Abstract
The immune response elicited after Mycobacterium tuberculosis (Mtb) infection is critically dependent on CD4 T cells during both acute and chronic infection. How CD4 T-cell responses are maintained throughout infection is not well understood, and evidence from other infection models has suggested that, under conditions of chronic antigen stimulation, T cells can undergo replicative exhaustion. These findings led us to determine whether subpopulations of CD4 T cells existed that displayed markers of terminal differentiation or exhaustion during murine Mtb infection. Analysis of antigen-specific effector CD4 T cells revealed that programmed death-1 (PD-1) and the killer cell lectin-like receptor G1 (KLRG1) delineated subpopulations of T cells. PD-1–expressing CD4 T cells were highly proliferative, whereas KLRG1 cells exhibited a short lifespan and secreted the cytokines IFNγ and TNFα. Adoptive transfer studies demonstrated that proliferating PD-1–positive CD4 T cells differentiated into cytokine-secreting KLRG1-positive T cells, but not vice versa. Thus, proliferating PD-1–positive cells are not exhausted, but appear to be central to maintaining antigen-specific effector T cells during chronic Mtb infection. Our findings suggest that antigen-specific T-cell responses are maintained during chronic mycobacterial infection through the continual production of terminal effector cells from a proliferating precursor population.
Tuberculosis presents a challenging worldwide public heath problem. Infection with Mycobacterium tuberculosis (Mtb) elicits humoral and cellular immune responses that normally control bacterial burden. However, bacteria are seldom, if ever, eradicated, and control of the infection requires continual effector T-cell responses. Consequently, either the depletion or suppression of T-cell responses results in disease reactivation (1). In the mouse model, Mtb causes chronic infection and control of infection is maintained by T cells essentially indefinitely (2).
Although a sustained T-cell response against Mtb infection is necessary, it is not understood how effector T cells are maintained. We have previously demonstrated that CD4 T-cell responses are associated with extensive proliferation throughout Mtb infection (3), suggesting that proliferation is a major mechanism required for the maintenance of T-cell responses. However, it is not clear how T-cell proliferation can be maintained during chronic infection, especially because effector T cells have been described to have a limited capacity for self-renewal (4).
Although the numbers of antigen-specific T cells are relatively stable during chronic Mtb infection, CD4 T cells proliferate extensively, suggesting that a high turnover of effector CD4 T cells occurs. This high level of turnover suggests that effector T cells become exhausted or terminally differentiated, and that the maintenance of the T-cell response depends on the continual replacement of effector T cells. The expression of several cell-surface receptors has been correlated with functional exhaustion and terminal differentiation in effector T cells (5, 6). These receptors include the programmed death-1 (PD-1) and the killer cell lectin-like receptor G1 (KLRG1). Blockade of PD-1–programmed death ligand 1 (PDL1) interactions in vivo during chronic infection with lymphocytic choriomeningitis virus (LCMV) clone 13 has been shown to increase the frequencies and numbers of antigen-specific CD8 T cells for LCMV epitopes, and to decrease viral titers (5). KLRG1 expression, during acute LCMV infection, has been associated with proliferative senescence and low expression of the IL-7R (7). Other studies of KLRG1-positive effector T cells during acute and chronic infections found the antigen-specific cells to be terminally differentiated, as they exhibited decreased multicytokine potential and were relatively short-lived (6).
In this study, we have addressed whether KLRG1 and PD-1 expression by CD4 T cells identifies functionally exhausted CD4 T cells during Mtb infection. We show that, unlike in other infection models, PD-1–expression identifies a population of activated effector T cells, and that PD-1–expressing cells transition into terminally-differentiated KLRG1-expressing effector cells. These data reveal that PD-1 and KLRG1 identify differentiating, not exhausted, CD4 T cells during Mtb infection.
Results
CD4 T Cells Undergo Continual Proliferation During Chronic Mtb Infection.
A substantial fraction of the T-cell response in C57BL/6 mice is specific for the early secreted antigenic target 6 (ESAT6)1–20/I-Ab epitope following aerosol challenge with a low dose of Mtb. We had previously shown that CD4 T cells in the lung proliferate extensively during both acute and chronic infection (3). However, these experiments used indirect techniques for the identification of ESAT6-specific CD4 T cells, an approach that did not allow us to directly correlate antigen-specific cells with proliferation. To circumvent this limitation, we took advantage of an ESAT64–17/I-Ab class II MHC tetramer reagent that allowed us to directly identify antigen-specific cells. The specificity of the tetramer was validated by demonstrating that the reagent detected antigen-specific CD4 T cells only from mice that were infected with ESAT6-expressing strains of Mycobacteria (Fig. S1).
