Abstract
The neuropeptide vasoactive intestinal peptide (VIP) has been shown to inhibit macrophage proinflammatory actions, promote a positive Th2/Th1 balance, and stimulate regulatory T-cell production. The fact that this peptide is highly efficacious in animal models of inflammatory diseases such as collagen-induced arthritis and experimental autoimmune encephalomyelitis (EAE) suggests that the endogenous peptide might normally provide protection against such pathologies. We thus studied the response of VIP-deficient (i.e., VIP KO) mice to myelin oligodendrocyte protein-induced EAE. Surprisingly, VIP KO mice were almost completely resistant to EAE, with delayed onset and mild or absent clinical profile. Despite this, flow cytometric analyses and antigen-rechallenge experiments indicated that myelin oligodendrocyte protein-treated VIP KO mice exhibited robust Th1/Th17 cell inductions and antigen-specific proliferation and cytokine responses. Moreover, adoptive transfer of lymphocytes from immunized VIP KO mice to WT recipients resulted in full-blown EAE, supporting their encephalitogenic potential. In contrast, transfer of encephalitogenic WT cells to VIP KO hosts did not produce EAE, suggesting that loss of VIP specifically affected the effector phase of the disease. Histological analyses indicated that CD4 T cells entered the meningeal and perivascular areas of VIP-deficient mice, but that parenchymal infiltration was strongly impaired. Finally, VIP pretreatment of VIP KO mice before immunization was able to restore their sensitivity to EAE. These results indicate that VIP plays an unanticipated permissive and/or proinflammatory role in the propagation of the inflammatory response in the CNS, a finding with potential therapeutic relevance in autoimmune neuroinflammatory diseases such as multiple sclerosis.
Keywords: neuropeptide, multiple sclerosis, inflammation
The 28-aa neuropeptide vasoactive intestinal peptide (VIP) was initially discovered in the lung and intestine, where it exhibits potent vasodilator activity (1). It belongs to the secretin family and has high homology to pituitary adenylyl cyclase-activating polypeptide, with which it shares its two high-affinity G protein-coupled receptors (VPAC1 and VPAC2) (2). Widely distributed in neurons in the CNS and periphery, VIP is also present in lymphocytes and innervation within lymphoid organs (3–5). In the immune system, VIP has multiple and complex actions on myeloid and lymphoid cells (6, 7). The fact that VIP inhibits the release of proinflammatory mediators by activated macrophages, promotes Th2 versus Th1 responses, and induces the generation of regulatory T cells (Tregs), suggested that its receptors may be targets for anti-inflammatory drugs. Accordingly, subsequent studies showed that VIP treatment inhibits ongoing inflammatory responses in murine models of septic shock, Crohn's disease, and rheumatoid arthritis (8–11). However, the role of the endogenous peptide in maintaining the balance between potentially harmful and beneficial actions of the immune system remains to be elucidated.
Numerous reports have shown that the expression of VIP is altered during pathological conditions. For example, it has been reported that patients with multiple sclerosis (MS) have reduced VIP immunoreactivity in the cerebral spinal fluid (12). Moreover, Th cells isolated from patients with MS have an altered profile of VIP receptors, and are resistant to an action of VIP to promote a positive Th2/Th1 balance (13). In contrast, an up-regulation of VIP levels was observed in the serum and joints of mice with collagen-induced arthritis (14) and in the serum and peritoneal suspensions of mice injected with LPS (8). Under these conditions, VIP might originate from neurons or immune cells. In this sense, it has been shown that VIP gene expression is induced in neurons by cytokines and inflammatory stimuli (15, 16), and that lymphocytes can synthesize and release VIP following activation in vitro (5).
In the past few years, several genetically engineered mouse models have been created to study the VIP/PACAP system. For example, we have shown that targeted mutation of the VIP gene leads to several behavioral and endocrine dysfunctions (17–21). These mice show inflammation in the lung in basal conditions and airway hyperresponsiveness to methacholine aerosols (20, 21), as well as pulmonary artery hypertension, right ventricular hypertrophy, and thickening of the walls of the pulmonary artery and branches (22). Also, the importance of the VIP/PACAP system in the modulation of Th responses in vivo has been inferred, because VPAC2 KO mice showed reduced immediate-type hypersensitivity but enhanced delayed-type hypersensitivity (23). This phenotype is consistent with the contention that VIP or PACAP promotes a positive Th2/Th1 balance.
