Abstract
The resurrection of endogenous retroviruses from inactive molecular fossils has allowed the investigation of interactions between extinct pathogens and their hosts that occurred millions of years ago. Two such paleoviruses, chimpanzee endogenous retrovirus-1 and -2 (CERV1 and CERV2), are relatives of modern MLVs and are found in the genomes of a variety of Old World primates, but are absent from the human genome. No extant CERV1 and -2 proviruses are known to encode functional proteins. To investigate the host range restriction of these viruses, we attempted to reconstruct functional envelopes by generating consensus genes and proteins. CERV1 and -2 enveloped MLV particles infected cell lines from a range of mammalian species. Using CERV2 Env-pseudotyped MLV reporters, we identified copper transport protein 1 (CTR1) as a receptor that was presumably used by CERV2 during its ancient exogenous replication in primates. Expression of human CTR1 was sufficient to confer CERV2 permissiveness on otherwise resistant hamster cells, and CTR1 knockdown or CuCl2 treatment specifically inhibited CERV2 infection of human cells. Mutations in highly conserved CTR1 residues that have rendered hamster cells resistant to CERV2 include a unique deletion in a copper-binding motif. These CERV2 receptor-inactivating mutations in hamster CTR1 are accompanied by apparently compensating changes, including an increased number of extracellular copper-coordinating residues, and this may represent an evolutionary barrier to the acquisition of CERV2 resistance in primates.
Keywords: endogenous retrovirus, copper transport
The ability of retroviruses to integrate into the genomes of target cells allows for the possibility of Mendelian inheritance of proviruses if integration into the host germ line occurs (1). This route of transmission results in an organism-wide presence of provirus in the genomes of progeny. Such endogenization events have occurred numerous times during primate evolution and many proviruses have become fixed in host populations (2–5). Endogenous retroviruses thus represent a record of ancient infections and these “paleoviruses” can provide information about the evolution of host–virus interactions (6, 7).
Endogenous γ-retroviruses are abundant in primate genomes and among them, chimpanzee endogenous retroviruses-1 and -2 (CERV1 and CERV2) and their relatives are the groups most closely related to the modern prototype γ-retrovirus, MLV (3, 4). CERV1 and -2 are assumed to be extinct, although without exhaustive sampling, it is nearly impossible to definitively demonstrate that intact exogenous relatives are not currently replicating in some modern primate species. They are of particular interest because of their peculiar absence from the human genome, although homologs exist in the genomes of chimpanzee, bonobo, gorilla, and Old World monkeys. Thus, both viruses apparently replicated after the divergence of the human and chimpanzee lineages approximately 6 million years ago, with zoonoses ultimately resulting in endogenization in diverse Old World primates, but not in the human lineage. Thus, it seems possible that a species-specific property protected human ancestors from CERV1 and -2 infection during the time that they were becoming endogenized in nonhuman primates, some 1 to 6 million years ago.
Previously, we investigated whether host antiretroviral factors that restrict modern retroviruses were able to target CERV1 and -2 during exogenous replication (6). TRIM5α is a restriction factor that blocks the replication of a variety of retroviruses, including certain MLV strains (8–11). However, we found no evidence that TRIM5α was involved in a protection of human ancestors from CERV1 or -2 infection. Conversely, inspection of CERV1 and -2 sequences revealed that many proviruses displayed G-to-A hypermutation within GG or GA dinucleotides (6, 7), characteristic of APOBEC3-mediated mutation (12–14). APOBEC3 proteins were therefore capable of acting on these viruses and may have been involved in limiting their host range.
In addition to restriction factors, cell-surface receptor usage is often a primary determinant of viral host range. Indeed, MLV tropism is partly determined by sequences in the viral envelope, which can direct the use of a variety of MLV cell-surface receptors, including cationic amino acid transporter-1, inorganic phosphate transporters, or xenotropic/polytropic receptor (15–22). To examine the host range and receptor usage of CERV1 and -2 during the time that they replicated, we reconstituted functional envelope genes and proteins. MLV particles carrying a reconstituted CERV2 envelope protein displayed a broad species tropism in cell culture and were used to identify copper transport protein 1 (CTR1) as a receptor that was likely used by CERV2 during ancient infections. The only mammalian species tested that was nonpermissive to CERV2 was hamster, and its resistance to infection was explained by mutations in the CTR1 extracellular domain. This work demonstrates that reconstruction of ancient endogenous viral envelope genes from molecular fossils can allow the discovery of host proteins that have been used as receptors by presumptively extinct viruses in prehistoric times.
Results
Reconstruction of Functional CERV1, CERV2, and RhERV2-A Envelope Genes and Proteins.
