E. coli K-12 MG1655, enterohemorrhagic E. coli (EHEC) O157:H7, enteropathogenic E. coli (EPEC) E2348/69, Salmonella Typhimurium, and Citrobacter rodentium, whereas virulence was abrogated in the T6SS-deficient mutant V52ΔvasK (Fig. 1 A and B). P. aeruginosa PAO1 was not susceptible to V52 bacterial virulence (Fig. 1A). V52 did not display T6SS-dependent virulence toward any of the Gram-positive species tested (Fig. S1A). Although V52 was virulent toward Bacillus subtilis, toxicity was not dependent on the T6SS because V52ΔvasK retained a wild-type level of virulence (Fig. S1A). This result is not surprising because V. cholerae produces a variety of bacteriocins that we suspect are toxic toward B. subtilis (15). Yeast prey Candida albicans and Saccharomyces cerevisiae grew well in the presence of both V52 and V52ΔvasK (Fig. S1 B and C). These results suggest that the T6SS enables V. cholerae to kill bacteria with a host range specificity limited to certain Gram-negative bacteria.
Fig. 1.
V. cholerae uses its T6SS to target bacteria with specificity toward Gram-negative species. Survival of rifampicin-resistant Gram-negative bacterial prey is shown: (A) E. coli K-12 MG1655 (red), S. Typhimurium (green), C. rodentium (blue), and P. aeruginosa (orange) and (B) E. coli K-12 MG1655 (red), EHEC (purple), and EPEC (orange) were determined by measuring cfu following exposure to the indicated rifampicin-sensitive predator listed on the x axis with each prey exposed to predatory self, V52, and V52ΔvasK. The data represent three independent experiments.
V. cholerae Is Highly Virulent Toward E. coli in a T6SS-Dependent Manner.
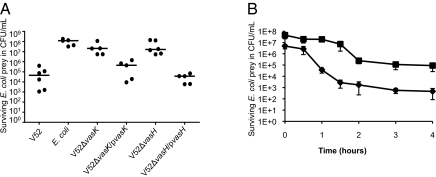
Because V52 exhibited the highest killing efficiency toward E. coli, we selected E. coli K-12 as experimental prey for future experiments. Rifampicin-resistant E. coli MG1655 prey was mixed with a rifampicin-sensitive predator, either self or V. cholerae strain V52, in a killing assay described in Materials and Methods. When E. coli was mixed with rifampicin-sensitive self, E. coli survival was on the order of 108 colony-forming units (cfu)·mL−1 (Fig. 2A). Remarkably, in the presence of the V52 predator, up to a 100,000-fold reduction in culturable E. coli was observed (Fig. 2A). The dramatic virulence exhibited by V52 was dependent on the T6SS, as deletion of vasK or vasH abrogated bacterial killing (Fig. 2A). T6SS-mediated virulence toward E. coli was restored in the complemented strains V52ΔvasK/pvasK and V52ΔvasH/pvasH (Fig. 2A).
Fig. 2.
V. cholerae is highly virulent toward E. coli in a T6SS-dependent manner. (A) Killing assay: Survival of rifampicin-resistant E. coli was determined by measuring cfu following exposure to the indicated rifampicin-sensitive predator listed on the x axis. The data represent three independent experiments. (B) Survival curve: Survival of E. coli prey was measured following varying lengths of exposure to V52. Predator and prey were mixed at an MOI of 1 (■) or 10 (◆). The results shown are the mean (±SD) of experimental duplicates.
To observe the kinetics of bacterial killing and further validate the previous results, E. coli survival was measured at multiple time intervals during exposure to V52 at a multiplicity of infection (MOI) of 1 and 10 (Fig. 2B). The resulting survival curves show a progressive reduction in E. coli survival due to extended exposure to wild-type V52 (Fig. 2B). Addition of V52 at a MOI of 10 and 1 caused 10,000- and 1,000-fold reductions in E. coli, respectively (Fig. 2B). Maximum killing occurred within 2 h, followed by the persistence of a smaller number of E. coli for the remaining exposure time (Fig. 2B).