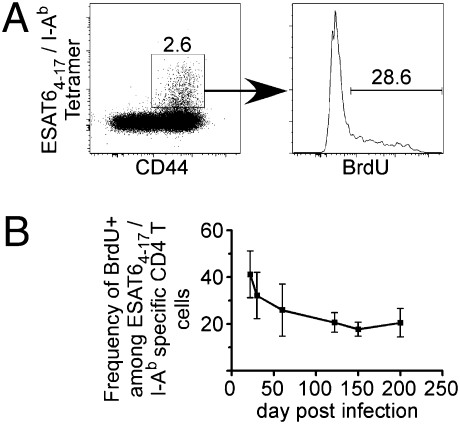
Similar to our previously reported findings (3), ≈40% of ESAT64–17/I-Ab tetramer-positive lung CD4 T cells incorporated BrdU within 24 h of administration, on day 21 postaerosol Mtb infection (Fig. 1). Thereafter, the percentage of T cells that divided in a 24-h interval declined by about twofold, and was maintained at ≈20% for at least 200 d. These data confirm that, in the mouse, the maintenance of protective immunity during Mtb infection is associated with a continual turnover of antigen-specific CD4 T cells. This finding contrasts the slow decline of CD4 memory T cells that occurs following acute infections, such as LCMV (8) and Listeria monocytogenes (9).
Fig. 1.
The proliferative capacity of antigen-specific cells diminishes during Mtb infection. (A) BrdU was administered to C57BL/6 mice on day 30 after low-dose aerosol infection with Mtb (strain H37Rv) and BrdU incorporation in ESAT64–17/I-Ab antigen-specific CD44hi cells gated on CD4hi T cells in the lung was measured 1 d later. (B) The percentages of ESAT64–17/I-Ab antigen-specific CD4 T cells that incorporated BrdU on the indicated days postinfection are shown. Data are presented from the lungs of five mice analyzed on each day. Error bars indicate SD from the mean, and are shown from two experiments.
KLRG1 and PD-1 Identify Subpopulations of Effector CD4 T Cells.
Although antigen-specific CD4 T cells undergo continual proliferation, total cell numbers remained relatively constant throughout the infection. This observation suggested that effector T cells within the lungs comprise subpopulations of proliferating, apoptotic, and perhaps exhausted cells. T-cell exhaustion has been demonstrated for CD8 T cells in several different chronic viral infections, and describes cells that fail to proliferate and produce cytokines (5, 10).
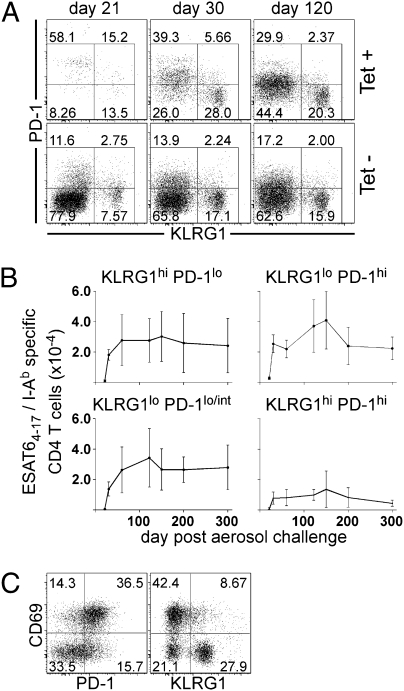
To address whether CD4 T cells undergo functional exhaustion during Mtb infection, we monitored the expression of surface proteins known to be associated with terminally differentiated and exhausted T cells, KLRG1 and PD-1 (5, 6). During Mtb infection we made the following three observations. First, on day 21 postinfection, a majority of ESAT64–17/I-Ab–specific CD4 T cells expressed high levels of surface PD-1 (Fig. 2A). Second, as the infection progressed, surface expression of PD-1 was reduced on the population of antigen-specific cells, such that by day 60 a significant population of these cells was found to express low-to-intermediate levels of PD-1 (Fig. 2B). This result was shown by a decrease in the mean fluorescent intensity (MFI) of PD-1 expression on days 21, 30, and 120 postinfection (MFI values of 2,056 ± 195, 1,357 ± 180, and 1,013 ± 74, respectively). Third, by day 30 postinfection, a population of antigen-specific CD4 T cells expressed KLRG1 but not PD-1 in the lungs of infected mice (Fig. 2 A and B). These three cell populations, PD-1hi, PD-1lo/int, and KLRG1hi could also be detected within the nontetramer-binding host CD4 T-cell population throughout Mtb infection.
Fig. 2.
