Abstract
The origin recognition complex (ORC) defines origins of replication and also interacts with heterochromatin proteins in a variety of species, but how ORC functions in heterochromatin assembly remains unclear. The largest subunit of ORC, Orc1, is particularly interesting because it contains a nucleosome-binding BAH domain and because it gave rise to Sir3, a key silencing protein in Saccharomyces cerevisiae, through gene duplication. We examined whether Orc1 possessed a Sir3-like silencing function before duplication and found that Orc1 from the yeast Kluyveromyces lactis, which diverged from S. cerevisiae before the duplication, acts in conjunction with the deacetylase Sir2 and the histone-binding protein Sir4 to generate heterochromatin at telomeres and a mating-type locus. Moreover, the ability of KlOrc1 to spread across a silenced locus depends on its nucleosome-binding BAH domain and the deacetylase Sir2. Interestingly, KlOrc1 appears to act independently of the entire ORC, as other subunits of the complex, Orc4 and Orc5, are not strongly associated with silenced domains. These findings demonstrate that Orc1 functioned in silencing before duplication and suggest that Orc1 and Sir2, both of which are broadly conserved among eukaryotes, may have an ancient history of cooperating to generate chromatin structures, with Sir2 deacetylating histones and Orc1 binding to these deacetylated nucleosomes through its BAH domain.
Keywords: heterochromatin, replication, SIR
The origin recognition complex (ORC) not only defines origins of replication but also interacts with heterochromatin proteins in a broad range of species. For example, human and Drosophila ORC bind to HP1 (heterochromatin protein 1) (1–4) and human ORC associates with telomeric and pericentromeric heterochromatin (5–7). Orc1 and the deacetylase Sir2 also repress genes near telomeres in the evolutionarily distant organism Plasmodium falciparum, which causes malaria (8). However, it remains unclear how ORC influences the assembly of heterochromatin. The largest subunit of ORC, Orc1, is particularly interesting because it contains a nucleosome-binding BAH domain, which potentially enables it to associate with chromatin. In addition, Orc1 gave rise to Sir3, a key silencing protein in Saccharomyces cerevisiae, via gene duplication, suggesting that before this duplication Orc1 had properties that predisposed it to become a silencing protein.
Gene duplication is an important force in evolution, as it provides new genetic material free of selective constraint. Preservation of a duplicated gene pair can result in conservation of function, neofunctionalization, or subfunctionalization (9). Neofunctionalization occurs when one duplicate gene evolves a new function, while the other copy retains the original function. In such a case, the gene with the new function is predicted to display a more rapid change in sequence, i.e., “accelerated evolution.” An alternative paradigm, subfunctionalization, predicts that if the ancestral gene had multiple, independent functions, those functions could be partitioned between the duplicates (10, 11). Although these models have been elaborated theoretically, there are relatively few duplicated gene pairs for which the path of divergence is known (12, 13). We previously demonstrated that the duplicated deacetylases Sir2 and Hst1 subfunctionalized (14, 15), and in this study, we propose that the SIR3–ORC1 gene pair subfunctionalized and then specialized after duplication.
In S. cerevisiae, SIR-mediated silencing occurs at telomeres and the mating-type loci, HMLα and HMRa (16). Transcriptional silencing of the mating-type loci is required to maintain cell-type identity in haploid cells. In contrast, SIR-silenced chromatin at the telomeres is thought to serve a structural role (17). The Sir proteins are recruited to the mating-type loci through silencer sequences that bind ORC, Rap1, and Abf1, which in turn recruit the Sir proteins. At telomeres, Sir proteins are recruited via multiple molecules of Rap1, whose binding sites are embedded within the telomere repeats (18). Once recruited, the Sir proteins spread along the chromosome to form an extended silenced domain. Sir2 is a NAD+-dependent deacetylase, and Sir3 and Sir4 bind preferentially to deacetylated histones H3 and H4 (19, 20). The deacetylation of nucleosomes by Sir2 is thought to create high-affinity binding sites for Sir3 and Sir4, which in turn recruit additional Sir2, enabling the propagation of Sir-silenced chromatin (21–23). A fourth Sir protein, Sir1, stabilizes the other Sir proteins at silencers by interacting with Orc1 but is not thought to spread (24–27).
Interestingly, both Sir2 and Sir3 have paralogs that arose in a whole-genome duplication ≈100 million years ago (28–30). The paralog of Sir2 is the deacetylase Hst1, which is part of the promoter-specific SUM1 repressor complex. The paralog of Sir3 is Orc1, the largest subunit of ORC. The sequences of Sir3 and Orc1 have diverged considerably, and these proteins cannot complement each other (31). However one domain, the nucleosome-binding BAH domain, is 50% identical and 65% similar between ScOrc1 and ScSir3 and has a highly conserved tertiary structure (31–33). Nonduplicated orthologs of Orc1 and Sir3 display more sequence similarity to ScOrc1 than to ScSir3, and this accelerated sequence divergence in Sir3 has led to the hypothesis that the silencing function of Sir3 arose through neofunctionalization (29). However, others have argued that Orc1 and Sir3 subfunctionalized (34).
Despite their common ancestry, Orc1 and Sir3 have distinct roles in the formation of silenced chromatin in S. cerevisiae. Orc1, as part of ORC, binds silencers (16), and the BAH domain of Orc1 interacts with Sir1 (26, 27) to stabilize other Sir proteins at silencers. This recruitment of Sir1 is thought to be the only function of ORC in silencing because ORC can be bypassed by tethering Sir1 to the silencer (27, 35). Interestingly, the BAH domain of Orc1 is not essential for DNA replication in S. cerevisiae (31), although it is conserved in most eukaryotic orthologs of Orc1. In contrast to Orc1, Sir3 is critical for the spreading of the SIR complex (21, 23), presumably due to its ability to bind histones through the BAH domain and a second C-terminal histone binding domain (19, 20).
