Abstract
Adding reagents to drops is one of the most important functions in droplet-based microfluidic systems; however, a robust technique to accomplish this does not exist. Here, we introduce the picoinjector, a robust device to add controlled volumes of reagent using electro-microfluidics at kilohertz rates. It can also perform multiple injections for serial and combinatorial additions.
Keywords: electrocoalescence, high-throughput biology, in vitro compartmentalization
In droplet-based microfluidics, drops are used as “test tubes” for reactions (1, 2); the drops can be formed (3, 4), filled with reagents (2), and sorted (5) at kilohertz rates. The advantage of droplet-based microfluidics is the separation between reagent containment and fluidic control; the reagent is contained within the drop, while fluidic control is achieved with the inert continuous phase. This ability proves useful for a range of applications, from single-cell studies and directed evolution (6) to genetic sequencing. To carry out complex experiments like these, the microfluidic devices must perform several functions on each drop; these include encapsulating beads, cells, and reagents within the drops and optically assaying their contents (7–10). Another essential function is the addition of reagent into each drop; this is particularly important when performing multistep reactions, which require mixing new reagents at different times. This can be accomplished by several methods: For example, using a T-junction device, reagents can be added to drops (11, 12); unfortunately, however, this cannot be used with stable emulsions, because the surfactants prevent reagents from entering the drops. This is a significant limitation, because stability is essential to ensure that drops in contact do not merge and their contents remain isolated. Alternatively, pairs of drops can be merged with electro-coalescence (13). In this elegant approach, a drop containing reagent is interspersed in the flow with a drop containing the target; the pairs are merged by applying an electric field. However, it is difficult to perform multiple additions with this technique, due to the need to synchronize several streams of drops. Thus, to realize the full potential of droplet-based microfluidics, a robust technique for adding reagents is needed.
In this paper we present a robust technique to add reagents to individual drops using electro-microfluidics. We use a pressurized channel to inject a controlled volume of reagent into each drop, triggered by an electric field. By adjusting the drop velocity and injection pressure, we control the volume injected with subpicoliter precision. By switching the electric field on and off, we inject drops selectively, at kilohertz rates. By using several picoinjectors in combination, each separately controlled by an electric field, we perform serial and combinatorial injections.
We combine the picoinjector with a spacer junction; the drops enter the device at high volume-fraction and pass through a narrow channel that forces them into single file (Fig. 1, left). Oil is added from a side channel, increasing the spacing between the drops. The picoinjector consists of a pressurized channel containing the reagent to be added and a positive and negative electrode (Fig. 1, center). When a drop flows by the picoinjector, the electrodes are energized; this destabilizes the water/oil interfaces, allowing the reagent to enter the drop. The interface ruptures due to an electrically induced thin-film instability (14, 15). The picoinjector is maintained at a high pressure, causing fluid to be injected into the drop, as shown in the image. As the drop continues in its flow, it remains connected to the orifice of the picoinjector by a narrow bridge of fluid. The bridge eventually becomes unstable and breaks, detaching the drop. Once away from the electric field the drops regain their stability, so that even when in contact they do not merge, as shown to the far right in Fig. 1.
Fig. 1.
Microfluidic device consisting of a droplet spacer and picoinjector. The spacer adds oil from a side channel to space the drops. The picoinjector injects fluid by merging the drops with a pressurized channel containing the reagent. Picoinjection is triggered by an electric field, which is applied by the electrodes.