Antigen-specific cells display markers of terminal differentiation or senescence during Mtb infection. (A) Flow cytometric gating strategy used to analyze KLRG1 and PD-1 expression on ESAT64–17/I-Ab antigen-specific CD4 T cells. (B) The number of KLRG1- and PD-1–expressing ESAT64–17/I-Ab antigen-specific CD4 T cells during Mtb infection. Data are presented from the lungs of five mice analyzed on each day. The error bars indicate the SD. The data are representative of two independent experiments. (C) The dot plots are representative of the CD69 and PD-1 or CD69 and KLRG1 expression on ESAT64–17/I-Ab antigen-specific CD4 T cells isolated from lungs of mice on day 30 postinfection.
The prevalence of large numbers of KLRG1- and PD-1–expressing cells suggested that a substantial fraction of the T cells were exhausted under steady-state conditions. We therefore examined several other activation markers (i.e., CD44, CD62L, CD27, and CD127) and found that they did not differ between the KLRG1- and PD-1–expressing cell populations during infection. However, the early activation marker, CD69 (11), was differentially expressed on PD-1 and KLRG1 subpopulations. By day 30 postinfection, most PD-1–positive cells, but few KLRG1-positive cells, expressed CD69 (Fig. 2C). Thus, cells expressing PD-1, a marker of chronic exhaustion, also express a marker found on recently activated effector T cells.
PD-1 Is Not a Marker of Chronically Exhausted T Cells in Mtb Infection.
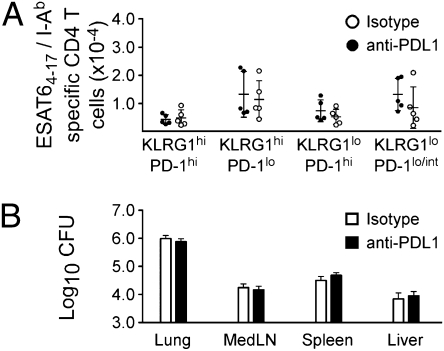
We next addressed whether PD-1 expression identified exhausted CD4 T cells by blocking PD-1/PDL1 interactions in vivo. In previous studies it has been demonstrated that blockading PD1/PDL1 interactions during LCMV chronic infection reversed CD8 T-cell exhaustion (12). We administered anti-PDL1 (clone 10F9.2, 200 μg) to mice beginning on day 30 postinfection, and at 3-d intervals thereafter, until day 45 postinfection. We validated the blocking antibody efficacy within Mtb-infected animals by confirming that a single administration of the anti-PDL1 antibody in vivo inhibited the detection of this ligand by flow cytometry (Fig. S2A). One day after the last antibody administration, no significant differences were observed in the number or phenotype KLRG1- and PD-1–expressing antigen-specific CD4 T-cell populations (Fig. 3A). Moreover, no differences were evident in the ability of the T cells to produce IFNγ or TNFα, following stimulation with antigen in vivo (Fig. S2B), and bacterial burden was unaffected following antibody treatment (Fig. 3B). Similarly, no differences were observed in the number (Fig. S2C) or function (Fig. S2D) of antigen-specific CD8 T cells following PD-1 blockade.
Fig. 3.
PD-1–PDL1 blockade does not affect the number or function of antigen-specific T cells. Mtb-infected mice were treated with anti-PDL1, from day 30 to day 45 postinfection. (A) The numbers of ESAT64–17/I-Ab antigen-specific CD4 T cells among each of the indicated populations of KLRG1- and PD-1–expressing cells in the lung are shown. Each datum represents an individual mouse. (B) Bacterial burdens in the lung, mediastinal lymph node, spleen, and liver, were determined on day 46 postinfection. The data are representative of two experiments of similar design.
The engagement of PD-1 and PDL1 following T-cell receptor stimulation leads to the inhibition of PI3K activity, as well as the down-regulation of the antiapoptotic protein Bcl-xL (13). However, evaluation of Bcl-xL expression was identical in KLRG1- and PD-1–expressing cell populations at several times postinfection (Fig. S3). These data indicate that, unlike its role in chronic viral infections, PD-1 expression on antigen-specific T cells during the chronic phase of Mtb infection does not identify a functionally exhausted T cell.
PD-1 Is Expressed on Effector CD4 T Cells Before Their Differentiation into KLRG1-Expressing Cells.