Next we tested whether the addition of fresh V52 predator at the 2-h time point could eliminate or reduce the number of persistent E. coli survivors. Exposing E. coli prey to a second dose of V52 at the 2-h time point did not drastically affect the survival of E. coli and 102 cfu·mL−1 remained after 6 h (Fig. S2).
E. coli Are Undetectable After Exposure to V. cholerae.
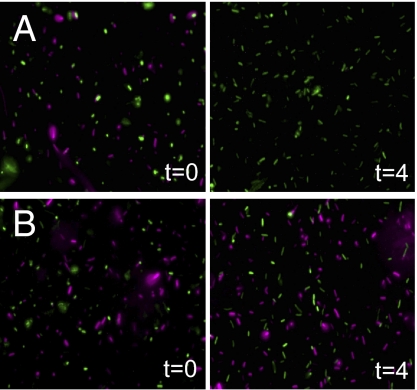
Fluorescence microscopy was used to determine the state of E. coli before and after exposure to V. cholerae strains V52 and V52ΔvasK, where V. cholerae and E. coli were distinguishable by fluorescence at separate wavelengths. V. cholerae and E. coli were incubated on Luria–Bertani medium (LB) agar for 4 h, at which time bacteria were harvested, washed, fixed, and inspected by fluorescence microscopy. E. coli was visibly detectable before exposure to V52 or V52ΔvasK, but was completely absent from the field of view after 4 h of exposure to V52 (Fig. 3A). The loss of fluorescent signal is indicative of cell lysis as released mCherry would have been lost during the washing step following the incubation on LB-agar plates. The lysis of E. coli can be attributed to the T6SS because the V52ΔvasK treatment had no effect on the visualization of E. coli (Fig. 3B).
Fig. 3.
E. coli cells are undetectable following exposure to V. cholerae. Detection of V. cholerae (green) and E. coli (red) by fluorescence microscopy before and after coincubation with V52 is shown. MG1655R/pTV-mCherry was mixed with V. cholerae strains V52/pBSW208lacP::GFP (A) and V52ΔvasK/pBSW208lacP::GFP (B) at an MOI of 0.1 and spotted onto LB for 4 h at 37 °C. Cells were visualized before (t = 0) and after (t = 4) coincubation by immobilization on glass slides.
Killing Requires Cell–Cell Contact.
On the basis of the current model for T6SS, substrates pass directly from the secreting cell into the cytoplasm of the target cell. To test whether T6SS-mediated antimicrobial activity requires contact between V. cholerae and E. coli, survival was measured with a 0.22-μm filter between V52 predator and E. coli prey to allow the passage of molecules but not bacterial cells (Fig. 4). The presence of the filter abrogated killing, made apparent by an increase in E. coli survival and cfu per milliliter values that were indistinguishable from those with no predator, V52ΔvasK, or E. coli as predator (Fig. 4).
Fig. 4.
Killing is contact-dependent. Contact-dependent killing assay: Survival of rifampicin-resistant E. coli was determined by measuring cfu following exposure to the indicated rifampicin-sensitive predator listed on the x axis with (green) or without (red) a 0.22-μm filter separating predator and prey. The data represent three independent experiments.
Actin-Cross-Linking Domain (ACD) of VgrG1 Is Not Required for Bacterial Killing.
T6SS-mediated killing of eukaryotic cells such as J774 macrophages and D. discoideum requires the activity of VgrG1 (3). Like the accessory toxin RtxA, VgrG1 carries a C-terminal ACD that, when exposed to a host cytosol, irreversibly cross-links host actin (16). We investigated whether the ACD could confer T6SS-mediated bacterial virulence by targeting MreB, a bacterial actin homolog capable of polymerizing into a protofilament similar to eukaryotic actin (17). Survival of E. coli prey was measured following exposure to V. cholerae predator strains V52, V52ΔvasK, and V52ΔACD in the killing assay, as described in Materials and Methods. V52ΔACD retained wild-type levels of E. coli killing, suggesting that MreB is not targeted in bacterial prey (Fig. 5).