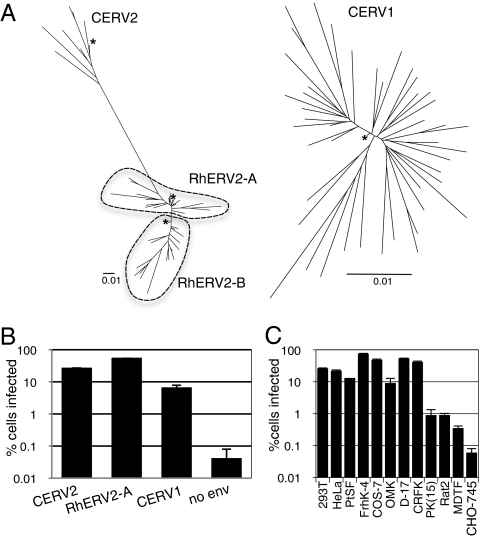
To investigate the tropism of the aforementioned extinct retroviruses, we attempted to reconstruct functional envelope genes and proteins. Specifically, consensus envelope sequences were derived by aligning all 50 CERV1 and 8 CERV2 env genes, which were identified by BLAST searches of the chimpanzee genome. Consensus env genes were also generated from CERV2 homologs present in the rhesus macaque genome, which fall into two distinct groups: RhERV2-A, consisting of 27 env genes, and RhERV2-B, consisting of 34 env genes. All env nucleotide sequences were unique, except for one pair of identical sequences in each of the RhERV2 groups. Phylogenies of CERV1 and CERV2/RhERV2 env genes and their respective consensus DNA sequences are shown in Fig. 1A. The CERV2/RhERV2 phylogentic tree was constructed using TM domain DNA sequences because only a small portion of the RhERV2-B SU showed significant homology to the CERV2 and RhERV2-A SU domains.
Fig. 1.
(A) Phylogenetic analyses of CERV2, RhERV2, and CERV1 envelope nucleotide sequences. CERV2/RhERV2 TM-encoding nucleotide sequences and complete CERV1 env nucleotide sequences were analyzed. Only those CERV1 (n = 45), CERV2 (n = 7), RhERV2A (n = 24), and RhERV2B (n = 17) proviruses that had complete env genes were included in the analyses. ClustalX software was used to derive neighbor-joining trees, which were formatted using FigTree software. The consensus sequences are indicated by asterisks. (Scale bars, 0.01 substitutions per nucleotide position.) (B) MLV particles (50 μL) carrying a GFP reporter and pseudotyped with the indicated envelope proteins were used to infect 104 293T cells. Cells were analyzed by FACS 2 d after infection. (C) CERV2 enveloped MLV-GFP particles were used to infect cell lines from a variety of species: human (293T, HeLa), chimpanzee (PtSF), rhesus macaque (FrhK-4), African green monkey (COS-7), owl monkey (OMK), dog (D-17), cat (CRFK), pig [PK(15)], rat (Rat2), mouse (MDTF), and hamster (CHO-745), as in B.
For each virus, nucleotides encoded by the majority of the env genes at each position in the alignment were included in the consensus sequence. Where no majority existed, which occurred in some situations where more than two nucleotide variants occurred at a given position, the nucleotide encoded by the largest fraction of individuals was selected. In-frame insertions or deletions were included in the consensus sequence if they were present in the majority of individual genes. In the case of RhERV2-B, 30 of the 34 sequences carried abundant G to A mutations within GG and GA dinucleotides, which were likely the result of APOBEC3-induced hypermutation. At these sites, G was assigned to the consensus sequence regardless of whether or not the majority carried G or A. In each case, the consensus sequence mapped close to the base of the tree or clade containing the corresponding extant sequences. The consensus CERV1, CERV2, RhERV2-A, and RhERV2-B DNA sequences were predicted to encode proteins of 557, 651, 626, and 465 amino acids, respectively. Each sequence contained features typical of γ-retroviral envelope proteins (Fig. S1), although both the consensus RhERV2-B env gene and all extant proviruses had a large deletion that removed the C-terminal portion of the SU protein. These consensus env genes were synthesized using panels of overlapping oligonucleotides and PCR-based synthesis and inserted into a mammalian expression vector (SI Materials and Methods).
Each consensus Env expression vector was cotransfected with an MLV Gag-Pol expression plasmid and an MLV vector carrying a GFP reporter (MLV-GFP) to generate putatively pseudotyped MLV particles. Three envelope proteins (CERV1, CERV2, and RhERV2-A) were functional, in that they supported pseudotype infection of human 293T cells (Fig. 1B), but the fourth (RhERV2-B, which encoded a large deletion in SU) did not. Further analyses revealed that CERV1 Env supported infection of several primate cell lines but did not support infection of cells from New World owl monkeys or from several rodent species (Fig. S2). CERV2 Env displayed a broad species tropism in that it supported infection of human, chimpanzee, rhesus macaque, African green monkey, owl monkey, dog, rat, and mouse cell lines (Fig. 1C). Notably, the ability of CERV1- and CERV2-enveloped particles to infect human cells argues against the notion that a lack of functional receptors is responsible for the absence of these viruses from the human lineage.