In the present study, we analyzed the response of VIP KO mice to experimental autoimmune encephalomyelitis (EAE), a model for MS, a chronic inflammatory and demyelinating disease of the CNS driven by autoreactive T cells (24). A beneficial effect of exogenously administered VIP on EAE has been previously shown. VIP given intraperitoneally ameliorated the clinical symptoms in myelin oligodendrocyte protein (MOG)-immunized mice, an effect that was associated with the down-regulation of the inflammatory cascade and enhanced Th2/Th1 balance (25). In addition, VIP treatment in a relapsing-remitting model of EAE resulted in the expansion of Tregs, enhancing their suppressive activity against encephalitogenic cells in vitro (26). Recently, we reported that mice deficient in PACAP, a peptide structurally and functionally highly homologous to VIP, develop an enhanced immunological response to EAE induction with enhanced Th1/Th17 cytokine profile and blunted expansion of Tregs (27). Given the similar observed actions of these peptides, we expected to also observe an enhanced incidence and severity of EAE in VIP KO mice. Surprisingly, the mice showed a near complete resistance to EAE, attributable to a defect in the effector phase of the disease, and demonstrating an essential permissive and/or proinflammatory role for VIP in this autoimmune disease model.
Results
VIP KO Mice Fail to Develop Clinical and Histopathological Features of EAE.
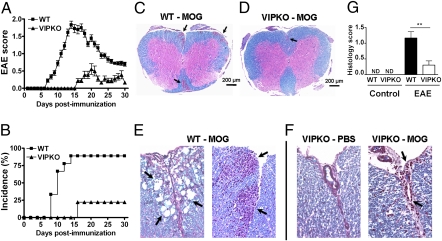
EAE was induced in female WT and VIP KO mice (C57BL/6J) (17) by s.c. immunization with MOG35–55 in complete Freund adjuvant (CFA) followed by pertussis toxin administration as described (27). Unexpectedly, the incidence and severity of the disease in VIP KO mice was strikingly lower in comparison with WT control mice (Fig. 1A). Whereas eight of nine WT mice developed typical EAE with a score of 2.0 or greater 14 d after immunization in this experiment, only two of the nine similarly immunized VIP KO mice developed any symptoms of EAE during the whole length of the study (Fig. 1A). In addition, the disease onset for the VIP KO animals was delayed to day 16 postimmunization, and the mean maximal disease grade in these mice was 0.56 ± 0.46 versus 1.83 ± 0.32 for WT. Two other experiments showed similar EAE resistance in female VIP KO mice, with only one of 21 VIP KO mice showing disease. Summation of these three experiments indicated that the incidence of EAE was 76% for WT mice, but only 15% for VIP KO mice (Fig. 1B).
Fig. 1.
Comparison of EAE in WT and VIP KO mice. Mice (n = 9–10 per group) were immunized s.c. with 100 μg of MOG35–55 in CFA and injected with pertussis toxin on days 0 and 2. (A) Representative experiment shows the mean clinical scores ± SEM in a range from 0 to 4 as described in Materials and Methods (*P < 0.05, Student t test). (B) Overall percent incidence of disease over time (summary of three experiments; *P < 0.05 by log-rank test). Histological changes in transverse sections of spinal cords from MOG-immunized WT and VIP KO mice. (C and D) Low-magnification photomicrographs (4×) of thoracolumbar spinal cord from WT (C) and VIP KO (D) mice. WT mice show multifocal inflammation in the meninges and white matter parenchyma. (E and F) Higher-magnification photomicrographs (20×) of spinal cords of WT and VIP KO mice, respectively. Whereas evidence of intense inflammation is present in the leptomeninges and white matter of the spinal cord of the WT mice (E), inflammation, when present, was much less severe in VIP KO mice, and was primarily in the leptomeninges, sometimes invading the anterior fissure (F). (G) Mean histological score of sections from WT and VIP KO mice ± SEM (**P < 0.01, Student t test). ND, no inflammation detected.