Fig. 5.
T6SS-mediated killing of E. coli does not involve actin cross-linking. Killing assay: Survival of rifampicin-resistant E. coli was determined by measuring cfu following exposure to the indicated rifampicin-sensitive predator listed on the x axis. The data represent three independent experiments.
Pandemic O1 Serogroups Do Not Kill E. coli.
To investigate the prevalence of T6SS-mediated bacterial virulence among V. cholerae, pandemic strains of the O1 serogroup were tested for virulence toward E. coli. Survival of E. coli prey was measured following exposure to V. cholerae predator strains V52, V52ΔvasK, El Tor N16961, and classical O395 in the killing assay, as described in Materials and Methods. The O1 serogroup strains N16961 and O395 were found to be avirulent toward E. coli as E. coli survival was comparable to V52ΔvasK exposure (Fig. 6). Furthermore, expression of the T6SS genes vasK and hcp was not detected in these pandemic strains (Fig. S3).
Fig. 6.
O1 pandemic strains of V. cholerae are not virulent toward E. coli. Killing assay: Survival of rifampicin-resistant E. coli was determined by measuring cfu following exposure to the indicated rifampicin-sensitive predator listed on the x axis. The data represent three independent experiments.
V. cholerae Is Resistant to V52 T6SS-Mediated Bacterial Virulence.
Another important question regarding T6SS-mediated bacterial virulence is whether V52 is capable of distinguishing self from nonself. The killing assay was performed using rifampicin-resistant isolates of selected V. cholerae serogroups as prey and measuring survival following exposure to a rifampicin-sensitive predator V52 and V52ΔvasK. As prey, V52 and V52ΔvasK were unaffected by wild-type V52 exposure, whereas E. coli suffered a reduction of >1,000-fold (Fig. 7). Survival of O1 serogroup representatives, including the seventh pandemic El Tor strains N16961 and C6706, and the classical O395 strain was not significantly affected by exposure to wild-type V52 (Fig. 7).
Fig. 7.
V. cholerae is resistant to T6SS-mediated bacterial virulence. Killing assay: Survival of the indicated rifampicin-resistant E. coli and V. cholerae prey as listed on the x axis was determined by measuring cfu following exposure to rifampicin-sensitive predator V52 or V52ΔvasK. Bars represent the mean cfu per milliliter of surviving E. coli and V. cholerae prey from three independent experiments (±SD).
Discussion
The T6SS has been linked to virulence in a broad range of bacteria and a variety of bacterial processes, including pathogenicity, quorum-sensing regulation, interbacterial interactions, and biofilm formation (5, 6, 18, 19). In V. cholerae specifically, the T6SS has been implicated in virulence toward J774 murine macrophages and D. discoideum (3, 16). Consistent with its homology to the T4 phage cell-puncturing device, the T6SS targeted multiple Gram-negative bacteria and conferred up to a 100,000-fold reduction in E. coli counts (Figs. 1 and 2A). The data presented here suggest that the T6SS confers interbacterial virulence at a remarkably high efficiency. The finding that the T6SS-deficient vasK and vasH mutants showed toxicity defects toward E. coli indicates that killing is T6SS dependent (Fig. 2A).
Hood et al. recently demonstrated that the T6SS encodes a toxin-immunity system in P. aeruginosa to target bacterial cells (5). V. cholerae and P. aeruginosa convey antimicrobial activity using different mechanisms. The P. aeruginosa T6SS relies on the toxin and immunity genes tse2 and tsi2, which are absent from V. cholerae genomes on the basis of BLAST searches. Additionally, the V. cholerae T6SS kills bacteria at a higher rate (∼10,000-fold higher compared with P. aeruginosa). Although the P. aeruginosa retS mutant has shown virulence toward a P. aeruginosa tsi2 mutant, interspecies virulence remains to be shown (5). Recently, the T6SS-1 of B. thailandensis was also implicated in bacterial interspecies competition (6). Additional support for the T6SS having a role in bacterial killing is given in Salmonella species by the VgrG protein SPI-21, which has a C-terminal extension homologous to S-type pyocins (20). Pyocins, produced by P. aeruginosa, are a group of bacteriocins that kill bacteria of the same species (21).