The evolution of SIR3 from ORC1 could indicate that before duplication, Orc1 functioned with Sir2 to generate heterochromatin and that this relationship is ancient, consistent with the association of both ORC and Sir2 with heterochromatin in many organisms. In this model, after the duplication, the replication and silencing functions of Orc1 were partitioned between ORC1 and SIR3. The alternative hypothesis is that the ability to assemble and spread with Sir2 was acquired by Sir3 after duplication. To distinguish between these models, we examined the function of Orc1 in the yeast Kluyveromyces lactis, which diverged from S. cerevisiae before the duplication. K. lactis is the only nonduplicated budding yeast species in which silencing has been examined experimentally. K. lactis has orthologs of SIR2 and SIR4, and deletion of either gene derepresses mating-type loci (15, 36–38). In addition, the SUM1 complex, which is not associated with Sir-silenced domains in S. cerevisiae, is essential for silencing both mating-type loci in K. lactis (15). Interestingly, the characterized silencers in K. lactis do not contain an ORC-binding sequence (39), and Sir1 is not identifiable in the K. lactis genome (40). Thus, KlOrc1 probably does not have the same function in silencing as ScOrc1.
We have examined the function of the single Orc1/Sir3 protein from K. lactis as a proxy for the ancestral Orc1 and found that KlOrc1 does indeed act in conjunction with the deacetylase Sir2 to generate silenced chromatin at telomeres and a mating-type locus.
Results
KlOrc1 Associated with the Silenced HMLα Locus.
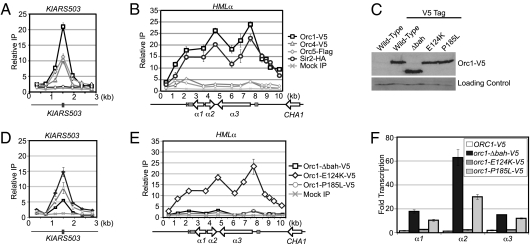
To determine whether the nonduplicated KlOrc1 (KLLA0B05016g) functions strictly as a replication factor, as predicted by the neofunctionalization model, or also functions as a silencing factor capable of spreading, as predicted by the subfunctionalization model, we assessed the association of KlOrc1 with the silenced mating-type locus HMLα and the known replication origin KlARS503 (41, 42) by chromatin immunoprecipitation. As anticipated, KlOrc1 associated with the origin, and its maximum enrichment coincided with the autonomously replicating sequence (Fig. 1A). Importantly, KlOrc1 also associated with HMLα and was distributed across the entire 6-kb locus in a pattern similar to that observed for other silencing factors, such as Sir2 (Fig. 1B). This result suggests either that KlOrc1 is a component of silenced chromatin at HMLα or that an unusually large replication origin occurs at HMLα. To distinguish between these possibilities, we assessed the association of two additional subunits of ORC, Orc4 (KLLA0C16984g) and Orc5 (KLLA0C02607g), with HMLα. If a replication origin occurs at HMLα, Orc4 and Orc5 should also associate with the locus. However, these subunits were only slightly enriched above background at HMLα (Fig. 1B), although they associated robustly with the origin KlARS503 (Fig. 1A). Therefore, KlOrc1 appears to have a role at HMLα that is independent of the entire ORC.
Fig. 1.
KlOrc1 associates with replication origins and HMLα. (A) The associations of KlOrc1–V5 (LRY2561), KlOrc4–V5 (LRY2711), KlOrc5–Flag (LRY2235), and KlSir2–HA (LRY2566) with the replication origin KlARS503 (previously known as KARS12) were assessed by chromatin IP followed by quantitative PCR. The gray box denotes the autonomously replicating sequence. The y axis represents the relative enrichment normalized to a control locus, RRP7, which is not detectably associated with KlOrc1, KlOrc4, KlOrc5, or KlSir2. Mock precipitations using the V5, HA, and Flag antibodies were conducted from a strain expressing untagged KlOrc1, KlOrc4, KlOrc5, and KlSir2 (CK213). (B) The associations of KlOrc1–V5, KlSir2–HA, and KlOrc5–Flag with HMLα were assessed using the same chromatin IP samples analyzed in A. The characterized silencer at HMLα is indicated by a dark gray box, and sequences conserved at all three mating-type loci are indicated in light gray. (C) KlOrc1–V5 (2561), KlOrc1–Δbah–V5 (LRY2562), KlOrc1–E124K–V5 (LRY2656), and KlOrc1–P185L–V5 (LRY2657) were detected by immunoblotting using an antibody against the V5 tag. A sample from a strain expressing untagged KlOrc1 (CK213) was also included. As a loading control, the same blot was probed with an antibody against H3-K4me3. (D) The associations of KlOrc1–Δbah–V5 (LRY2562), KlOrc1–E124K–V5 (LRY2656), and KlOrc1–P185L–V5 (LRY2657) with KlARS503 were analyzed as for A. (E) The associations of KlOrc1–Δbah–V5, KlOrc1–E124K–V5, and KlOrc1–P185L–V5 with HMLα were assessed using the same chromatin IP samples analyzed in D. (F) Quantitative RT-PCR analysis of HMLα1, HMLα2, and HMLα3 mRNA derived from the same strains analyzed in A–E. The amount of cDNA was first normalized to the control locus ACT1 and then compared with the untagged ORC1 strain.
BAH Domain of KlOrc1 Was Required for Silencing but Not Viability.