A unique and valuable property of the picoinjector is that it can be switched at kilohertz speeds. This is possible due to the design of the picoinjection channel. The channel abruptly narrows to a small orifice; as a result, the interface between the reagent and the oil has a high curvature, creating a pressure differential between the injector and the oil channels. This pressure differential is approximately determined by the Laplace pressure,
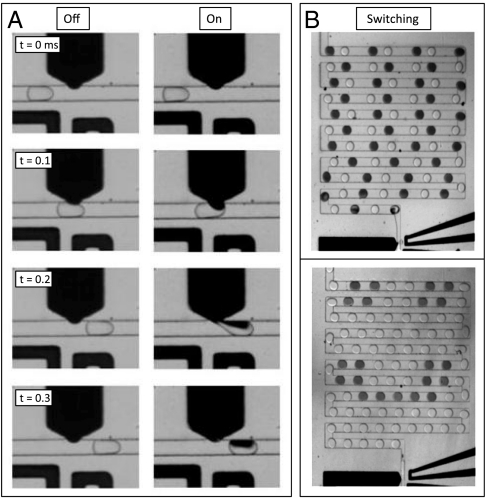
where Pin is the pressure of the injection fluid, Pout the pressure in the flow channel, γ the water/oil surface tension, and r the radius of curvature of the interface in the orifice. The forces on the interface balance, maintaining a static equilibrium. If the reagent pressure is increased, it bulges farther into the oil, adopting a shape of higher curvature; this increases the Laplace pressure so that static equilibrium is maintained. Because the pressure fluctuates as the drops flow through the channel (16, 17), the interface must move slightly to maintain the equality of the Laplace law. Such a quasi-static description is justified provided surface tension is large compared to flow stresses. To compare surface tension to inertial effects, we calculate the Weber of the injection phase We = ρv2l/γ, where ρ is the fluid density, v its velocity, and l the amplitude of the oscillation. For the peak velocity of the interface during picoinjection, We ∼ 10-5, indicating that inertial effects are negligible. Similarly, to compare surface tension to viscous shear effects, we calculate the Capillary number of the oil phase Ca = μv/γ, where μ is the fluid viscosity. During picoinjection Ca ∼ 10-2, indicating that viscous effects are also small. When the electric field is not activated, the drop slides over the interface without reagent injection, as shown in Fig. 2A and in Movie S1. However, when the electric field is activated, the interface is ruptured and the drop and reagent fluid merge, as shown in Fig. 2A and in Movie S2. Immediately upon injection, the bulge returns to its equilibrium position; this ensures injection of identical volumes into each drop; moreover, the drops need not enter at regular intervals.
Fig. 2.
The picoinjector can be switched on and off. This is possible because the injector channel begins wide and narrows to a small orifice; to pass through the orifice, the fluid must adopt a bulge with high curvature. This creates a Laplace pressure that balances the pressure differential between the injection and oil channels. (A) When the electrode is off, the drop slides over the bulge without injection, because surfactants prevent merger. By contrast, (B) when the electric field is activated, the drop merges with the reagent, causing fluid to be injected. By switching the field on and off, drops can be injected selectively. This can be used to inject every other drop (Upper) or to inject a more complex pattern (Lower).
The control afforded by the electric field enables high-speed switching, allowing a single drop within the flow to be injected. We demonstrate this by injecting every other drop, as shown in Fig. 2B, Upper. The switching is well enough controlled that more complex patterns can also be injected, as demonstrated in Fig. 2B, Lower (see also Movies S3 and S4). We can control injection at rates up to approximately 10 kHz; this is ultimately limited by the droplet detection speed.
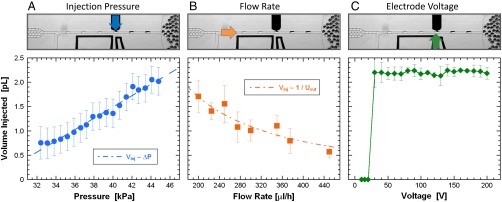
We quantify the volume injected by measuring the change in the drop size. We record images of the drops during injection and measure the distance between their leading and trailing interfaces. Modeling them as cylinders with rounded ends, we determine the change in volume. This allows us to measure the volume injected to a precision of 0.1 pL, limited by our ability to optically resolve the interfaces. The volume injected is proportional to the injection velocity and injection time. By increasing the injection pressure, we increase the injection velocity, leading to a linear increase in the volume injected, as shown in Fig. 3A. By increasing the oil flow rate, we increase the velocity of the drops, thereby shortening the injection time. This leads to an inverse dependence of injection volume on oil flow rate, as shown in Fig. 3B. The upper and lower limits to the injection volume result from the precision with which the interface can be balanced in the orifice. For our device and for the fluids used here, we are able to inject a minimum volume of 0.1 pL and a maximum volume of 3 pL; these limits can be expanded by changing the dimensions of the microfluidic device. The electric field can also be used to control injection. When the voltage is less than 30 V, the water/oil interfaces are stable and no injection occurs; however, if it is increased above this threshold value, the interfaces are unstable so that a constant volume is injected, as shown in Fig. 3C. To track sources of injection variability, we measure the standard deviation in the volume injected into approximately 100 drops, plotted as error bars in Fig. 3. The standard deviation is constant as a function of injection pressure, as shown by constant error bar length. As a function of continuous phase flow rate the injection variability decreases slightly; this may be due to the operation of our pumps, which tend to become more precise at higher flow rates. We also track variability resulting from differences in the initial drop size but do not find a correlation; this may be due to the small range of sizes, resulting from the monodispersity of our drops. With a larger range of sizes, a correlation may become evident, but this would not be representative of normal device operation. Thus, the volume injected can be controlled to subpicoliter precision by adjusting the injection pressure and oil flow rate; injection can be switched on and off by adjusting the electric field.
Fig. 3.