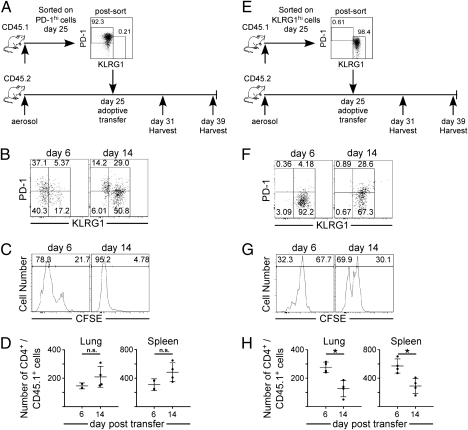
We next examined whether PD-1–expressing cells comprised a stable cell population, or if they underwent changes in phenotype during infection. To address this question, PD-1hi KLRG1lo CD4 T cells were purified by flow cytometric cell sorting from Mtb-infected B6.SJL-Ptprca Pep3b/BoyJ mice on day 25 postinfection. Purified T cells were transferred to C57BL/6J-infected mice, and donor T cells in the recipient mice were analyzed 6 and 14 d later (Fig. 4A). PD-1 expression was down-regulated on donor cells within 6 d after transfer, and by day 14 posttransfer, 80% of the donor T cells expressed KLRG1 (Fig. 4B). This differentiation of PD-1–expressing cells into KLRG1 was not seen when cells were adoptively transferred into naive mice (Fig. S4). The changes in cell-surface phenotype was accompanied by cell division, as most PD-1–expressing donor cells had diluted their 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) within 6 d of transfer, and ∼95% had proliferated by day 14 posttransfer (Fig. 4C). Consistent with these findings, the donor cells increased in number during this period (Fig. 4D). These studies demonstrate that PD-1 expression identifies a population of early activated and proliferating T cells that undergo phenotypic transition to KLRG1-expressing cells.
Fig. 4.
Differentiation and proliferation of PD-1– and KLRG1-expressing CD4 T cells. (A and E) B6.SJL-Ptprca Pep3b/BoyJ mice were infected, lymphocytes from the spleen, mediastinal lymph node, and lung were isolated on day 25 postinfection, and PD-1– or KLRG1-expressing cells were purified by flow cytometric cell sorting. CFSE-labeled PD-1–expressing cells (2.3 × 104) or KLRG1-expressing cells (2.5 × 104) were transferred on day 25 to infected congenic C57BL/6 recipient mice. (B and F) Representative dot plots of KLRG1 and PD-1 expression on CD4+/CD45.1+ donor cells in the lung on days 6 and 14 following transfer. Gates were set on the host population CD4 T cells. (C and G) A representative histogram of CFSE staining on CD4+/CD45.1+ donor T cells in the lung on days 6 and 14 after transfer. (D and H) The number of CD4+/CD45.1+ donor cells in the lung and spleen are shown. Differences with a P value <0.05 were considered significant and are denoted by an asterisk.
KLRG1 Expression Defines a Population of Terminally Differentiated T Cells.
Previous studies suggested that KLRG1 expression defined a population of terminally differentiated, nondividing T cells that were capable of cytokine production (6, 7). Moreover, engagement of KLRG1 by its ligand, E-cadherin, has been shown to mitigate T-cell receptor signaling and to lead to decreased functional activity (14). Because approximately one-third of the antigen-specific T cells express KLRG1 throughout Mtb infection, we hypothesized that this cell population may be a terminally differentiated population of effector cells with reduced proliferative potential.
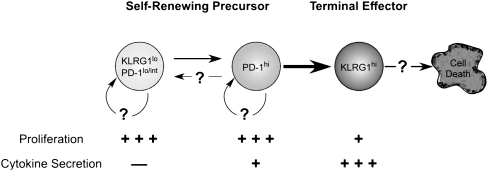
To address this question, we purified lung-resident KLRG1hi PD-1lo CD4 T cells from infected mice by flow cytometry and transferred the cells to recipient mice on day 25 postinfection (Fig. 4E). In these studies, the donor KLRG1-positive T cells, unlike the PD-1–expressing cells, retained KLRG1 expression for at least as long as 14 d (Fig. 4F). The KLRG1-expressing cells proliferated, although not to the extent observed in the PD-1 population (i.e., only 32 and 70% had proliferated within 6 and 14 d, respectively) (Fig. 4G). In contrast to the studies of the PD-1–expressing cells, the KLRG1-positive cells also decreased in number following transfer (Fig. 4H). These studies demonstrate that, although the KLRG1-expressing cells retain some proliferative potential, surface expression of KLRG1 ultimately marks short-lived T cells during Mtb infection. Therefore, the KLRG1 cell population is likely maintained via the proliferation and differentiation of PD-1–expressing cells (Fig. 5).
Fig. 5.