To determine whether KlOrc1 contributes to transcriptional silencing at HMLα, we sought a separation-of-function mutation that would disrupt the silencing but not the essential replication properties of KlOrc1. We focused on the nucleosome-binding BAH domain, which is essential for the silencing functions of both ScSir3 and ScOrc1 but is dispensable for DNA replication in S. cerevisiae (26, 31, 43). We truncated the genomic copy of KlORC1, removing the sequence encoding the first 217 amino acids, which correspond to the BAH domain. KlOrc1–Δbah was expressed at a level comparable to wild-type KlOrc1 (Fig. 1C), and cells harboring orc1–Δbah as the only copy of ORC1 were viable and displayed no growth defects or gross perturbations of the cell cycle (Figs. S1 and S2), indicating that the replication function of KlOrc1 was largely intact. Moreover, KlOrc1–Δbah still associated with the origins KlARS503 (Fig. 1D) and KlARS406 (Fig. S3), although its enrichment was reduced, consistent with observations in S. cerevisiae (44). In contrast, KlOrc1–Δbah no longer associated with HMLα (Fig. 1E).
To determine whether the loss of KlOrc1 at HMLα affects silencing, we measured the amount of the α1, α2, and α3 transcripts in wild-type and orc1–Δbah strains by quantitative RT-PCR. In the absence of the BAH domain, all three genes were derepressed (Fig. 1F), demonstrating that KlOrc1 contributes to the transcriptional silencing of HMLα.
BAH Domain of KlOrc1 Contributes to Silencing in a Sir3-Like Manner.
The BAH domains of ScSir3 and ScOrc1 contribute to silencing in different ways. The ScSir3 BAH domain enables the spreading of the Sir complex by binding nucleosomes (45, 46), whereas the ScOrc1 BAH domain recruits Sir1 to silencers. Given that there is no identifiable homolog of Sir1 in K. lactis (40), it is more likely that KlOrc1 acts at HMLα in a manner analogous to ScSir3. To explore this hypothesis, we generated two point mutations in KlORC1. The P185L mutation is analogous to a mutation in ScSir3 that disrupts nucleosome binding and the ability of Sir proteins to spread (46), and the E124K mutation occurs in the H domain, which interacts with Sir1 in S. cerevisiae (26) (Fig. S4). If KlOrc1 acts similarly to ScSir3, the P185L mutation should reduce its ability to associate with and maintain repression of HMLα, whereas if KlOrc1 acts similarly to ScOrc1 and interacts with a currently unknown Sir1-like protein, the E124K mutation might be disruptive. We found that the association of KlOrc1 with HMLα was greatly reduced by the P185L mutation but was only slightly affected by the E124K mutation (Fig. 1E). In addition, the P185L mutation derepressed HMLα, whereas the E124K mutation had only a slight effect (Fig. 1F). Both proteins were expressed at levels similar to wild-type Orc1 (Fig. 1C) and still associated with replication origins (Fig. 1D and Fig. S3), indicating that the observed phenotypes are not due to instability of the proteins. Therefore, KlOrc1 most likely contributes to silencing of HMLα by binding to nucleosomes in a Sir3-like manner.
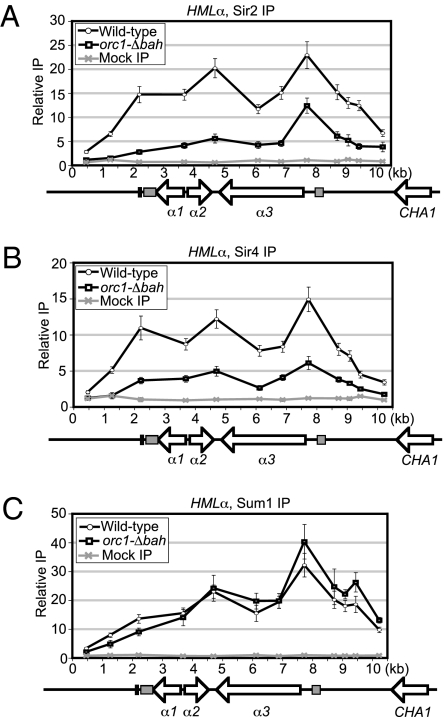
KlOrc1 BAH Domain Promoted the Distribution of Sir2 and Sir4 Across HMLα.
To investigate the mechanism by which KlOrc1 contributes to transcriptional silencing, we determined whether KlOrc1 was required for the association of other silencing factors. If KlOrc1 acts similarly to ScSir3, it would not be required for the initial recruitment of other silencing proteins to HMLα, but it would be required for them to spread. In contrast, if KlOrc1 acts like ScOrc1, it would be required for the recruitment of other proteins to silencers. In the absence of the BAH domain of KlOrc1, the association of Sir2 (KLLA0F14663g) was reduced across most of HMLα, although a peak of enrichment remained at the α3 promoter (Fig. 2A). The association of Sir4 (KLLA0F13420g) was also reduced across the HMLα locus (Fig. 2B). In contrast, there was little change in the association of Sum1 (KLLA0C14696g) in the absence of the KlOrc1 BAH domain (Fig. 2C). Therefore, all three proteins associated with HMLα, at least to some extent, in the absence of the BAH domain, inconsistent with KlOrc1 acting as a primary recruiting factor. However, the BAH domain of KlOrc1 was important for Sir2 and Sir4 to spread across HMLα, consistent with KlOrc1 acting similarly to ScSir3.
Fig. 2.
KlSir2, KlSir4, and KSum1 associate with HMLα in an orc1–Δbah strain. (A) The association of KlSir2–HA with HMLα in ORC1 (LRY2566) and orc1–Δbah (LRY2563) strains. (B) The association of KlSir4–Flag with HMLα in ORC1 (LRY2566) and orc1–Δbah (LRY2563) strains. (C) The association of myc–KlSum1 with HMLα in ORC1 (LRY2566) and orc1–Δbah (LRY2563) strains.