Identification of a Receptor for CERV2 and RhERV2-A.
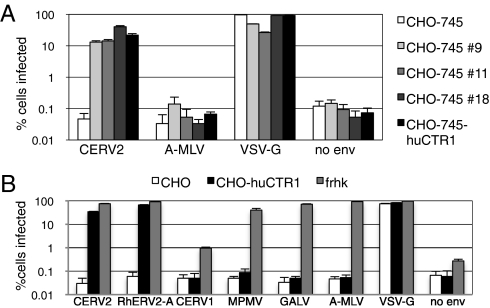
MLV particles bearing the resurrected CERV2 envelope could efficiently infect human cells, yielding an infectious titer that was four to seven times higher than particles carrying CERV1 envelope (Fig. 1 B and C and Fig. S2). Therefore, we used CERV2-enveloped MLV particles to screen a HeLa cDNA library for genes encoding receptors that were used by CERV2. Moreover, Chinese hamster ovary (CHO)-derived CHO-745 cells were resistant to CERV2 infection and provided a suitable target cell with which to perform such a screen. Twenty independent pools of a HeLa cDNA-IRES-Zeo retroviral library (105 clones per pool) were separately introduced into CHO-745 cells yielding 20 pools of zeocin selected, library-expressing cells. These cells were challenged, in duplicate, with CERV2-enveloped virions carrying a neomycin-resistance gene. All but one of these 40 infections yielded G418-resistant colonies (from 1 to 24 colonies per 2 × 105 challenged cells). The G418-resistant colonies were replated in 20 dishes that corresponded to the original 20 pools of the cDNA library and were challenged in a second round of selection with CERV2-enveloped virions carrying a hygromycin-resistant MLV vector. Numerous hygromycin resistant colonies (too many to count) were obtained in 3 of the 20 G418-resistant pools. These three G418 and hygromycin-resistant pools displayed a >100-fold increase in permissivity, relative to unmanipulated CHO-745 cells, to a CERV2-pseudotyped MLV vector that carried a DsRED reporter gene (Fig. 2A). Importantly, resistance to virions carrying amphotropic MLV envelope (A-MLV) was maintained in these selected populations of cells, suggesting that their increased permissivity was envelope-dependent and -specific.
Fig. 2.
HuCTR-1 expression confers sensitivity to CERV2 Env-mediated infection in hamster cells. (A) Following two rounds of selection using CERV2 pseudotyped MLV, cells from three separate pools of cDNA library transduced CHO-745 cells (#9, #11, and #18) were tested for sensitivity to infection by MLV particles pseudotyped with the indicated envelope and carrying a DsRED reporter construct. Unmanipulated CHO-745 cells, as well as cells stably transduced with a huCTR1 expressing retroviral vector, are included for comparison. (B) Unmanipulated CHO cells, CHO stably transduced with huCTR1, and FRHK cells were infected as in A with MLV particles pseudotyped with the indicated Env proteins.
PCR primers directed to the retroviral library vector were used to obtain a 1.7-kb amplicon from all three CERV2-permissive cell lines that was not amplified when DNA from unmanipulated CHO-745 cells was used. Sequence analysis revealed that, in all three cases, these DNAs encoded human copper transport protein 1 (huCTR1) (23). Next, the isolated coding region of huCTR1 was inserted into a retroviral vector and independently transduced into CHO-745 cells. This manipulation rendered CHO-745 cells as permissive to CERV2 infection as the CHO-745 cells that were derived by library transduction and selection (Fig. 2A). Notably, expression of huCTR1 also rendered CHO cells permissive to an MLV pseudotype carrying the RhERV2-A consensus envelope (Fig. 2B). In contrast, huCTR1 did not render CHO cells permissive to CERV1-, MPMV-, GALV-, or A-MLV–pseudotyped virions. Thus, huCTR1 specifically induced permissivity to CERV2- and RhERV2-A-pseudotyped vector infection.
Inhibition of CERV2 Infection by CTR1 Depletion or Copper, but Not by Endosome Acidification.
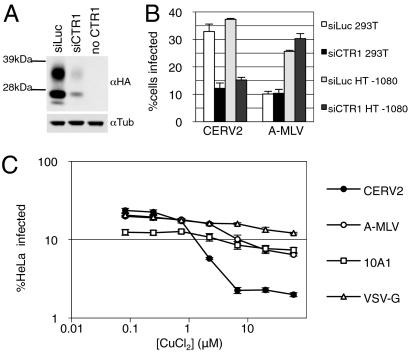
To determine whether CTR1 is necessary for CERV2 infection, two different human cells lines were transfected with an siRNA pool targeting huCTR1 or a control luciferase-specific siRNA. The activity of the huCTR1 siRNA pool was confirmed by knockdown of tagged huCTR1 ectopically expressed in 293T cells (Fig. 3A). The huCTR1 siRNA transfection reduced CERV2 infection of 293T or HT1080 cells by approximately threefold compared with cells transfected with control siRNA, but susceptibility to A-MLV–enveloped MLV was unaffected (Fig. 3B). To further investigate the requirement for huCTR1 in CERV2 infection, we tested whether its normal ligand, copper, could inhibit its function as a viral receptor. Copper II chloride treatment did in fact inhibit CERV2 infection of HeLa cells by up to 10-fold and only had minor effects on infection by vesicular stomatitis virus (VSV)-G and MLV enveloped virions (Fig. 3C). These data strongly suggest that CTR1 is the major receptor used by CERV2, but do not formally exclude the possibility that another receptor could also be used at lower efficiency.