The picoinjector allows the injection volume to be controlled. (A) By increasing the injection channel pressure, more fluid is injected because the flow velocity into the drop is larger. (B) By increasing the spacer oil flow rate, less reagent is injected because the drop spends less time by the picoinjector. (C) Electrode voltage can switch picoinjection on and off, not injecting below 30 V, and injecting a constant volume above. The error bars correspond to the standard deviation in the volume injected, as measured for 100 injections.
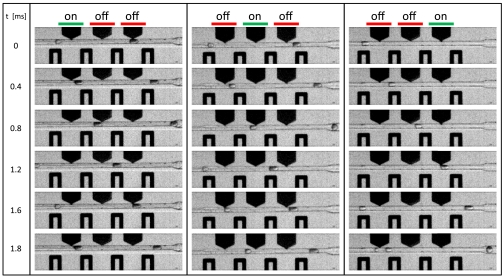
The picoinjector is compact, requiring a space of only a few hundred microns along the channel. This makes it easy to integrate the picoinjector with other components, such as drop formers and sorters or even additional picoinjectors. We demonstrate this with a device composed of three picoinjectors in series, as shown in Fig. 4. Each picoinjector can be controlled independently: When the first is activated but the second and third are not, only the first injects, as shown in Fig. 4. Similarly, we can activate each of the other two picoinjectors independently, as shown in Fig. 4 (see also Movie S5). This can be used to perform serial and combinatorial injections. Upon detecting a drop, a control computer can activate the picoinjectors in a specific combination. Subsequent drops can be injected with a different combination of reagent. Switching of the electrodes can be accomplished at rates of several kilohertz; thus, this serial array of picoinjectors can be used for rapid combinatorial screening.
Fig. 4.
Picoinjectors can be used in combination for serial and combinatorial injection. In this example, three picoinjectors are used in series. Each is activated by the electrode opposite, while ground electrodes on either side shield the picoinjectors nearby. This allows each to be actuated independently, to inject different combinations.
The picoinjector is a robust and controlled device for injecting reagents into drops. It is fast, operating at several kilohertz. This makes it of general use for droplet-microfluidic applications, especially those that require high throughput. It should also be useful for applications requiring multiple additions or additions of different reagent combinations. This could be useful for chemical and biological screens and genetic sequencing (18–22).
Materials and Methods
Fabrication of the Microfluidic Devices.
Our picoinjector devices are fabricated in poly(dimethylsiloxane) (PDMS) using the techniques of soft lithography (23). The devices have channel heights between 25 and 50 μm. The picoinjectors require electrodes to trigger injection. To make the electrodes, we use a simple liquid-solder approach (24). We fabricate empty microchannels in the shape of the electrodes; we heat the device and inject into these channels a low melting-point liquid solder. By cooling the device we solidify the solder, producing a metallic electrode in the shape of the channel. This allows fabrication of high-resolution electrodes.
Reagents.
We inject into aqueous drops dispersed in an inert carrier oil. For the oil we choose the fluorocarbon oil HFE-7500, due to its inertness to proteins. To stabilize the drops, we use a triblock biocompatible fluoro-surfactant (25) donated by RainDance Technologies. The surfactant has a hydrophilic poly(ethyleneglycol) (PEG) block flanked by two perfluorinated tails. The perfluorinated tails stabilize the drops against coalescence, while the PEG block coats the inner surface of the drop with a biocompatible layer that is resistant to protein adsorption. The surfactant is dissolved in the oil at 1.8% by weight. To visualize the amount of fluid injected, we inject a solution of bromphenol blue aqueous at 5 mM concentration. We also illuminate the sample with green light that is strongly absorbed by the dye; this makes the injected fluid appear dark black while the drops are clear.
Droplet Detection and Injection.
We automate injection using a computer. The microfluidic device is placed on an inverted fluorescent microscope with a photo-multiplier tube (PMT) detector attached to its epifluorescence port. A laser is directed into the back of the microscope so that it is focused by the objective in front of the picoinjector. When a fluorescent drop passes though the laser spot, it emits light that is transmitted to the PMT. The PMT measures the fluorescence, outputting a voltage proportional to the intensity. The computer analyzes the peaks in the signal, to detect when drops are present. When a drop is detected the computer activates a high-voltage amplifier, outputting a voltage between 0–1000 V at a frequency of 20 kHz. This is applied to the electrodes, to trigger injection.
Supplementary Material
Acknowledgments.
We thank Tobias M. Schneider for helpful discussions. This work was supported by the Massachusetts Life Sciences Center, the National Science Foundation (DMR-1006546), and the Harvard Materials Research Science and Engineering Centers (DMR-0820484). P.M. thanks Saint-Gobain Recherche for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006888107/-/DCSupplemental.