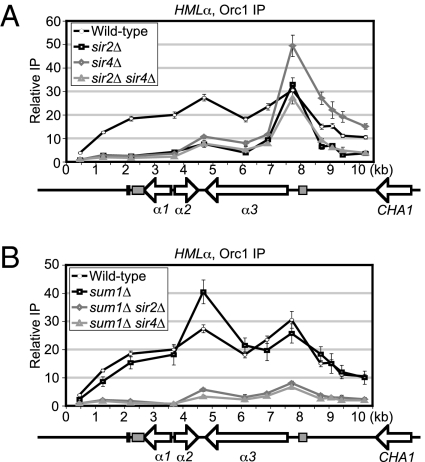
Sir2 and Sir4 Were Important for the Distribution of KlOrc1 Across HMLα.
In S. cerevisiae, ORC binds directly to silencers independently of the Sir proteins. In contrast, ScSir3 depends on silencer binding proteins and Sir4 for recruitment. To determine whether KlOrc1 requires known silencing proteins for recruitment to HMLα, KlOrc1 was immunoprecipitated from strains lacking Sir4, Sir2, or Sum1. In the absence of Sir2 or Sir4, individually or in combination, there was a severe reduction of KlOrc1 over most of the HMLα locus, except for the α3 promoter (Fig. 3A). These results suggest that KlOrc1 is recruited independently of Sir2 and Sir4 to a putative silencer, but requires Sir2 and Sir4 to spread over the rest of the locus.
Fig. 3.
KlSir2 and KlSir4, but not KlSum1, facilitate the spreading of KlOrc1 across HMLα. (A) The association of KlOrc1–V5 with HMLα in wild-type (LRY2561), sir2Δ (LRY2572), sir4Δ (LRY2573), and sir2Δ sir4Δ (LRY2577) strains. (B) The association of KlOrc1–V5 with HMLα in wild-type (LRY2561), sum1Δ (LRY2574), sum1Δ sir2Δ (LRY2578), and sum1Δ sir4Δ (LRY2576) strains. The wild-type data are the same as in Fig. 1B.
In contrast, the deletion of Sum1 did not result in a reduction of KlOrc1 with any region of HMLα (Fig. 3B). To determine whether Sir2/Sir4 and Sum1 act in parallel pathways to recruit KlOrc1, the association of KlOrc1 with HMLα was examined in sum1Δ sir2Δ and sum1Δ sir4Δ strains. In both strains, KlOrc1 was reduced over the entire locus (Fig. 3B). Therefore, KlOrc1 did not associate with HMLα independently of other silencing proteins, as S. cerevisiae ORC does, but instead required either the SUM1 complex or the Sir4/Sir2 complex for recruitment. Thus, KlOrc1 is recruited to HMLα in a manner resembling that of ScSir3.
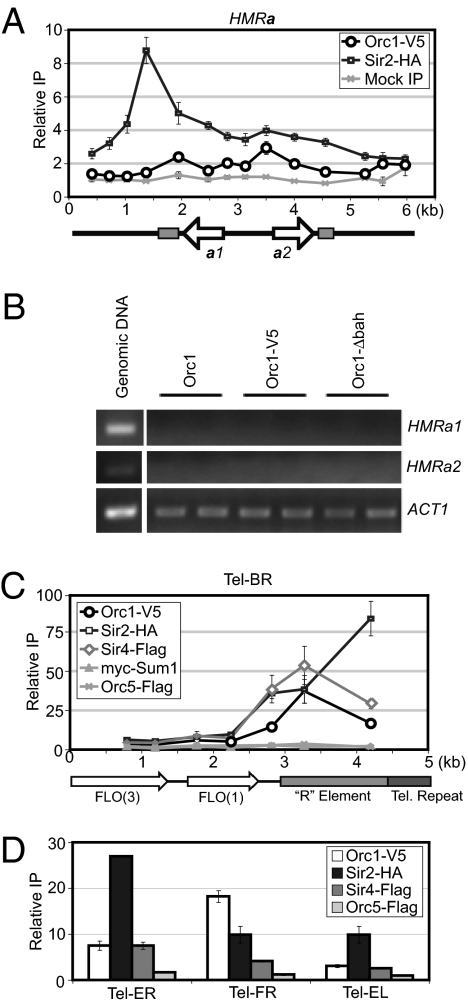
KlOrc1 Was Detected at Telomeres but Not the Silenced Mating-Type Locus HMRa.
To investigate whether KlOrc1 contributes to silencing at other genomic loci, we examined the association of KlOrc1 with HMRa and telomeres, loci associated with Sir proteins in S. cerevisiae. KlHMRa is silenced by Sir2 and Sum1 but not by Sir4 (15). Similarly, KlOrc1 was not associated with KlHMRa (Fig. 4A). Furthermore, there was no detectable change in expression of HMRa1 or a2 when the BAH domain was deleted (Fig. 4B), indicating that KlOrc1 does not contribute to the silencing of HMRa. Thus, in contrast to S. cerevisiae, the two silenced mating-type loci in K. lactis are repressed by different mechanisms, with the Sum1–Sir2 complex repressing both HMRa and HMLα and KlOrc1 and Sir4 acting only at HMLα.
Fig. 4.