Model of antigen-specific CD4 T-cell differentiation and self-renewal during Mtb infection. We hypothesize that PD-1–expressing effector CD4 T cells differentiate into KLRG1-expressing cells, as delineated by the bold arrow. In this model, a self-renewing population of either PD-1lo/int– or PD-1hi–expressing effector cells maintains antigen-specific T-cell numbers. PD-1lo/int cells can differentiate into cells that express higher levels of PD-1. Presently, it is unclear if the surface expression of PD-1 on antigen-specific CD4 T cells, once up-regulated, can be down-regulated to a low or intermediate phenotype. The KLRG1-expressing T cells secreted both IFNγ and TNFα, underwent only moderate proliferation, and exhibited a shorter life span, all characteristics of terminally differentiated cytokine-secreting cells.
KLRG1- and PD-1–Expressing Cells Exhibit Different Capacities for Cytokine Production.
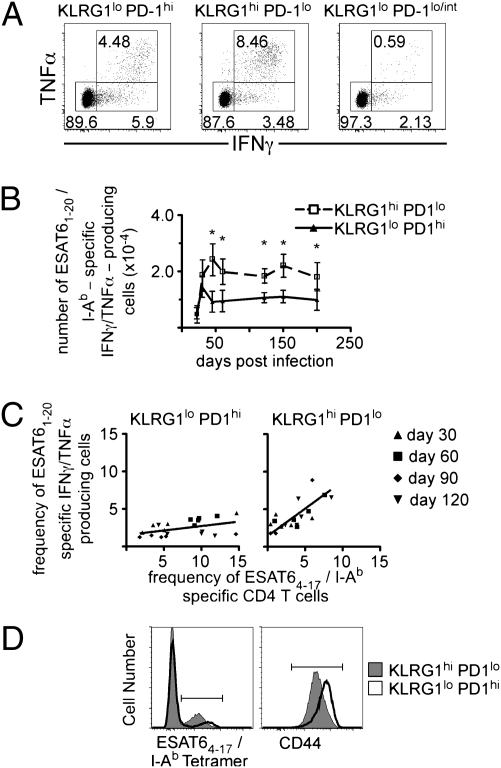
To determine if effector cell differentiation correlated with functional differences, we examined the capacity of PD-1– and KLRG1-expressing cells to produce inflammatory cytokines ex vivo. Both KLRG1hi- and PD-1hi–expressing cells produced both IFNγ and TNFα in vitro in response to stimulation with ESAT61–20 peptide. However, compared with the PD-1hi cells, approximately twice as many KLRG1hi cells were capable of producing both cytokines; this pattern persisted for at least 200 d following Mtb infection (Fig. 6 A and B). In these studies, we confirmed that at a population level, surface expression of KLRG1 and PD-1 remained unchanged during the 5-h in vitro culture, and that cell numbers remained stable after peptide treatment. We also observed a nearly one-to-one relationship between the frequency of KLRG1-expressing ESAT6 tetramer-positive cells and the frequency of ESAT6-specific IFNγ- and TNFα-producing T cells within the KLRG1-expressing CD4 subset, independent of the day postinfection when the cells were harvested (Fig. 6C). We interpret these data to mean that nearly all of the KLRG1-expressing cells were cytokine-producing cells. In contrast, only a minority of PD-1–expressing cells were capable of producing cytokines.
Fig. 6.
KLRG1 expression identifies terminally differentiated cytokine secreting cells. Lymphocytes isolated from the lung of Mtb-infected mice were stimulated with ESAT61–20 peptide for 5 h in the presence of brefeldin A. (A) Representative dot plots of IFNγ-and TNFα-producing KLRG1lo PD-1hi, KLRG1hiPD-1lo, and KLRG1lo PD-1lo/int CD4 T cells on day 60 postinfection. (B) The number of ESAT6-specific IFNγ- and TNFα-producing KLRG1- or PD-1–expressing CD4 T cells is shown for the indicated days postinfection. Data are presented from the lungs of five mice analyzed on each day. The error bars indicate the SD. The analysis was conducted from two experiments of similar design. (C) The frequencies of ESAT64–17/I-Ab tetramer-positive cells within the KLRG1- or PD-1–expressing populations were plotted against the frequency of ESAT61–20/I-Ab IFNγ- and TNFα-producing cells within these same populations in each mouse. The best-fit line was generated using least-squares regression analysis. (D) A representative histogram of ESAT64–17/I-Ab tetramer expression and CD44 expression on KLRG1 (filled)- or PD-1 (open)–expressing CD4 T cells, on day 60 postinfection. The MFI of ESAT64–17/I-Ab tetramer-positive cells was 2,147 ± 371 for PD-1–expressing cells and 1,280 ± 439 for KLRG1-expressing cells. The MFI of CD44 was 6,555 ± 935 for PD-1–expressing cells and 2,617 ± 352 for KLRG1-expressing cells. The data are representative of mice analyzed at several different time points in two experiments. Differences with a P value <0.05 were considered significant and are denoted by an asterisk.