To corroborate the clinical results, histological analyses of spinal cords were performed on day 30 postimmunization, combining H&E and Luxol fast blue staining to visualize immune cell infiltration and demyelination, respectively. Whereas pronounced infiltration was observed in the meninges and CNS parenchyma of WT mice, smaller numbers of inflammatory cells were found in the CNS of VIP KO mice; these were always confined to the anterior median fissure and/or surrounding meninges (Fig. 1 C–F). In addition, the WT spinal cords exhibited large areas of demyelination, a phenomenon that was absent in the MOG-immunized VIP KO mice. Overall, the mean histological score was significantly higher for WT (1.2 ± 0.2) than for VIP KO mice (0.3 ± 0.1; P < 0.01; Fig. 1G). Consistent with the reduced pathology, gene expression of the proinflammatory cytokines TNFα, IL-6, IFNγ, IL-17, and IL-23 was significantly reduced in spinal cord extracts of VIP KO mice, as was that of cytokines generally considered to be anti-inflammatory: IL-10, TGFβ, and IL-4 (Fig. S1). Moreover, the induced expressions of multiple chemokine genes were dramatically attenuated in the CNS of MOG-immunized VIP KO compared with WT mice, including those purported to be relatively specific for Th1 (MIG/CXCL9 and IP-10/CXCL10) and Th17 (MIP-3α/CCL20) cells (Figs. S2 and S3). In summary, VIP KO mice showed an unpredicted resistance to MOG-induced EAE, reflected in a mild EAE clinical profile, relative absence of histopathological signs, and decreased cytokine and chemokine gene expression in the CNS.
Immunized VIP KO Mice Exhibit Robust MOG-Specific T-Cell Responses in Vivo and ex Vivo.
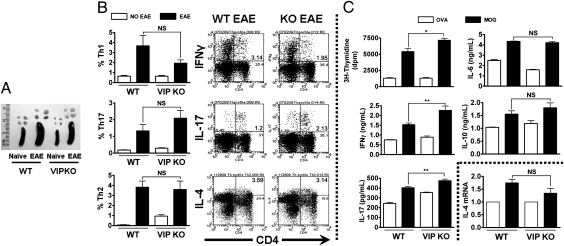
Despite their apparent lack of EAE development, VIP KO mice exhibited enlarged spleens and draining lymph nodes after MOG administration, similar to WT mice (Fig. 2A), suggesting that MOG immunization did, in fact, elicit a strong immunological response. To compare the T-cell responses in MOG-immunized WT and VIP KO mice, we performed cytokine intracellular staining of lymph node cells and ex vivo recall assays. We first measured by flow cytometry the proportions of IFNγ+ (Th1), IL-17+ (Th17), and IL-4+ (Th2) cells in both WT and VIP KO mice lymph node suspensions 14 d after EAE induction. MOG immunization triggered robust increases in Th1, Th17, and Th2 cells in WT and VIP KO mice (Fig. 2B), with no statistically- significant alterations in VIP-deficient mice. Moreover, no significant differences between genotypes were observed in the proportions of Tregs (CD4+CD25+Foxp3+; Table S1). Other immune cell subsets such as cytotoxic T cells (CD3+CD8+) and natural killer T cells (CD3+NK1.1+) were not different between the two genotypes, but elevated numbers of NK cells were observed in lymph nodes of VIP KO mice (Table S2).
Fig. 2.
Th profiles and MOG-induced proliferative and cytokine responses in lymph node cultures in WT versus VIP KO mice. (A) Photograph of WT and VIP KO spleen and draining lymph nodes (DLN) 14 d after EAE induction. Cell suspensions from DLN from EAE-induced WT and VIP KO mice (day 14) were prepared. For flow cytometry Th profile analyses (B), cells were stimulated for 5 h with PMA/ionomycin/brefeldin, and then stained for CD4 and IFNγ, IL-17, or IL-4. For ex vivo studies (C), cells were stimulated with MOG35–55 peptide or ovalbumin (10 μg/mL). Proliferation was measured 72 h later by [3H]thymidine incorporation. Cytokines released to the culture supernatant were measured by ELISA 48 h after stimulation with MOG35–55. IL-4 expression was detected in cell extracts by real-time PCR. Data shown are representative of three independent experiments (*P < 0.05 and **P < 0.01, Student t test). In WT mice, all parameters were significantly induced by MOG (*P < 0.05). NS, not significant.