KlOrc1 associates with telomeres, but not with HMRa. (A) The associations of KlOrc1–V5 (LRY2581) and KlSir2–HA (LRY2285) with HMRa. A mock precipitation using the V5 antibody was conducted from a strain expressing untagged KlOrc1 (SAY538). Sequences found at HMLα, MAT, and HMRa are represented by light gray boxes. Data for KlSir2–HA were previously reported (15). (B) RT-PCR analysis of HMRa1, HMRa2, and ACT1 in KlOrc1 (SAY538), KlOrc1–V5 (LRY2581) and KlOrc1–Δbah–V5 (LRY2709) strains. (C) The association of KlOrc1–V5 (LRY2561), KlOrc5–Flag (LRY2235), KlSir2–HA (LRY2239), KlSir4–Flag (LRY2239), and myc–KlSum1 (LRY2239) with Tel–BR. Telomeres in K. lactis contain a 1.5- to 2-kb element, termed the R element, immediately adjacent to the telomeric repeat (58). The R element, as well as the telomeric repeat sequence and the ORFs FLO3 and FLO1 are indicated. (D) Association of KlOrc1–V5, KlOrc5–Flag, KlSir2–HA, and KlSir4–Flag with Tel–ER, Tel–FR, and Tel–EL was assessed using the same chromatin IP samples as in C.
We also examined the association of KlOrc1 with subtelomeric regions, focusing on the right arm of chromosome B, which has a unique sequence. We observed a gradient of Sir2 enrichment, with the greatest association nearest the telomere (Fig.4C), consistent with the pattern of Sir protein association at S. cerevisiae telomeres. KlOrc1 and Sir4 were also associated with this telomere, but peaked at an internal site. As was observed at HMLα, KlOrc1 was recruited to this telomere independently of the entire ORC, as Orc5 was not detected (Fig. 4C). Interestingly, Sum1 was also absent from this telomere (Fig. 4C). We also observed Sir2 adjacent to three other telomeres, and KlOrc1 and Sir4 associated with two of these telomeres (Fig. 4D). Thus, KlOrc1, Sir2, and Sir4 colocalize at subtelomeres.
Discussion
This study demonstrates that KlOrc1, a subunit of the origin recognition complex, functions in the formation of silenced chromatin at HMLα and telomeres. Interestingly, the role of KlOrc1 in silencing is more like that of its duplicated homolog ScSir3 than like ScOrc1. In particular, KlOrc1 associated with silenced loci independently of the entire ORC and was distributed across the entire HMLα locus. Moreover, the BAH domain was required for the spreading of KlOrc1, KlSir2, and KlSir4 across the locus, and a single point mutation predicted to disrupt nucleosome binding perturbed transcriptional repression as well as the association of KlOrc1 with HMLα. In contrast, KlOrc1 did not appear to be a silencer binding protein, as it required other silencing proteins to associate with HMLα.
Data presented here and previously (15) suggest the existence of two distinct repressive modules acting at HMLα. One module, akin to the S. cerevisiae SIR complex, is composed of KlOrc1, Sir2, and Sir4 and associates with telomeres as well. These three proteins depend on one another to assemble on the telomere-proximal side of HMLα. The other module, akin to the S. cerevisiae SUM1 complex, is composed of Sum1, Sir2, and Rfm1 and also associates with HMRa. Interestingly, these two modules appear to assemble independently of one another at HMLα, as Sum1 still associated with HMLα in the orc1–Δbah strain, and Orc1 still associated in the sum1Δ strain (Figs. 2C and 3B).
The capacity of KlOrc1 to spread and promote the spreading of other silencing proteins implies that the common ancestor of KlOrc1 and ScSir3 also had this ability and that subfunctionalization of the replication and spreading functions occurred after duplication. There are different ways duplicated genes can subfunctionalize. In the duplication, degeneration, and complementation (DDC) model, duplicated genes each lose one of the original functions and together retain the entire set of ancestral functions (47, 48), whereas in the specialization model the divergence of functions among paralogs also involves the accumulation of advantageous mutations in at least one of the duplicated genes, enabling it to outperform the ancestral gene (10, 11, 49, 50). An earlier study investigating the nonduplicated Orc1 from Saccharomyces kluyveri concluded that Orc1 subfunctionalized through the DDC pathway, based on the ability of SkOrc1 to complement both orc1 and sir3 mutations in S. cerevisiae (34). However, we suggest that although the SIR3–ORC1 gene pair did subfunctionalize, it is more likely a case of specialization. In particular, the accelerated sequence divergence of SIR3 compared with ORC1 implies that SIR3 continued to evolve after duplication. A prediction of the specialization model is that the ancestral gene did not function as well as the duplicated genes. Although it is difficult to compare the silencing efficiencies of KlOrc1 and ScSir3, the requirement for Sum1 to silence the mating-type loci of K. lactis indicates that the KlOrc1–Sir4–Sir2 complex cannot maintain transcriptional repression on its own and therefore may be less efficient than the ScSIR complex.
The observation that KlOrc1 does not appear to have a silencing function similar to that of ScOrc1, which interacts with Sir1 (25–27) is best explained by the absence of SIR1 in K. lactis (40, 51). The only nonduplicated species in which an ortholog of SIR1 has been detected is Zygosaccharomyces rouxii (51), which is thought to be closer to the duplication event than K. lactis is. It will be interesting to determine whether ZrOrc1 has both the capacity to bind nucleosomes like ScSir3 and the capacity to recruit Sir1 (called ZrKos3), as ScOrc1 does. If so, the ORC1 gene that became duplicated most likely also had both properties. In species such as K. lactis that lack Sir1, other mechanisms must exist to recruit silencing proteins to the chromosome, and indeed two silencer-binding proteins distinct from those that act in S. cerevisiae, KlReb1 and KlUme6, have been described (39, 52). The recent discovery of RNAi in budding yeasts (53) also raises the possibility that small RNAs could play a role in the formation of heterochromatin, as occurs in Schizosaccharomyces pombe, although neither argonaute nor dicer-like proteins have been detected in K. lactis.