Fig. 3.
CTR1 knockdown or copper treatment inhibits CERV2 pseudotype infection of human cells. (A) 293T cells were cotransfected with plasmids expressing huCTR1 with an N-terminal HA tag and siRNAs targeting CTR1 or luciferase, as indicated, and subjected to Western blot analysis. Blots were probed with anti-HA (Upper) or anti-tubulin (Lower). Glycosylated forms of CTR1 can be observed above the nonglycosylated protein. (B) 293T or HT1080 cells were transfected twice with siRNAs targeting CTR1 or luciferase before infection with MLV particles carrying a GFP reporter and CERV2 or A-MLV Env proteins. (C) HeLa cells were incubated for 2 h in CuCl2 before overnight infection, at the same CuCl2 concentration, with the indicated MLV pseudotypes.
The protein huCTR1 is naturally endocytosed in response to copper treatment (24), and it was possible that endocytosis might be required for CERV2 infection. Therefore, we asked whether endosome acidification was required for CERV2 entry. Pretreatment of cells and infection in the presence of the endosome acidification inhibitor, ammonium chloride, demonstrated that infection by CERV2 enveloped MLV was endosomal pH-independent in both CHO-huCTR1 and human TE671 cells (Fig. S3). This finding suggests that, unlike VSV-G–mediated entry, CERV2 infection does not require the low pH environment of endocytic compartments.
Human CTR1 Induces Attachment of CERV2 Enveloped Particles to Cells.
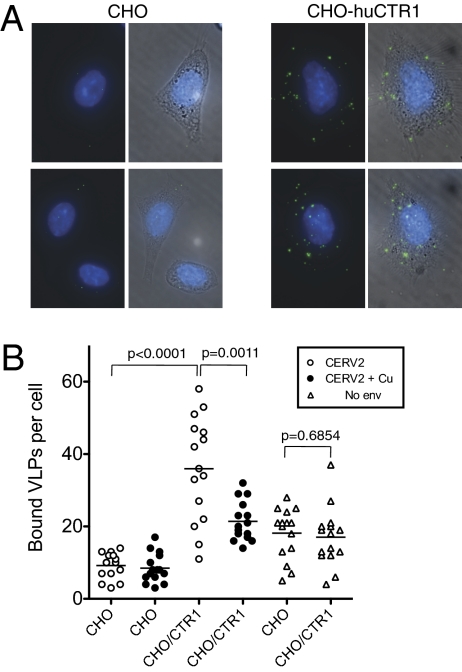
To determine whether CTR1 facilitates CERV2 Env-mediated attachment to target cells, GFP-labeled virus-like particles (VLPs) were generated by expressing MLV Gag-GFP in the presence or absence of the CERV2 envelope. These fluorescent VLPs were applied to CHO cells, or a derivative expressing huCTR1 (CHO-huCTR1) and bound VLPs were enumerated by microscopic examination. CERV2-enveloped VLPs bound to unmanipulated CHO cells only at low levels, below which particles lacking a viral envelope glycoprotein bound (Fig. 4). Conversely, approximately four times as many CERV2-enveloped VLPs bound to CHO-huCTR1 cells (P < 0.0001), and treatment of CHO-huCTR1 cells with CuCl2 inhibited this binding by approximately twofold (P = 0.011) (Fig. 4B). Attachment of VLPs that lacked Env protein to CHO cells, presumably mediated by interactions between plasma membrane components, was not affected by huCTR1 (P = 0.6854). Overall, these data demonstrate that huCTR1 confers on CHO cells the ability to bind CERV2 enveloped VLPs, and therefore suggest that CTR1 served to mediate both attachment and infection of CERV2.
Fig. 4.
HuCTR1 promotes binding of CERV2 particles to CHO cells. (A) Images of CERV2 particles bound to CHO cells or a derivative expressing huCTR1, as indicated. A single image representing a projection of a z-series of fluorescent images that capture the entire thickness of the cell monolayer is shown (Left), and a merge of fluorescence and phase-contrast images is also shown (Right). (B) Quantitation of the number of MLV Gag-GFP particles with (circles) or without (triangles) CERV2 Env protein that bound to CHO or CHO-huCTR1 cells, as indicated. Particles were incubated with cells in the absence (open symbols) or presence (filled symbols) of CuCl2. For quantitation, 15 individual cells under each condition were chosen at random and the number of GFP particles per cell was counted. Each symbol represents an individual cell and the mean number of particles per cell is indicated by a horizontal line. P values were calculated using the unpaired, two-tailed t test (Graphpad Prism).