For ex vivo antigen recall assay, we isolated draining lymph nodes cells 14 d after EAE immunization, and stimulated them for 3 d with MOG or ovalbumin. As expected, WT cells displayed high proliferation rates and cytokine production specifically in response to MOG (Fig. 2C). Interestingly, the proliferative response was even stronger in cells from VIP KO compared with WT mice. In addition, medium from VIP KO cells contained higher levels of IFNγ and IL-17, suggesting an increased Th1/Th17 polarization, whereas no differences were found in IL-6 or IL-10. As IL-4 protein levels were not detectable by ELISA, we performed real-time PCR analysis of IL-4 mRNA in cells stimulated with MOG (Fig. 2C). No differences in IL-4 gene expression were found between WT versus VIP KO cell cultures. In summary, an antigen-specific response was clearly observed ex vivo in VIP KO mice despite their inability to develop EAE.
Adoptive Transfer Studies Indicate a Defect in the Effector Phase of EAE.
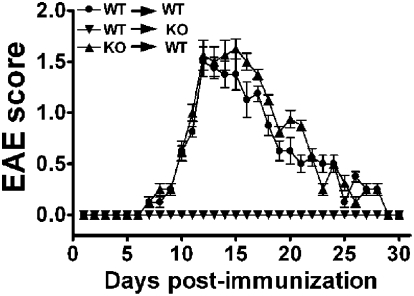
The aforementioned studies indicated that T cells from MOG-immunized VIP KO mice express a proinflammatory phenotype and respond ex vivo to secondary MOG exposure. Thus, it was of interest to determine if these cells would be competent to produce EAE in a WT genetic background. Hence, draining lymph nodes and spleen cells were harvested from WT and VIP KO mice on day 14 postimmunization, restimulated ex vivo with MOG35–55 for 3 d, and then transferred i.v. into naive WT or VIP KO mice. As expected, transfer of WT cells reproduced EAE in WT hosts, with an incidence of 100%, an onset of symptoms at approximately day 7, a peak on day 12, and a recovery period starting on day 18 (Fig. 3). Similarly, when MOG-stimulated cells from VIP KO mice were transferred to WT mice, the recipients developed EAE, with severity and kinetics comparable to those receiving WT cells. In contrast, when cells from immunized WT mice were transferred to naive VIP KO mice, no EAE symptoms were observed. The latter demonstrates that competent encephalitogenic cells are not able to propagate an immune response in the CNS of VIP KO mice. This implies that the defect in VIP KO mice does not lie in the initial priming of T cells in the lymph nodes, but on the subsequent steps consolidating the effector phase of disease, such as T-cell entry and/or in amplification steps.
Fig. 3.
Adoptive transfer of immune cells from WT or VIP KO MOG-injected mice triggered EAE in WT but not in VIP KO recipients. Encephalitogenic cells were prepared by immunizing WT or VIP KO mice and culturing their spleen and lymph node cells harvested on day 14 in the presence of 30 μg/mL of MOG for 3 d. Cells were injected i.v. into WT and/or VIP KO C57BL/6 mice, and EAE clinical scores were assessed as described in Fig. 1A. Data are shown as a mean clinical score ± SEM (n = 4 per group). One of three similar experiments is shown.
Immune Cell Infiltration Is Impaired in VIP KO Mice.