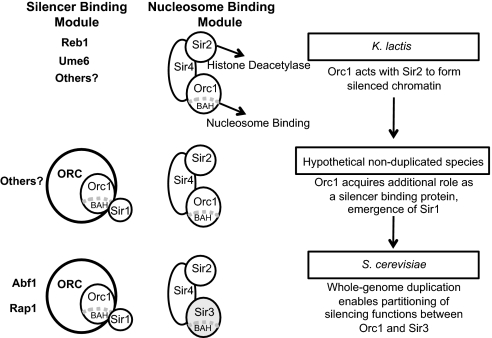
In summary, we propose that two important transitions occurred in the mechanism by which yeast Orc1/Sir3 functions in silencing (Fig. 5). In the ancestral state, as in K. lactis, Orc1 acted with Sir2 to generate extended silenced domains, with Sir2 deacetylating histones and Orc1 binding to these deacetylated nucleosomes through its BAH domain. As Sir2 and Orc1 are both associated with heterochromatin in a variety of eukaryotes, this type of cooperation may be quite ancient. The first transition was the acquisition of a Sir1-like protein, which could interact with the BAH domain of Orc1. Thus, Orc1 gained the ability to act as a silencer-binding protein. Subsequently, a whole-genome duplication enabled the partitioning of these two silencing functions between the ORC1 and SIR3 paralogs, as observed in S. cerevisiae. Once Sir3 was no longer constrained to act in DNA replication, it may have acquired additional beneficial properties. For example, the second histone-binding domain in the C terminus of Sir3 (20) or the modification of the ATPase domain to bind the Sir2 product O-acetyl-ADP ribose (54) could have arisen after duplication. It is also possible that even after subfunctionalization, Orc1 retained some ability to propagate along chromatin, as recently suggested (55).
Fig. 5.
Proposed sequence of events in the evolution of Orc1/Sir3. In the ancestral state (Top) Orc1 acted with Sir2 to generate extended silenced domains. The first transition (Middle) was the acquisition of a Sir1-like protein and the emergence of a role for Orc1 as a silencer-binding protein. Subsequently (Bottom), a whole-genome duplication enabled the partitioning of functions between Orc1 and Sir3.
Materials and Methods
Strains and Media.
Strains used in this study were derived from CK213 and SAY538 (Table S1) and were grown at 30° in YPD medium containing 1% yeast extract, 2% peptone, and 2% glucose.
Gene Expression Analysis.
RNA was isolated from two independent logarithmically growing cultures of each strain using a hot phenol method (56), and cDNA was synthesized as previously described (14). The resulting cDNA was quantified by real-time PCR in the presence of SYBR Green on a Bio-Rad iCycler. A standard curve was generated with genomic DNA isolated from the wild-type strain (CK213). Oligonucleotide sequences are provided in Table S2. Transcript levels of queried genes were first normalized to the KlACT1 mRNA for each genetic background. The fold induction was then calculated by normalizing to the wild-type strain. The SE measurement (SEM) was calculated from the differences in fold induction of two or more independent cultures from the mean.
Chromatin Immunoprecipitation.
Chromatin immunoprecipitations were performed as previously described (15) using 10 optical density equivalents of cell lysate and 4 μL anti-HA tag antibody (Sigma, H6908), anti-myc tag antibody (Upstate Biotechnology, 06–549), anti-FLAG antibody (Sigma, F3165), or anti-V5 antibody (Millipore, AB3792). Dimethyl adipimidate was used as a second cross-linking agent (57). The amounts of immunoprecipitated DNA at experimental loci and a control locus, KlRRP7, were quantified by real-time PCR relative to a standard curve prepared from input DNA, and the relative enrichments of the experimental loci compared with the control locus were calculated. Oligonucleotide sequences are provided in Table S2. The data presented represent results from two or more independent cultures of each strain, and the SEM was calculated from differences in the relative enrichment from the mean.
Immunoblotting.
Protein samples were prepared from 40 optical density equivalents of cells fixed in 10% trichloroacetic acid for 20 min. Cell pellets were washed with 1 M Tris pH 8.0 and lysed by vortexing 5 min in the presence of glass beads in 40 μL lysis buffer (10 mM hepes pH 7.9, 150 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT, 10% glycerol, protease inhibitors). The volume of the lysate was increased to 160 μL, proteins were denatured by the addition of 3× sample buffer (30% glycerol, 15% β-mercaptoethanol, 6% SDS, 200 mM Tris pH 6.8, 0.08 mg/mL bromophenol blue) at 95° for 5 min, and the samples were clarified by centrifugation. Samples were fractionated on 7.5% polyacrylamide-SDS gels, transferred to nitrocellulose membranes (Amersham), and probed with rabbit α-V5 (Millipore, AB3792).
Supplementary Material
Acknowledgments
We thank S. Haase for help with the FACS analysis and D. L. Bailey, H. Chang, and K. Cocce for technical assistance. This research was supported by a grant from the National Institutes of Health (GM073991) (to L.N.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006436107/-/DCSupplemental.