Determinants of CTR1 Receptor Function.
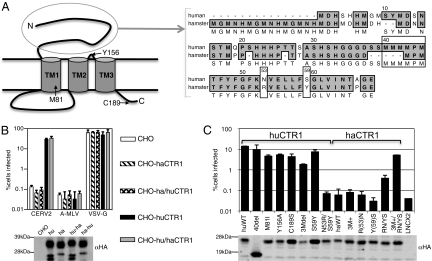
Next, we sought to explain why CHO cells were nonpermissive, and human cells were permissive to CERV2 infection. Presumably, this could be explained either by sequence differences between primate and hamster CTR1 (haCTR1), or by low or absent expression of haCTR1 in CHO cells. CTR1 has three transmembrane helices, a 64 amino acid (in the case of huCTR-1) variable extracellular N-terminal domain, and a small invariant three amino acid extracellular loop between helices 2 and 3 (25–27) (Fig. 5A). Notably, stable expression of haCTR1 in CHO cells did not confer CERV2 permissivity, indicating that low CTR1 expression could not explain the nonpermissivity of CHO cells (Fig. 5B). However, CHO cells that were engineered to stably express a chimeric haCTR1 in which the N-terminal extracellular domain was replaced with its huCTR1 counterpart were as permissive to CERV2 infection as CHO cells expressing the full-length huCTR1; cells expressing the reciprocal chimera were nonpermissive (Fig. 5B). A C-terminal HA tag was appended to the human, hamster, and chimeric CTR1 proteins and a Western blot analysis demonstrated that all four CTR1 proteins were equivalently expressed (Fig. 5B). Thus, the N-terminal extracellular domain of CTR1 is a key determinant of CERV2 receptor function.
Fig. 5.
Determinants of CTR1 viral receptor function. (A) An Illustration of CTR1 topology with residues that are crucial to normal CTR1 transporter function indicated. An amino acid alignment of human and hamster CTR1 N-terminal extracellular domains is shown to the right. The MMMMXM copper coordination motif and two other residues that contribute to CERV2 receptor function are indicated by boxes. (B) CERV2 pseudotype infection of CHO cell lines expressing C-terminally HA tagged huCTR1 (hu), haCTR1 (ha), or chimeras in which the N-terminal extracellular domains were exchanged (hu-ha and ha-hu). Expression of CTR1 was detected on Western blots probed with an αHA antibody (Lower). (C) CHO cells stably expressing N-terminally HA-tagged CTR1 proteins were infected with CERV2 pseudotypes (chart), and were analyzed by Western blotting with anti-HA antibodies (Lower). Mutations of huCTR1 (huWT) or haCTR1 (haWT) were introduced, as indicated, including a deletion of 40 amino acids at the N terminus of huCTR1 (40del), deletion of methionine residues 41 to 43 in huCTR1 (3Mdel), or addition of three methionine residues at the orthologous position in haCTR1 (ham 3M+).
Alignment of CTR1 amino acid sequences from primates revealed a high degree of conservation (Fig. S4). CTR1 proteins from human and chimpanzee have identical amino acid sequences, but the rhesus macaque CTR1 has two amino acid differences. Both changes occur within the 40-residue N-terminal portion of the 68-residue extracellular domain that precedes an MMMMXM motif (positioned at human CTR1 residues 40–45) that is essential for copper uptake (28). In fact, inspection of CTR1 sequences from a wide range of mammals, frog, and zebrafish revealed considerable variability in this 40-residue N-terminal region, although the remainder of the protein is highly conserved in these diverse species (Fig. S4). Notably, a mutant huCTR1 with a 40 amino acid N-terminal truncation was able to confer CERV2 permissivity to a similar degree as the intact huCTR-1 (Fig. 5C). Thus, the most divergent region of CTR1 is completely dispensable for CERV2 receptor function. Rather, CERV2 appears to target the conserved membrane proximal portion of the extracellular domain.
Consistent with this notion, haCTR1 mutants that were altered to mimic huCTR1 in the conserved membrane proximal portion of the extracellular domain were functional CERV2 receptors (Fig. 5 A and C). Specifically, combined introduction of R(53)N and Y(59)S mutations (numbered according to huCTR1) into haCTR1 resulted in partial receptor function. Notably, haCTR1 is unique among the CTR1 proteins examined in that it lacks three methionines from the conserved MMMMXM motif (Fig. S4). The addition of three methionines to reconstitute this motif in an otherwise unaltered haCTR1 did not result in a functional receptor, however this change did significantly improve the receptor function of the R(53)N/Y(59)S haCTR1 mutant (Fig. 5 A and C). The reciprocal mutations in huCTR1 inhibited CERV2 receptor function. Specifically, a three-methionine deletion in the huCTR1 MMMMXM motif decreased receptor function and the N53R/S59Y mutant huCTR1 was inactive. An N-terminal HA tag was included in all CTR1 proteins tested and expression in transduced CHO cells was confirmed by Western blot and FACS analysis (Fig. 5C and Table S1). Modest variation in expression levels did not correlate with CERV2 receptor function. Thus, mutations in the conserved extracellular portion of haCTR1 relative to primate CTR1 confer resistance to CERV2.