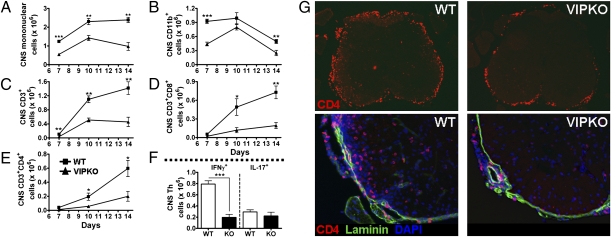
To evaluate potential anomalies in immune cell recruitment over the course of EAE, we isolated and quantified the number of mononuclear cells in the CNS at different stages of EAE induction: days 7 (asymptomatic phase), 10 (onset), and 14 (peak) postimmunization. Total numbers of immune cell infiltrating the CNS of VIP KO mice were found to be lower than those in the WT at all times examined (Fig. 4 A–F). This suggests a defect in migration to the CNS, manifested as early as day 7 after immunization. Although reduced in VIP-deficient mice, the total numbers of immune cells in the CNS increased in parallel in both WT and VIP KO mice between days 7 and 10. However, from day 10 to day 14, the numbers continued to increase in WT mice, whereas they failed to accumulate further in VIP KO mice. The latter suggests that subsequent CNS amplification may be impaired in mice deficient in VIP. To characterize the immune infiltrates, we stained isolated CNS mononuclear cells with CD11b, CD3, CD4, and CD8 antibodies and determined the total number of different cell subsets after flow cytometry analysis. The quantities of all immune cell types studied were lower in VIP KO mice (Fig. 4 A–E). For example, the number of CD11b+ cells was strikingly reduced in VIP-deficient mice on day 7 after EAE compared with the WT mice, and remained lower, although differences were less pronounced on days 10 and 14. More importantly, VIP KO mice exhibited a diminished abundance of CD3+ cells (including both CD4+ and CD8+ cells) on day 7 postimmunization and their numbers did not increase with the course of the disease like those of WT mice. Analysis of CD4 cytokine phenotype on day 14 revealed the presence of large numbers of CD4+IFNγ+ (Th1) cells and CD4+IL-17+ (Th17) cells in the CNS of WT mice, as reported by others (28). In contrast, the total number of Th1 cells was reduced in the VIP KO mice, whereas the number of Th17 cells was not significantly different (Fig. 4F). Thus, despite the fact that encephalitogenic T cells are generated in VIP KO mice, the numbers of those—at least one subtype (Th1)—that reach the CNS is reduced, suggesting that their ability to enter and/or expand in the CNS parenchyma is impaired in mice chronically deficient in VIP.
Fig. 4.
Immune cell infiltration is impaired in VIP KO mice. EAE was induced to WT and VIP KO mice as in Fig. 1 legend, and CNS tissues were collected on days 7, 10, and 14. (A–E) Total numbers of mononuclear cells, as well as CD11b, CD3, CD4, and CD8 cells. (F) Total numbers of Th1 and Th17 cells in the CNS on day 14. The number of infiltrating immune cells was remarkably lower in VIP KO mice (*P < 0.05, **P < 0.01, and ***P < 0.001; Student t test). (G) Photomicrographs at magnifications of 4× (Upper) and 20× (Lower) of spinal cord sections from WT (Left) and VIP KO mice (Right) collected 14 d after immunization and stained by immunofluorescence for CD4 (Alexa 594), laminin (FITC), and DAPI. Thoracic level is shown. Top: CD4 staining only. Lower: Triple staining of laminin, CD4, and DAPI. Note in particular the high abundance of CD4+ cells in the parenchyma of WT but not VIP KO mice (lower two panels), and their apparent nonassociation with laminin-positive blood vessels. Similar results were found at all levels of spinal cords of four sets of WT and VIP KO mice.
CD4+ T Cells Accumulate in the Meninges but Fail to Enter the CNS Parenchyma of VIP KO Mice.
To determine if the spatial distribution of T cells in CNS differed between WT and VIP KO mice immunized with MOG, CD4 immunofluorescence assays were performed on CNS sections. These studies revealed abundant CD4+ cells in the meninges and parenchyma of WT spinal cords, whereas these cells were less abundant in cords of VIP KO mice, and almost exclusively confined to the meninges and subarachnoid space (Fig. 4G). Similar results were obtained in selected regions of the brain, where CD4+ cells were copious in the meninges, the parenchymal and perivascular areas of the cerebellum and hippocampus, as well as proximal to the ventricles in the WT mice, but reduced and restricted to the meninges in the KO mice (Fig. S4). Thus, whereas significant, albeit lower, numbers of CD4+ cells reach the meningeal area of the CNS of VIP KO mice, their penetration into the CNS parenchyma was severely impaired.
VIP KO Mouse Resistance Is Reversed by VIP Pretreatment.