References
- 1.Auth T, Kunkel E, Grummt F. Interaction between HP1alpha and replication proteins in mammalian cells. Exp Cell Res. 2006;312:3349–3359. doi: 10.1016/j.yexcr.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Lidonnici MR, et al. Subnuclear distribution of the largest subunit of the human origin recognition complex during the cell cycle. J Cell Sci. 2004;117:5221–5231. doi: 10.1242/jcs.01405. [DOI] [PubMed] [Google Scholar]
- 3.Pak DT, et al. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell. 1997;91:311–323. doi: 10.1016/s0092-8674(00)80415-8. [DOI] [PubMed] [Google Scholar]
- 4.Prasanth SG, Prasanth KV, Siddiqui K, Spector DL, Stillman B. Human Orc2 localizes to centrosomes, centromeres and heterochromatin during chromosome inheritance. EMBO J. 2004;23:2651–2663. doi: 10.1038/sj.emboj.7600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng Z, Dheekollu J, Broccoli D, Dutta A, Lieberman PM. The origin recognition complex localizes to telomere repeats and prevents telomere-circle formation. Curr Biol. 2007;17:1989–1995. doi: 10.1016/j.cub.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 6.Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell. 2009;35:403–413. doi: 10.1016/j.molcel.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasanth SG, Shen Z, Prasanth KV, Stillman B. Human origin recognition complex is essential for HP1 binding to chromatin and heterochromatin organization. Proc Natl Acad Sci USA. 2010;107(34):15093–15098. doi: 10.1073/pnas.1009945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancio-Silva L, Rojas-Meza AP, Vargas M, Scherf A, Hernandez-Rivas R. Differential association of Orc1 and Sir2 proteins to telomeric domains in Plasmodium falciparum. J Cell Sci. 2008;121:2046–2053. doi: 10.1242/jcs.026427. [DOI] [PubMed] [Google Scholar]
- 9.Ohno S. Evolution by Gene Duplication. New York: Springer; 1970. p. 160. [Google Scholar]
- 10.Conant GC, Wolfe KH. Turning a hobby into a job: How duplicated genes find new functions. Nat Rev Genet. 2008;9:938–950. doi: 10.1038/nrg2482. [DOI] [PubMed] [Google Scholar]
- 11.Hahn MW. Distinguishing among evolutionary models for the maintenance of gene duplicates. J Hered. 2009;100:605–617. doi: 10.1093/jhered/esp047. [DOI] [PubMed] [Google Scholar]
- 12.Des Marais DL, Rausher MD. Escape from adaptive conflict after duplication in an anthocyanin pathway gene. Nature. 2008;454:762–765. doi: 10.1038/nature07092. [DOI] [PubMed] [Google Scholar]
- 13.Hittinger CT, Carroll SB. Gene duplication and the adaptive evolution of a classic genetic switch. Nature. 2007;449:677–681. doi: 10.1038/nature06151. [DOI] [PubMed] [Google Scholar]
- 14.Hickman MA, Rusche LN. Substitution as a mechanism for genetic robustness: The duplicated deacetylases Hst1p and Sir2p in Saccharomyces cerevisiae. PLoS Genet. 2007;3:e126. doi: 10.1371/journal.pgen.0030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman MA, Rusche LN. The Sir2-Sum1 complex represses transcription using both promoter-specific and long-range mechanisms to regulate cell identity and sexual cycle in the yeast Kluyveromyces lactis. PLoS Genet. 2009;5:e1000710. doi: 10.1371/journal.pgen.1000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rusche LN, Kirchmaier AL, Rine J. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu Rev Biochem. 2003;72:481–516. doi: 10.1146/annurev.biochem.72.121801.161547. [DOI] [PubMed] [Google Scholar]
- 17.Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: Reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 18.Longtine MS, Wilson NM, Petracek ME, Berman J. A yeast telomere binding activity binds to two related telomere sequence motifs and is indistinguishable from RAP1. Curr Genet. 1989;16:225–239. doi: 10.1007/BF00422108. [DOI] [PubMed] [Google Scholar]
- 19.Carmen AA, Milne L, Grunstein M. Acetylation of the yeast histone H4 N terminus regulates its binding to heterochromatin protein SIR3. J Biol Chem. 2002;277:4778–4781. doi: 10.1074/jbc.M110532200. [DOI] [PubMed] [Google Scholar]
- 20.Hecht A, Laroche T, Strahl-Bolsinger S, Gasser SM, Grunstein M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell. 1995;80:583–592. doi: 10.1016/0092-8674(95)90512-x. [DOI] [PubMed] [Google Scholar]
- 21.Hoppe GJ, et al. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol Cell Biol. 2002;22:4167–4180. doi: 10.1128/MCB.22.12.4167-4180.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo K, Vega-Palas MA, Grunstein M. Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 2002;16:1528–1539. doi: 10.1101/gad.988802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rusché LN, Kirchmaier AL, Rine J. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2207–2222. doi: 10.1091/mbc.E02-03-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chien CT, Buck S, Sternglanz R, Shore D. Targeting of SIR1 protein establishes transcriptional silencing at HM loci and telomeres in yeast. Cell. 1993;75:531–541. doi: 10.1016/0092-8674(93)90387-6. [DOI] [PubMed] [Google Scholar]
- 25.Gardner KA, Rine J, Fox CA. A region of the Sir1 protein dedicated to recognition of a silencer and required for interaction with the Orc1 protein in Saccharomyces cerevisiae. Genetics. 1999;151:31–44. doi: 10.1093/genetics/151.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou Z, Bernstein DA, Fox CA, Keck JL. Structural basis of the Sir1-origin recognition complex interaction in transcriptional silencing. Proc Natl Acad Sci USA. 2005;102:8489–8494. doi: 10.1073/pnas.0503525102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triolo T, Sternglanz R. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature. 1996;381:251–253. doi: 10.1038/381251a0. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich FS, et al. The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science. 2004;304:304–307. doi: 10.1126/science.1095781. [DOI] [PubMed] [Google Scholar]
- 29.Kellis M, Birren BW, Lander ES. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature. 2004;428:617–624. doi: 10.1038/nature02424. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe KH, Shields DC. Molecular evidence for an ancient duplication of the entire yeast genome. Nature. 1997;387:708–713. doi: 10.1038/42711. [DOI] [PubMed] [Google Scholar]