Some mice had higher frequencies of ESAT6-specific IFNγ- and TNFα-producing T cells, relative to ESAT6 tetramer-positive cells within the KLRG1 population. We hypothesized that this difference was a result of T-cell receptor down-regulation, perhaps as a consequence of chronic antigen stimulation. T-cell receptor down-regulation on antigen-specific effector CD8 T cells has been reported in human chronic hepatitis B virus infection (15). Similarly, we observed that KLRG1-expressing cells exhibited decreased surface receptor expression of both T-cell receptors, as measured by intensity of ESAT64–17/I-Ab tetramer binding and CD44 (Fig. 6D). These findings suggest that the down-regulation of the T-cell receptor on KLRG1-expressing cells during Mtb infection is caused by stimulation by antigen within the lung of infected mice.
Taken together, our results demonstrate that the T-cell response during Mtb infection is highly dynamic, involving a linear process of T-cell maturation. We find that a less differentiated but highly proliferative population of PD-1–expressing T cells matures into a terminally differentiated cytokine-producing population of KLRG1-expressing T cells. This linear progression of T-cell differentiation is a major pathway by which T-cell responses are maintained during infection with Mtb.
Discussion
Although many studies have examined the kinetics of the CD4 T-cell responses at the population level during Mtb infection, the phenotypic heterogeneity of the CD4 T-cell population had not been investigated in depth. Our studies have focused on two phenotypic markers commonly associated with exhaustion or terminal differentiation in peripheral T cells, PD-1 and KLRG1. We have shown that effector CD4 T cells identified by these markers do not exhibit characteristics of exhausted T cells. Both PD-1– and KLRG1-expressing cells proliferated during infection and produced cytokines, although the two subsets exhibited differing capacities for these functions. Instead, PD-1 and KLRG1 expression define functionally distinct subsets of effector CD4 T cells. PD-1 expression identifies activated effector cells that exhibit a higher proliferative capacity than the KLRG1-expressing cells, but a lower capacity for cytokine production. In contrast, KLRG1-expressing T cells produce cytokines with a higher efficiency. These findings, along with the observation that PD-1–expressing cells transition into KLRG1–expressing cells, suggest that PD-1–positive T cells are a less-differentiated CD4 T-cell subset, and that additional signals are required for their maturation into terminally differentiated cytokine-producing effector cells. A model that summarizes our findings, and a proposed scheme for effector cell differentiation during Mtb infection, is shown in Fig. 5. The differences we have observed between PD-1– and KLRG1-expressing T-cell subsets suggest that yet additional heterogeneity exists within effector CD4 T-cell populations in vivo. Such heterogeneity may include differences in the ability of the cell subsets to localize in and around granulomas, or even within specific lymph nodes or peripheral sites.
Although previous studies have demonstrated that PD-1–expressing CD8 T cells exhibited decreased proliferative potential and cytokine production, PD-1 has commonly been used as a marker of T-cell functional exhaustion in both mouse and human infections (16) and in autoimmunity (17). Our data demonstrate that PD-1 surface expression on CD4 T cells, during chronic Mtb infection, is not a marker of exhausted T cells during Mtb infection. Support for this conclusion comes from the PD-1/PDL1 blockade studies, where no effect was observed on either bacterial burden or antigen-specific T-cell responses. PDL1 was expressed in the lungs during Mtb infection, indicating that the ligand was likely available to T cells during infection. Our results demonstrate that cells that express PD-1 are less differentiated, and have the capacity to differentiate into antigen-specific cells with other effector functions.
These data raise questions regarding the role of PD-1 on T cells. One possible explanation for the observation that PD-1 is not a marker of exhaustion in CD4 T cells is that previous studies focused on antigen-specific CD8 T cells. Lineage-specific differences may result from the utilization of different signaling pathways in the T-cell subsets. An alternative explanation is that signal transduction via the PD-1 pathway during chronic viral infections are inherently different from those that occur during chronic bacterial infections. In light of our findings, careful consideration should be used when associating the expression of PD-1 on antigen-specific cells with functional exhaustion.