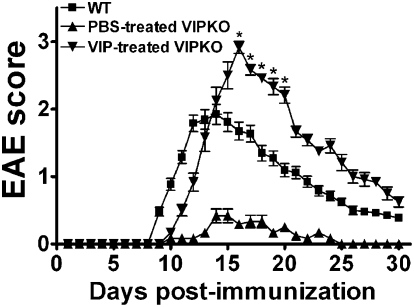
As previous studies showed that administration of VIP ameliorated clinical symptoms and decreased inflammatory parameters in EAE (25), the finding that VIP KO mice are resistant to these diseases was unexpected. One potential explanation is that, whereas acute short-term administration of VIP during disease is able to exert an anti-inflammatory action, chronic loss of VIP might actually impair the process of inflammation. One way to determine if the EAE resistance in VIP KO mice is, in fact, a result of prior chronic loss of VIP would be to pretreat these mice with VIP to determine if EAE can be restored. To test this, we selected a dose regimen of VIP (10 nmoles i.p. per day) which has been repeatedly shown to be pharmacologically active in mice (26), and arbitrarily chose a 2-wk pretreatment period. VIP administration was halted 1 d before MOG immunization to avoid potential confounding anti-inflammatory actions of administered peptide. EAE symptoms were scored for 30 d after MOG administration for WT, PBS-treated VIP KO, and VIP-treated VIP KO mice. Strikingly, only WT and VIP-treated VIP KO mice were found to be highly sensitive to MOG-induced EAE. Interestingly, VIP-treated VIP KO mice showed more severe symptoms than WT controls (mean EAE score slightly lower than 3.0 ± 0.2 for VIP-treated VIP KO vs. 2.0 ± 0.1 for WT mice; P < 0.01) and delayed recovery (Fig. 5). The data indicate that a 2-wk period of VIP preadministration is capable of restoring EAE in VIP KO mice, demonstrating the reversibility of the phenotype and providing strong evidence that the EAE resistance was caused by the selective loss of VIP.
Fig. 5.
Pretreatment of VIP KO mice with VIP restores their sensitivity to EAE. Mice were injected i.p. with 10 nmoles of PBS solution or VIP every day for 2 wk, and treatment was stopped 1 d before EAE immunization. One of three representative experiments is shown. Scores in VIP-treated VIP KO mice were higher than in PBS-treated VIP KO mice on days 10 to 30 (*P < 0.05, Student t test) and higher than in WT mice on days 16 to 20.
Discussion
The anti-inflammatory properties of VIP have been extensively reported in the literature (6, 7, 29). The fact that cytokines and inflammatory conditions trigger VIP expression in neurons and lymphocytes (5, 15, 16), provided a clue that VIP might play a protective role against excess inflammation, and that its gene deletion might lead to enhanced disease. Indeed, Hamidi et al. found that lungs of VIP KO mice were inflamed in basal conditions (21). Unlike this result, and contrary to our expectations, VIP KO mice were found to be remarkably resistant to active and passive EAE induction, with almost absent clinical and histological features of the disease, and little or no induction of cytokine expression in the spinal cord. Importantly, the phenotype of VIP KO mice could be corrected by VIP preadministration during a period of 2 wk, providing strong evidence that the phenotype was a result of the selective loss of VIP.
Because of the complexity of EAE, there are many steps at which the loss of a regulatory molecule such as VIP might affect the disease. During the initial MOG immunization phase, antigen presenting cells in the draining lymph nodes take up antigen and induce the differentiation of MOG-specific T effector cells primarily toward Th1/Th17 phenotypes (30). Antigen recall assays indicated that Th activation (proliferation as well as IFNγ and IL-17 production) was actually enhanced in VIP KO mice compared to WT mice. This indicates that immunization to MOG was indeed robust in VIP KO mice. Moreover, T cells isolated from EAE-induced VIP KO mice induced EAE when adoptively transferred to naive WT hosts, demonstrating their competence to induce EAE. In contrast, encephalitogenic T cells generated in MOG-immunized WT mice failed to produce EAE in VIP KO mice, indicating that the defect in the KO mice occurred at steps downstream of the immunization step.
The T-cell localization studies provided some insights into potential mechanisms of the EAE resistance in VIP KO mice. These revealed that significant numbers of CD4+ T cells accumulated in the subarachnoid space of VIP KO mice, but that they failed to enter the CNS parenchyma. The inability to penetrate the CNS parenchyma might have occurred as a result of a failure in the local reactivation of these T cells in the CNS (31), or in other mechanisms regulating T-cell/macrophage penetration across the blood–brain barrier. With respect to the latter, the impaired induction in endothelial, astroglial, or myeloid cell expression of any of several molecules implicated in T-cell penetrance of the blood–brain barrier (32) might explain the lack of EAE in VIP KO mice. These molecules include metalloproteases, chemokines, and cell adhesion molecules. Indeed, the potential for VIP to stimulate metalloprotease activity (33) and to modulate chemokine production (34) has been shown. In addition, published studies demonstrated that VIP can directly stimulate macrophage and T-cell adherence and chemotaxis in vitro, indicating a possible role for locally released VIP in the homing and tissue infiltration of these cells (35–37). Although inductions of spinal cord chemokine gene expression were attenuated in immunized VIP KO mice, this reduction could have occurred simply as consequence of reduced neuroinflammation. Nonetheless, the chemokine reduction in VIP KO mice warrants further exploitation with respect to time after MOG administration and cellular sources.