References
- 1.Rotman B. Measurment of activity of single molecule of β–D-Galactosidase. Proc Natl Acad Sci USA. 1961;47:1981–1991. doi: 10.1073/pnas.47.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song H, Chen DL, Ismagilov RF. Reactions in droplets in microfluidic channels. Angew Chem Int Ed Engl. 2006;45:7336–7356. doi: 10.1002/anie.200601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorsen T, Roberts RW, Arnold FH, Quake SR. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys Rev Lett. 2001;86:4163–4166. doi: 10.1103/PhysRevLett.86.4163. [DOI] [PubMed] [Google Scholar]
- 4.Anna SL, Bontoux N, Stone HA. Formation of dispersions using “flow focusing” in microchannels. Appl Phys Lett. 2003;82:364–366. [Google Scholar]
- 5.Ahn K, et al. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl Phys Lett. 2006;88:024104. [Google Scholar]
- 6.Agresti JJ, et al. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc Natl Acad Sci USA. 2010;107:6550–6550. doi: 10.1073/pnas.0910781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chabert M, Viovy JL. Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells. Proc Natl Acad Sci USA. 2008;105:3191–3196. doi: 10.1073/pnas.0708321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clausell-Tormos J, et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem Biol. 2008;15:875–875. doi: 10.1016/j.chembiol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Koster S, et al. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip. 2008;8:1110–1115. doi: 10.1039/b802941e. [DOI] [PubMed] [Google Scholar]
- 10.Huebner A, et al. Quantitative detection of protein expression in single cells using droplet microfluidics. Chem Commun. 2007;12:1218–1220. doi: 10.1039/b618570c. [DOI] [PubMed] [Google Scholar]
- 11.Gerdts CJ, et al. Time-controlled microfluidic seeding in nL-volume droplets to separate nucleation and growth stages of protein crystallization. Angew Chem Int Ed Engl. 2006;45:8156–8160. doi: 10.1002/anie.200602946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song H, Li HW, Munson MS, Van Ha TG, Ismagilov RF. On-chip titration of an anticoagulant argatroban and determination of the clotting time within whole blood or plasma using a plug-based microfluidic system. Anal Chem. 2006;78:4839–4849. doi: 10.1021/ac0601718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn K, Agresti J, Chong H, Marquez M, Weitz DA. Electrocoalescence of drops synchronized by size-dependent flow in microfluidic channels. Appl Phys Lett. 2006;88:264105. [Google Scholar]
- 14.Herminghaus S. Dynamic instability of thin liquid films between conducting media. Phys Rev Lett. 1999;83:2359–2361. [Google Scholar]
- 15.Priest C, Herminghaus S, Seemann R. Controlled electrocoalescence in microfluidics: Targeting a single lamella. Appl Phys Lett. 2006;89:134101. [Google Scholar]
- 16.Zien TF. Hydrodynamics of bolus flow—an analytical approach to blood flow in capillaries. B Math Biophys. 1969;31:681–694. doi: 10.1007/BF02477781. [DOI] [PubMed] [Google Scholar]
- 17.Vanapalli SA, Banpurkar AG, van den Ende D, Duits MHG, Mugele F. Hydrodynamic resistance of single confined moving drops in rectangular microchannels. Lab Chip. 2009;9:982–990. doi: 10.1039/b815002h. [DOI] [PubMed] [Google Scholar]
- 18.Chen DL, Ismagilov RF. Microfluidic cartridges preloaded with nanoliter plugs of reagents: An alternative to 96-well plates for screening. Curr Opin Chem Biol. 2006;10:226–231. doi: 10.1016/j.cbpa.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, et al. Nanoliter microfluidic hybrid method for simultaneous screening and optimization validated with crystallization of membrane proteins. Proc Natl Acad Sci USA. 2006;103:19243–19248. doi: 10.1073/pnas.0607502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kritikou E. It’s cheaper in the Picolab. Nat Rev Genet. 2005;6:668. [Google Scholar]
- 21.Sauer S, et al. Miniaturization in functional genomics and proteomics. Nat Rev Genet. 2005;6:465–476. doi: 10.1038/nrg1618. [DOI] [PubMed] [Google Scholar]
- 22.Tewhey R, et al. Microdroplet based PCR enrichment for large scale targeted sequencing. Nat Biotechnology. 2009;27:1025–1031. doi: 10.1038/nbt.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane) Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 24.Siegel AC, Bruzewicz DA, Weibel DB, Whitesides GM. Microsolidics: Fabrication of three-dimensional metallic microstructures in poly(dimethylsiloxane) Adv Mater. 2007;19:727–733. [Google Scholar]
- 25.Holtze C, et al. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab Chip. 2008;8:1632–1639. doi: 10.1039/b806706f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.