We identified a direct relationship between the dose of V. cholerae and the number of E. coli recovered (Fig. 2B). The dose dependency suggested that V. cholerae directly reduced E. coli survival. However, because the killing assay measured survival on the basis of viability, it could not be established whether E. coli was in fact killed or transformed to a viable but nonculturable (VBNC) state (bacteria, including E. coli, can enter a VBNC state due to natural stress) (22). Fluorescence microscopy results were consistent with E. coli lysis (Fig. 3).
The lack of virulence following the placement of a 0.22-μm filter between V52 and E. coli, which is expected to disable direct contact while allowing the diffusion of secreted substrates, suggests that V52 directly contacts E. coli to enable bacterial killing and refutes the possibility that V52 secretes a diffusible substrate with toxicity toward E. coli (Fig. 4). These findings are consistent with the current T6SS model where the substrates are directly secreted into the target cell and suggest that the T6SS apparatus punctures the target bacterial cell, requiring contact, and injects one or more substrate(s) directly into the target cell.
The remarkable flexibility of the T6SS is demonstrated by its ability to target both eukaryotic and prokaryotic cells. T6SS-mediated virulence of V52 toward eukaryotic cells is known to depend on the ACD (16). Although it is plausible that the ACD could use virulence toward E. coli by targeting MreB, the ACD was not found to be essential for V52 T6SS-mediated bacterial virulence (Fig. 5). This result suggests that MreB is not a target of the T6SS and that the T6SS likely accommodates separate virulence factors depending on the target cell.
Although V52 was found to be highly virulent toward E. coli, representative pandemic strains N16961 and O395 from the O1 serogroup did not kill E. coli (Fig. 6). This result could be due to a difference in regulation and expression of the T6SS in the O1 serogroup strains compared with V52, where N16961 and O395 did not express the T6SS under laboratory conditions (Fig. S3). Lack of T6SS expression could explain the absence of T6SS-dependent phenotypes, including bacterial killing and virulence toward D. discoideum (3). The fact that all four V. cholerae serogroups tested were resistant to the T6SS's antimicrobial properties implies that the system is specific toward non-V. cholerae bacteria (Fig. 7). Intraspecies resistance may be due to a specific immunity protein, similar in idea to the toxin-immunity system encoded by the P. aeruginosa T6SS but encoded by unique toxin and immunity genes (5).
It was also apparent that maximum killing left a small number of E. coli survivors and a second dose of fresh V52 was unable to eliminate the prey organisms (Fig. 2B and Fig. S2). We are currently exploring explanations for why E. coli prey was not completely eliminated. Possible explanations include the development of resistance among a small group of E. coli prey; alternatively, the minority of prey following extensive bacterial reduction could make contact extremely unlikely, allowing for a small number of E. coli to evade predation.
On the basis of the 10 species tested as bacterial prey, the killing host range was found to be limited to selected Gram-negative coliforms. Because these coliforms and V. cholerae alike colonize the host gastrointestinal tract and inhabit an aquatic environment, we hypothesize that V. cholerae uses the T6SS as a means of competitive exclusion. Interspecies competition for nutrients and colonization of a favorable niche are an effective survival mechanism used by bacteria inhabiting harsh environments (23). By creating a niche free of other bacteria, V. cholerae could readily adhere to copepods and other abiotic surfaces and thus benefit from increased nutrient acquisition in a nutrient-poor environment (13). The persistence of V. cholerae in the environment during periods between epidemics is crucial to disease recurrence in some parts of the world. If the T6SS were able to promote intraspecies survival in the environment, it would have implications in the prevention and elimination of the disease cholera.