- 31.Bell SP, Mitchell J, Leber J, Kobayashi R, Stillman B. The multidomain structure of Orc1p reveals similarity to regulators of DNA replication and transcriptional silencing. Cell. 1995;83:563–568. doi: 10.1016/0092-8674(95)90096-9. [DOI] [PubMed] [Google Scholar]
- 32.Connelly JJ, et al. Structure and function of the Saccharomyces cerevisiae Sir3 BAH domain. Mol Cell Biol. 2006;26:3256–3265. doi: 10.1128/MCB.26.8.3256-3265.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Hayashi MK, Merkel O, Stillman B, Xu R-M. Structure and function of the BAH-containing domain of Orc1p in epigenetic silencing. EMBO J. 2002;21:4600–4611. doi: 10.1093/emboj/cdf468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Hoof A. Conserved functions of yeast genes support the duplication, degeneration and complementation model for gene duplication. Genetics. 2005;171:1455–1461. doi: 10.1534/genetics.105.044057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox CA, Ehrenhofer-Murray AE, Loo S, Rine J. The origin recognition complex, SIR1, and the S phase requirement for silencing. Science. 1997;276:1547–1551. doi: 10.1126/science.276.5318.1547. [DOI] [PubMed] [Google Scholar]
- 36.Aström SU, Kegel A, Sjöstrand JO, Rine J. Kluyveromyces lactis Sir2p regulates cation sensitivity and maintains a specialized chromatin structure at the cryptic alpha-locus. Genetics. 2000;156:81–91. doi: 10.1093/genetics/156.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aström SU, Rine J. Theme and variation among silencing proteins in Saccharomyces cerevisiae and Kluyveromyces lactis. Genetics. 1998;148:1021–1029. doi: 10.1093/genetics/148.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen XJ, Clark-Walker GD. sir2 mutants of Kluyveromyces lactis are hypersensitive to DNA-targeting drugs. Mol Cell Biol. 1994;14:4501–4508. doi: 10.1128/mcb.14.7.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjöstrand JOO, Kegel A, Aström SU. Functional diversity of silencers in budding yeasts. Eukaryot Cell. 2002;1:548–557. doi: 10.1128/EC.1.4.548-557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabre E, et al. Comparative genomics in hemiascomycete yeasts: Evolution of sex, silencing, and subtelomeres. Mol Biol Evol. 2005;22:856–873. doi: 10.1093/molbev/msi070. [DOI] [PubMed] [Google Scholar]
- 41.Irene C, et al. DNA elements modulating the KARS12 chromosomal replicator in Kluyveromyces lactis. Mol Genet Genomics. 2007;277:287–299. doi: 10.1007/s00438-006-0188-7. [DOI] [PubMed] [Google Scholar]
- 42.Liachko I, et al. A comprehensive genome-wide map of autonomously replicating sequences in a naive genome. PLoS Genet. 2010;6:e1000946. doi: 10.1371/journal.pgen.1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou Z, et al. Phylogenetic conservation and homology modeling help reveal a novel domain within the budding yeast heterochromatin protein Sir1. Mol Cell Biol. 2009;29:687–702. doi: 10.1128/MCB.00202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller P, et al. The conserved bromo-adjacent homology domain of yeast Orc1 functions in the selection of DNA replication origins within chromatin. Genes Dev. 2010;24:1418–1433. doi: 10.1101/gad.1906410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onishi M, Liou G-G, Buchberger JR, Walz T, Moazed D. Role of the conserved Sir3-BAH domain in nucleosome binding and silent chromatin assembly. Mol Cell. 2007;28:1015–1028. doi: 10.1016/j.molcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Buchberger JR, et al. Sir3-nucleosome interactions in spreading of silent chromatin in Saccharomyces cerevisiae. Mol Cell Biol. 2008;28:6903–6918. doi: 10.1128/MCB.01210-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Force A, et al. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154:459–473. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He X, Zhang J. Rapid subfunctionalization accompanied by prolonged and substantial neofunctionalization in duplicate gene evolution. Genetics. 2005;169:1157–1164. doi: 10.1534/genetics.104.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch M, Katju V. The altered evolutionary trajectories of gene duplicates. Trends Genet. 2004;20:544–549. doi: 10.1016/j.tig.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Gallagher JEG, Babiarz JE, Teytelman L, Wolfe KH, Rine J. Elaboration, diversification and regulation of the Sir1 family of silencing proteins in Saccharomyces. Genetics. 2009;181:1477–1491. doi: 10.1534/genetics.108.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barsoum E, Sjöstrand JOO, Aström SU. Ume6 is required for the MATa/MATα cellular identity and transcriptional silencing in Kluyveromyces lactis. Genetics. 2010;184:999–1011. doi: 10.1534/genetics.110.114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drinnenberg IA, et al. RNAi in budding yeast. Science. 2009;326:544–550. doi: 10.1126/science.1176945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gasser SM, Cockell MM. The molecular biology of the SIR proteins. Gene. 2001;279:1–16. doi: 10.1016/s0378-1119(01)00741-7. [DOI] [PubMed] [Google Scholar]
- 55.Ozaydin B, Rine J. Expanded roles of the origin recognition complex in the architecture and function of silenced chromatin in Saccharomyces cerevisiae. Mol Cell Biol. 2010;30:626–639. doi: 10.1128/MCB.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt ME, Brown TA, Trumpower BL. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18:3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurdistani SK, Robyr D, Tavazoie S, Grunstein M. Genome-wide binding map of the histone deacetylase Rpd3 in yeast. Nat Genet. 2002;31:248–254. doi: 10.1038/ng907. [DOI] [PubMed] [Google Scholar]
- 58.Nickles K, McEachern MJ. Characterization of Kluyveromyces lactis subtelomeric sequences including a distal element with strong purine/pyrimidine strand bias. Yeast. 2004;21:813–830. doi: 10.1002/yea.1119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.