Finally, to determine if the use of CTR1 as a viral receptor is directly related to its cellular function, mutants that have been previously shown to be deficient in copper transport were tested for viral receptor function. Two huCTR1 mutations (M81I and Y156A) were previously shown to inhibit transport function (29), and a third (C189S) has been shown to decrease huCTR1 trimer stability (27) (Fig. 5A). None of these point mutations affected the ability of huCTR1 to confer CERV2 permissivity to CHO cells (Fig. 5C). Therefore, the ability of CTR1 to serve as a receptor for CERV2 can be uncoupled from its copper transport function.
Discussion
Determining the tropism of extinct ancient viruses requires the reconstitution of functional viral proteins from molecular fossils. By resurrecting a functional CERV2 envelope we were able to determine the identity of a receptor for a presumptively extinct virus. Specifically, we determined that CERV2, and its relative RhERV2-A, used the copper transporter CTR1 as its receptor during their exogenous replication millions of years ago. HuCTR1 expression was sufficient to allow CERV2- and RhERV-2A–pseudotyped MLV particle binding and infection in otherwise resistant hamster cells; siRNAs directed against CTR1, or copper treatment, inhibited CERV2 infection of human cells. Copper treatment caused a 10-fold decrease in HeLa cell infection and a twofold decrease in CHO-hCTR1 cell binding. This difference in magnitude is likely the result of differing dynamic ranges in the binding and infection assays, but it remains possible that copper can inhibit entry without entirely preventing virion binding.
The exploitation of transport proteins for cell entry is a common feature of γ-retrovirus biology. MLV, gibbon ape leukemia virus, feline leukemia virus (FeLV), porcine endogenous retrovirus, and the baboon endogenous retrovirus/RD114 feline endogenous retrovirus/simian type D retrovirus interference group all use transporters as receptors (15–18, 30–41). Interestingly, FeLV-A recurrently evolves into FeLV-B or -C, which display shifted tropisms because of their use of different transport proteins. Aside from their expression on the surface of a wide variety of cells, no common property of transport proteins has been shown to promote their ability to serve as receptors for viruses. Indeed, in the case of CTR1, we demonstrated that viral receptor activity could be uncoupled from its normal transport function, as has previously been shown for the ecotropic MLV receptor (42).
The ability of CERV2 pseudotypes to infect human cells and to use huCTR1 as a receptor indicates that species-specific envelope:receptor incompatibility does not explain the absence of endogenous copies of CERV2 from the human genome. Additionally, huCTR1 is broadly expressed (43), which is to be expected given the use of copper ions by crucial cellular enzymes (e.g., superoxide dismutase and cytochrome c oxidase). In particular, the expression of CTR1 in human testes, ovaries, and fetal tissues implies that a lack of endogenization is not explained by the absence of receptor expression in the germ line. Although we cannot exclude the possibility that huCTR1 reverted from a CERV2-resistant form at a time subsequent to exogenous CERV2 replication, this evolutionary course is unlikely, given the perfect nucleotide sequence identity in the extracellular regions of human and chimpanzee CTR1.
Other plausible explanations for the absence of CERV1 and -2 from human DNA include the possibilities that human ancestors were ecologically isolated from viral reservoirs during the time of its exogenous replication, or that endogenous proviruses were lost during passage through genetic bottlenecks. It is also conceivable that behavioral changes in nascent humanity limited acquisition or dissemination of these viruses. The possibility also remains that human ancestors were protected from these γ-retroviruses by cytidine deaminases (6), other unknown restriction factors, or effectively suppressive adaptive immune responses.