Materials and Methods
Bacterial Strains and Cell Cultures.
Bacterial strains were grown in LB broth and supplemented with 50 μg·mL−1 rifampicin where indicated. A V. cholerae V52 (O37 serogroup) derivative strain lacking hlyA, rtxA, and hapA was the background strain for all T6SS deletion mutants and was used as the wild-type strain in all experiments (3). The V52ΔACD mutant was provided by John Mekalanos (Harvard Medical School, Cambridge, MA) (16). E. coli K-12 strain MG1655 (genotype F−, λ−, rph-1) was provided by Tracy Raivio (University of Alberta). mCherry was designed as described in ref. 24. Plasmid pTV-mCherry was a gift from Lieke B. van Alphen (University of Alberta). Spontaneous rifampicin-resistant strains were isolated by growth on 50 μg·mL−1 rifampicin.
Killing Assay.
Bacterial strains were grown as lawns on LB-agar plates with the appropriate antibiotics. Rifampicin-resistant prey and rifampicin-sensitive predator were harvested and mixed at an MOI of 10 with volumes normalized by OD600 readings. Twenty-five microliters of the mixed bacterial culture was spotted onto prewarmed LB agar and incubated at 37 °C for 1–4 h, except for S. cerevisiae, which was incubated at 30 °C. Bacterial spots were harvested and the cfu per milliliter of surviving prey and predator were measured by serial dilution and selective growth on agar containing 50 μg·mL−1 rifampicin and 100 μg·mL−1 streptomycin, respectively. Complementation experiments were performed by cloning the respective genes into a pBAD24 vector with arabinose-induced expression on LB-agar plates containing 0.1% arabinose.
Reinfection Assay.
V52 predator and E. coli prey were mixed and treated as described in the killing assay at an MOI of 10. After 2 h the bacterial spot was harvested and resuspended in 1 mL of LB. A second dose of fresh kanamycin-resistant V52 was added equal to the initial number of cells added. The cfu per milliliter of surviving prey and predator were measured as previously described, with the cfu per milliliter of the surviving second dose of V52 determined by selective growth on agar containing 100 μg·mL−1 kanamycin.
Contact-Dependent Assay.
Predator and prey were mixed and treated as described in the killing assay at an MOI of 10 with the addition of a sterile 0.22-μm filter (Millipore). The filter was placed either directly on the LB-agar plate or on top of the predator cells. Prey were spotted on top of the filter. After 4 h incubation, cells were harvested by suspending the filter in 1 mL LB and vortexing for 10 s. The cfu per milliliter of surviving prey were determined by serial dilution and growth on selective media as described above.
Fluorescence Microscopy.
V. cholerae strains V52/pBSW208lacP::GFP and V52ΔvasK/pBSW208lacP::GFP were grown overnight on LB agar containing 100 μg·mL−1 ampicillin and 1.2 mM isopropyl-β-d-1-thio-galactopyranoside (IPTG). MG1655/pTV-mCherry was grown overnight on LB agar containing 100 μg·mL−1 ampicillin. Predator and prey were mixed as described in the killing assay at an MOI of 0.1. Twenty-five microliters of the bacterial mixture was spotted onto prewarmed LB agar containing 100 μg·mL−1 ampicillin and 1.2 mM IPTG and incubated at 37 °C for 4 h. Bacterial spots were washed in 1× PBS once and fixed in 4% paraformaldehyde for 1 h at room temperature. For microscopic examination, 1.5 μL of liquid culture was spotted onto a glass slide. Samples were examined using a Zeiss Axioskop2 plus fluorescent microscope and imaged with an AxioCam MRc camera, using Zeiss AxioVision software (AxioVs40 V 4.6.1.0).