It has been demonstrated that KLRG1 expression identifies short-lived, terminally differentiated effector T cells with minimal proliferative capacity (6, 7, 18). Our results are in partial agreement with these previous studies, in that the surface expression of KLRG1, once expressed on T cells, is maintained. Moreover, KLRG1-expressing cells transferred into Mtb-infected mice failed to persist, a finding that is similar to that reported by Joshi et al. in their studies of short-lived effector cells (6). However, we have observed that cytokine production differs between KLRG1-expressing and non–KLRG1-expressing cells, indicating that additional functional differences demarcate these cell populations. Furthermore, in our studies the KLRG1-expressing cells retained a limited capacity for cell division, although they did not proliferate to the same extent as the PD-1–expressing cells. One explanation for the ability of KLRG1-expressing cells to proliferate is that KLRG1 cells had recently transitioned from PD-1–expressing cells and, for a limited period, retain their proliferative ability. Alternatively, the survival or maintenance of KLRG1 cells during Mtb infection may require proliferation because of expression of other coreceptors induced during infection or from external cytokine stimulation within local microenvironments of infected lung tissue.
Antigen-specific T-cell numbers, which are quite stable throughout chronic Mtb infection, must be maintained by a source of new effector cells. Our previous studies have revealed that replenishment of the effector T-cell pool does not appear to require new thymic emigrants (3), which would suggest that newly derived T cells do not appear to provide a major mechanism whereby chronic T-cell responses are maintained during Mtb infection. Rather, these data demonstrate that a precursor population must exist that continually produces new terminal effectors throughout the infection. The origin or location of such a pool of proliferating effector cells is not known, although possible sources of T cells may include secondary lymph nodes, spleen, or other organs (Fig. 5). Such a putative self-renewing effector T-cell population may bear some similarity to “memory stem cells” that have been described recently (19). The existence of such a population of self-renewing effector T cells during chronic infections may have importance for understanding the mechanisms of T-cell persistence for not only human Mtb, but for other chronic infections.
Materials and Methods
Animals.
C57BL/6J and B6.SJL-Ptprca Pep3b/BoyJ (CD45.1) mice were purchased from The Jackson Laboratory. C57BL/6 mice were maintained in specific pathogen-free facilities at the Trudeau Institute (Saranac Lake, NY) and at the University of Washington (Seattle, WA). Experimental mice were age- and gender-matched, and were infected between 8 and 12 wk of age. Mice were used in accordance with the Institutional Animal Care and Use Committee guidelines of the National Research Council, the Trudeau Institute, and the University of Washington.
Infections.
Aerosol infections were preformed with a low dose of bacteria (∼75 CFU), using a Glas-Col airborne infection system, as described previously (20). Bacterial burden was measured by serial dilutions of whole-organ homogenates (20).
Lymphocyte Isolation and Flow Cytometry.
Lung tissue was prepared by injecting intact lungs with a 0.5 mg/mL solution of collagenase (Roche) and DNase (Sigma Aldrich); the tissue was coarsely chopped and incubated for 30–45 min at 37 °C. Single-cell suspensions were prepared from lung tissue, lymph nodes, or spleens by dispersing the tissues through a 70-μm nylon tissue strainer (BD Falcon). The cell suspensions were treated with Gey's solution to remove erythrocytes. Lymphocytes were enriched from lung tissue by differential centrifugation, using a gradient of 40/80% Percoll. Cells were stained with fluorochrome-labeled antibodies for anti-CD4 (clone RM4-5), anti-CD8 (clone 5H10), anti-CD45.2 (104) and anti-CD69 (H1.2F3), anti-BrdU, streptavidin-PerCP-Cy5.5 (all from BD Biosciences); anti-PDL1 (10F.9G2), anti-CD45.1 (A20), anti–PD-1 (RPM1) (all from BioLegend); anti-KLRG1 (2F1) (Southern Biotech); anti–Bcl-XL (H-5) (Santa Cruz Biotechnology); and anti-PDL1 (HI5I), anti-CD44 (IM7) (eBioscience). The samples were analyzed on a LSRII flow cytometer (BD Biosciences). For the adoptive transfer studies, the low numbers of adoptively transferred KLRG1- and PD-1–expressing T cells required an enrichment method to detect them within recipient mice, as has been previously described (21). The flow cytometry data were analyzed using FlowJo software (Tree Star).
Intracellular Cytokine Detection.
Lymphocytes isolated from infected mice were incubated in a 96-well plate, at a concentration of 3 × 106 cells per well. Cells were incubated in the presence of ESAT-61–20 or Sendai HN421–436 peptide (5 μg/mL each) for 2 h at 37 °C; Brefeldin A (50 μg/mL) was added, and the incubation was continued for an additional 4 h. Surface staining for CD4, CD8, PD-1, and KLRG1 was performed, as described previously (22), and the cells were fixed and permeabilized using a Cytofix/Cytoperm Fixation/Permeabilization kit (BD Biosciences). For detection of intracellular IFNγ and TNFα, the cells were incubated for 30 min in Perm/Wash Buffer (BD Biosciences) with anti-IFNγ (clone XMG1.2) and anti-TNFα (clone MP6-XT22); the cells were analyzed as described above.