Our data demonstrate that primate CTR1 did not evolve to resist CERV2 and, furthermore, CTR1 conservation coupled with the identity of residues critical to its viral receptor function may explain why this evolutionary course was unlikely to occur. Interestingly, the vast majority of CTR1 sequence diversity among diverse animals is concentrated in its N-terminal extracellular domain, particularly the extreme N-terminal 40 amino acids that we found to be dispensable for virus receptor function (Fig. 5C and Fig. S4). That CERV2 targets the extracellular domain's most conserved portion likely explains why cells from diverse species are susceptible to CERV2 pseudotype infection (Fig. 1C). The extracellular copper-binding residues that are the most crucial to CTR1’s transport function are found in the highly conserved MMMMXM motif (28), which is positioned at the N-terminal boundary of the extracellular domain that is critical for CERV2 receptor function. Although this motif is conserved from human to zebrafish, haCTR1 is very unusual in that it has only a vestigial MPM motif in the orthologous position and, perhaps by way of compensation, has an extended N terminus that encodes several additional putative copper-coordinating residues (Fig. S4). We found that the three-methionine deletion, along with point mutations at two other conserved residues, are responsible for the resistance of hamster cells to CERV2 infection. Possibly, these changes could represent the result of selection in the hamster lineage by a pathogenic CERV2 relative. Alternatively, it is possible that haCTR1 diverged in response to changes in copper availability or requirement, and loss of CERV2 receptor function is a fortuitous by-product. In any case, the divergence observed in the haCTR1 N-terminal domain may compensate for mutations in the primary copper coordination motif, which represents an evolutionary hurdle that a primate host may have needed to overcome (and apparently did not) to acquire CERV2 resistance through receptor evolution, without forfeiting optimal CTR1 function. Thus, the work described here demonstrates that paleovirology has the potential to both identify novel host factors that were usurped by ancient viruses, as well as indicate the limitations that hosts may have faced in avoiding such exploitation.
Materials and Methods
Generation of Consensus Envelope Genes.
Envelope sequences were collected from Ensembl using TBLASTN and were used to derive majority consensus sequences. Overlapping oligonucleotides used to synthesize consensus genes were designed using Genedesign from The Johns Hopkins University (http://www.genedesign.org). For envelope gene construction, two sequential PCR reactions were carried out and the resulting products were cloned into pCAGGS (see SI Materials and Methods for a detailed description).
Receptor Screen.
Twenty pools of 2 × 105 pgsA-745 cells were transduced with a retroviral HeLa cDNA library constructed in the LM8iresZeo MLV vector. The library-transduced cells underwent two rounds of challenge and selection with CERV2 Env-pseudotyped MLV virions. Genomic DNA was extracted from surviving cells and used as PCR template with primers designed to anneal to DNA flanking the LMN8iresZeo cloning site (see SI Materials and Methods for a detailed description).
VLP Binding Assay.
GFP-labeled virus particles were generated by cotransfection of 293T cells with MLV Gag-GFP and CERV2 envelope-expression plasmids. CHO cells, or a derivative expressing huCTR1, were incubated in medium either with or without CuCl2 followed by VLP-containing supernatent supplemented with or without CuCl2. The cells were washed, fixed, DAPI stained, and visualized by microscopy (see SI Materials and Methods for a detailed description).
RNA Interference.
A Dharmacon siGENOME smart pool was used to knockdown huCTR-1 expression. Small interfering RNA transfections were done twice, and cells were infected the day after the second transfection. To validate siRNA activity, siRNAs were cotransfected with a plasmid expressing HA-CTR1 whose expression was assessed by Western blot analysis (see SI Materials and Methods for a detailed description).
Supplementary Material
Acknowledgments
We thank Theodora Hatziioannou for the cDNA library construction protocol, and Chetankumar Tailor (The Hospital for Sick Children, Toronto, Canada) and various members of the P.D.B. laboratory for helpful discussions and reagents. This work was supported by National Institutes of Health Grant R01AI64003 (to P.D.B.)
Footnotes
The authors declare no conflict of interest.
Data deposition: The hamster CTR1 sequence reported in this paper has been deposited in the GenBank database (accession no. HQ290320).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012344107/-/DCSupplemental.
References
- 1.Weiss RA, Payne LN. The heritable nature of the factor in chicken cells which acts as a helper virus for Rous sarcoma virus. Virology. 1971;45:508–515. doi: 10.1016/0042-6822(71)90351-5. [DOI] [PubMed] [Google Scholar]
- 2.Martin MA, Bryan T, Rasheed S, Khan AS. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci USA. 1981;78:4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polavarapu N, Bowen NJ, McDonald JF. Identification, characterization and comparative genomics of chimpanzee endogenous retroviruses. Genome Biol. 2006;7:R51. doi: 10.1186/gb-2006-7-6-r51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jern P, Sperber GO, Blomberg J. Divergent patterns of recent retroviral integrations in the human and chimpanzee genomes: Probable transmissions between other primates and chimpanzees. J Virol. 2006;80:1367–1375. doi: 10.1128/JVI.80.3.1367-1375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderssen S, Sjøttem E, Svineng G, Johansen T. Comparative analyses of LTRs of the ERV-H family of primate-specific retrovirus-like elements isolated from marmoset, African green monkey, and man. Virology. 1997;234:14–30. doi: 10.1006/viro.1997.8590. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Caballero D, Soll SJ, Bieniasz PD. Evidence for restriction of ancient primate gammaretroviruses by APOBEC3 but not TRIM5alpha proteins. PLoS Pathog. 