Supplementary Material
Acknowledgments
We thank Daniele Provenzano, Tracy Raivio, Jon Dennis, and members of the S.P. laboratory for helpful discussions and Shahna Tariq and Karen Gahir for technical assistance. We thank Marcia Craig for critically reviewing the manuscript, and Kim Ellison and Randy Irvin (University of Alberta) for providing us with bacterial strains. Work in the S.P. laboratory is supported by Canadian Institute for Health Research Operating Grant MOP-84473 and by Alberta Innovates–Health Solutions (funded by the Alberta Heritage Foundation for Medical Research Endowment Fund). S.P. is the recipient of the Alberta Heritage Foundation for Medical Research Scholar Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012931107/-/DCSupplemental.
*Meeting on the Potential Role of New Cholera Vaccines in the Prevention and Control of Cholera outbreaks during Acute Emergencies. World Health Organization, Geneva, February 13–14, 1995, Document CDR/GPV/95.1.
References
- 1.Bryce J, Boschi-Pinto C, Shibuya K, Black RE WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 2.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 3.Pukatzki S, et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci USA. 2006;103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer F, et al. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genetic resources? BMC Genomics. 2009;10:104–117. doi: 10.1186/1471-2164-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood RD, et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 2010;7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz S, et al. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 2010;6:1–14. doi: 10.1371/journal.ppat.1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leiman PG, et al. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci USA. 2009;106:4154–4159. doi: 10.1073/pnas.0813360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci USA. 2007;104:15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pukatzki S, McAuley SB, Miyata ST. The type VI secretion system: Translocation of effectors and effector-domains. Curr Opin Microbiol. 2009;12:11–17. doi: 10.1016/j.mib.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Ballister ER, Lai AH, Zuckermann RN, Cheng Y, Mougous JD. In vitro self-assembly of tailorable nanotubes from a simple protein building block. Proc Natl Acad Sci USA. 2008;105:3733–3738. doi: 10.1073/pnas.0712247105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pell LG, Kanelis V, Donaldson LW, Howell PL, Davidson AR. The phage lambda major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system. Proc Natl Acad Sci USA. 2009;106:4160–4165. doi: 10.1073/pnas.0900044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam MS, Drasar BS, Sack RB. The aquatic flora and fauna as reservoirs of Vibrio cholerae: A review. J Diarrhoeal Dis Res. 1994;12:87–96. [PubMed] [Google Scholar]
- 13.Huq A, et al. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol. 1983;45:275–283. doi: 10.1128/aem.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field M, Fromm D, al-Awqati Q, Greenough WB., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972;51:796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakrabarty AN, Adhya S, Basu J, Dastidar SG. Bacteriocin typing of Vibrio cholerae. Infect Immun. 1970;1:293–299. doi: 10.1128/iai.1.3.293-299.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma AT, McAuley S, Pukatzki S, Mekalanos JJ. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe. 2009;5:234–243. doi: 10.1016/j.chom.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Ent F, Amos LA, Löwe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001;413:39–44. doi: 10.1038/35092500. [DOI] [PubMed] [Google Scholar]
- 18.Weber B, Hasic M, Chen C, Wai SN, Milton DL. Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environ Microbiol. 2009;11:3018–3028. doi: 10.1111/j.1462-2920.2009.02005.x. [DOI] [PubMed] [Google Scholar]
- 19.Aschtgen MS, Bernard CS, De Bentzmann S, Lloubès R, Cascales E. SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli. J Bacteriol. 2008;190:7523–7531. doi: 10.1128/JB.00945-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blondel CJ, Jiménez JC, Contreras I, Santiviago CA. Comparative genomic analysis uncovers 3 novel loci encoding type six secretion systems differentially distributed in Salmonella serotypes. BMC Genomics. 2009;10:354. doi: 10.1186/1471-2164-10-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel-Briand Y, Baysse C. The pyocins of Pseudomonas aeruginosa. Biochimie. 2002;84:499–510. doi: 10.1016/s0300-9084(02)01422-0. [DOI] [PubMed] [Google Scholar]
- 22.Oliver JD. The viable but nonculturable state in bacteria. J Microbiol. 2005;43(Spec No):93–100. [PubMed] [Google Scholar]
- 23.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: Surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.