Generation of the MHC Class II ESAT64–17/I-Ab Tetramer Reagent.
The ESAT64–17/I-Ab tetramer was generated in the laboratory of Dr. M. Jenkins, University of Minnesota, as described by Moon et al. (23). In brief, a set of overlapping oligonucleotides encoding the ESAT6 peptide sequence (amino acid residues 4–17) was clone into the expression plasmid pRMHa3 I-Ab. The plasmids were transfected into Drosophila S2 cells, using the DES Drosophila Expression System (Invitrogen). Transfected S2 cells were flow cytometrically sorted for the highest surface expression of the ESAT64–17 I-Ab tetramer. ESAT64–17 I-Ab tetramer expression was induced in the transfected S2 cells using CuSO4. Soluble I-Ab heterodimers were purified from cell cultures supernatants using nickel affinity chromatography and purified by size-exclusion chromatography. Biotinylated heterodimers were tetramerized using streptavidin-PE, and the labeled tetramers were separated from heterodimers using size-exclusion chromatography.
BrdU Incorporation.
BrdU (4 mg/mL in PBS; Sigma-Aldrich) was injected into the peritoneum of mice 24 h before the assay (200 μL per mouse). Single-cell suspensions were stained with the ESAT6 tetramer, followed by surface staining. BrdU incorporation was detected using a BrdU Flow Kit (BD Biosciences).
Isolation of KLRG1- and PD-1–Expressing CD4 Effector T Cells.
Lymphocytes were isolated from spleen, mediastinal lymph nodes, and lungs, and CD4 T cells were enriched using a CD4+ T cell-isolation kit (Miltenyi Biotec). Enriched CD4 T cells were stained with anti–KLRG1-PE and anti–PD-1-biotin, followed by streptavidin-PerCP-Cy5.5. The cells were flow cytometrically sorted into two subsets, KLRG1hi PD-1lo and KLRG1lo PD-1hi. Sorted cells were labeled with 0.5 μM CF(DA)SE and were transferred i.v. into day 25 Mtb-infected congenic mice. Cell sorting was performed on a FACSVantage cell sorter (BD Biosciences).
In Vivo PDL1 Antibody Blockade.
In vivo antibody treatment of infected mice was preformed as described previously (5). Anti-mouse PDL1 (clone 10F.9G2; BioXCell) or a rat IgG2b isotype control Ig (200 μg each) were administered every third day, beginning on day 30 postinfection, for 15 d.
Statistic Analysis.
Statistical analysis was preformed with Prism5 GraphPad software using a two-tailed student's t test. Differences with a P value <0.05 were considered significant and are denoted by an asterisk.
Supplementary Material
Acknowledgments
We thank Dr. M. Blackman for critical review of the manuscript and Weldon Debusk for assistance with cell sorting. This work was supported by Public Health Service Grants RO1AI073564 (to D.L.W. and G.M.W.), R21AI077531 (to G.M.W. and D.L.W.), RO1AI 076327-01 (to K.B.U.), a Burroughs-Wellcome Fund Career Award in the Biological Sciences (to K.B.U.), and R01AI39614 (to M.K.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006298107/-/DCSupplemental.
References
- 1.Scanga CA, et al. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 3.Winslow GM, Roberts AD, Blackman MA, Woodland DL. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J Immunol. 2003;170:2046–2052. doi: 10.4049/jimmunol.170.4.2046. [DOI] [PubMed] [Google Scholar]
- 4.Hayflick L. Human cells and aging. Sci Am. 1968;218:32–37. doi: 10.1038/scientificamerican0368-32. [DOI] [PubMed] [Google Scholar]
- 5.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 6.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voehringer D, et al. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 8.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 9.Pepper M, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 11.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 12.Ha SJ, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosshart S, et al. Interaction of KLRG1 with E-cadherin: New functional and structural insights. Eur J Immunol. 2008;38:3354–3364. doi: 10.1002/eji.200838690. [DOI] [PubMed] [Google Scholar]
- 15.Reignat S, et al. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med. 2002;195:1089–1101. doi: 10.1084/jem.20011723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 17.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts A, et al. In: Methods in Microbiology. Kaufmann S, Kabelitz D, editors. London: Academic Press; 2002. pp. 433–462. [Google Scholar]
- 21.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiley WW, et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc Natl Acad Sci USA. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.