2008;4:e1000181. doi: 10.1371/journal.ppat.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YN, Bieniasz PD. Reconstitution of an infectious human endogenous retrovirus. PLoS Pathog. 2007;3:e10. doi: 10.1371/journal.ppat.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatziioannou T, Perez-Caballero D, Yang A, Cowan S, Bieniasz PD. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc Natl Acad Sci USA. 2004;101:10774–10779. doi: 10.1073/pnas.0402361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keckesova Z, Ylinen LM, Towers GJ. The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc Natl Acad Sci USA. 2004;101:10780–10785. doi: 10.1073/pnas.0402474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stremlau M, et al. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 11.Yap MW, Nisole S, Lynch C, Stoye JP. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc Natl Acad Sci USA. 2004;101:10786–10791. doi: 10.1073/pnas.0402876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishop KN, et al. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 13.Harris RS, et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 14.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 15.Albritton LM, Tseng L, Scadden D, Cunningham JM. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 16.Miller DG, Edwards RH, Miller AD. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Zeijl M, et al. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoye JP, Coffin JM. The four classes of endogenous murine leukemia virus: Structural relationships and potential for recombination. J Virol. 1987;61:2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YL, et al. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nat Genet. 1999;21:216–219. doi: 10.1038/6005. [DOI] [PubMed] [Google Scholar]
- 20.Tailor CS, Nouri A, Lee CG, Kozak C, Kabat D. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc Natl Acad Sci USA. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elder JH, et al. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci USA. 1977;74:4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischinger PJ, Nomura S, Bolognesi DP. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci USA. 1975;72:5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou B, Gitschier J. hCTR1: A human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA. 1997;94:7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem. 2003;278:9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- 25.Klomp AE, et al. The N-terminus of the human copper transporter 1 (hCTR1) is localized extracellularly, and interacts with itself. Biochem J. 2003;370:881–889. doi: 10.1042/BJ20021128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc Natl Acad Sci USA. 2009;106:4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisses JF, Kaplan JH. Molecular characterization of hCTR1, the human copper uptake protein. J Biol Chem. 2002;277:29162–29171. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- 28.Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem. 2002;277:26021–26030. doi: 10.1074/jbc.M202547200. [DOI] [PubMed] [Google Scholar]
- 29.Eisses JF, Kaplan JH. The mechanism of copper uptake mediated by human CTR1: A mutational analysis. J Biol Chem. 2005;280:37159–37168. doi: 10.1074/jbc.M508822200. [DOI] [PubMed] [Google Scholar]
- 30.O'Hara B, et al. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990;1:119–127. [PubMed] [Google Scholar]
- 31.Kavanaugh MP, et al. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendoza R, Anderson MM, Overbaugh J. A putative thiamine transport protein is a receptor for feline leukemia virus subgroup A. J Virol. 2006;80:3378–3385. doi: 10.1128/JVI.80.7.3378-3385.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ericsson TA, et al. Identification of receptors for pig endogenous retrovirus. Proc Natl Acad Sci USA. 2003;100:6759–6764. doi: 10.1073/pnas.1138025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quigley JG, et al. Cloning of the cellular receptor for feline leukemia virus subgroup C (FeLV-C), a retrovirus that induces red cell aplasia. Blood. 2000;95:1093–1099. [PubMed] [Google Scholar]
- 35.Tailor CS, Willett BJ, Kabat D. A putative cell surface receptor for anemia-inducing feline leukemia virus subgroup C is a member of a transporter superfamily. J Virol. 1999;73:6500–6505. doi: 10.1128/jvi.73.8.6500-6505.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi Y, et al. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J Virol. 1992;66:1219–1222. doi: 10.1128/jvi.66.2.1219-1222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 1997;389:681–682. doi: 10.1038/39489. [DOI] [PubMed] [Google Scholar]
- 38.Shalev Z, et al. Identification of a feline leukemia virus variant that can use THTR1, FLVCR1, and FLVCR2 for infection. J Virol. 2009;83:6706–6716. doi: 10.1128/JVI.02317-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marin M, Tailor CS, Nouri A, Kabat D. Sodium-dependent neutral amino acid transporter type 1 is an auxiliary receptor for baboon endogenous retrovirus. J Virol. 2000;74:8085–8093. doi: 10.1128/jvi.74.17.8085-8093.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tailor CS, Nouri A, Zhao Y, Takeuchi Y, Kabat D. A sodium-dependent neutral-amino-acid transporter mediates infections of feline and baboon endogenous retroviruses and simian type D retroviruses. J Virol. 1999;73:4470–4474. doi: 10.1128/jvi.73.5.4470-4474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasko JE, Battini JL, Gottschalk RJ, Mazo I, Miller AD. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc Natl Acad Sci USA. 1999;96:2129–2134. doi: 10.1073/pnas.96.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Kavanaugh MP, Kabat D. A critical site in the cell surface receptor for ecotropic murine retroviruses required for amino acid transport but not for viral reception. Virology. 1994;202:1058–1060. doi: 10.1006/viro.1994.1439. [DOI] [PubMed] [Google Scholar]
- 43.Su AI, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.