Although most published studies indicate that VIP actions are anti-inflammatory, some data suggest that VIP can exert actions considered to be proinflammatory, thus providing other potential mechanisms to explain the EAE resistance of VIP-deficient mice. For example, VIP was reported to stimulate IL-6 release from resting peritoneal macrophages in vitro, and a single i.p. injection of VIP resulted in a significant increase in mice IL-6 serum levels (38). However, another study reported that VIP strongly inhibited the release of proinflammatory cytokines after LPS, suggesting that VIP effects on myeloid cells might be context-dependent (39). Recently, Yadav et al. showed that VIP induced purified mouse CD4 T cells to differentiate in vitro to a Th17-like phenotype in a TGFβ-rich environment (40). Although the significance of these potential proinflammatory actions of VIP in the context of the EAE model is unknown, they provide some potential clues to the unexpected resistance we observed in VIP KO mice.
Overall, the findings obtained here with VIP-deficient mice indicate that VIP plays an unexpected permissive and/or proinflammatory action in the development of EAE. As short-term administration of VIP has been shown to ameliorate the clinical symptoms and pathology of EAE, collagen-induced arthritis and other model inflammatory diseases, this potential proinflammatory action of VIP revealed by genetic loss of VIP needs to be given careful consideration before translation of prior findings to human disease. In the aforementioned studies, VIP was administered either during or several days after the induction of the disease. In our model, mice are chronically deficient in VIP. Perhaps therapies can be designed to optimize response by proper timing of treatment and/or targeting specific VIP receptor subtypes with agonists and/or antagonists. In any case, the mechanisms by which VIP depletion protects against EAE remain to be elucidated in future studies.
Materials and Methods
Induction of EAE and Histological Analysis.
Female VIP KO mice (6–8 wk old, backcrossed >12 generations to C57BL/6J mice) (17), and age-matched female WT C57BL/6J mice from the same colony were housed and fed ad libitum in a specific pathogen-free animal facility. In all studies, the recommendations for animal use and welfare, as dictated by the University of California, Los Angeles, Division of Laboratory Animals and the guidelines from the National Institutes of Health, were followed. To induce EAE, mice were immunized by s.c. injection of 100 μg of MOG35–55 (GLBiochem) in 100 μL of a solution containing 50% of CFA with 5 mg/mL of Mycobacterium tuberculosis (Difco) in the two flanks. Mice also received i.p. 200 ng of pertussis toxin (Sigma) in PBS solution on days 0 and 2 after immunization. For VIP pretreatment studies, mice were injected i.p. for 2 wk with 10 nmoles of VIP in PBS solution (GLBiochem) until 1 d before EAE induction. From day 0, mice were monitored on a daily basis for clinical signs of EAE, and scored on a four-point severity scale on which 0 represents no signs of disease, 1 represents tail limpness, 2 represents wobbly gait, 3 represents hind limb paralysis, and 4 represents moribund condition or death. For histopathological studies, stained sections distributed along the cervical and thoracic cord were evaluated for immune cell infiltration and demyelination, and scored as follows: 1 represents a few inflammatory cells and little demyelination, 2 represents organized perivascular infiltrates and a few areas of demyelination, and 3 represents increasing severity of perivascular cuffing with extension into adjacent tissue with large areas of demyelination.
Preparation of Cell Suspensions and Flow Cytometry Staining.
Draining lymph nodes and spleen cell suspensions were prepared as described (27). To isolate mononuclear cells from PBS-perfused spinal cord and brain, tissues were digested in collagenase and DNase I (Roche) 30 min at 37 °C and cells were separated on a 40%/80% Percoll gradient by centrifuging at 500 × g for 30 min. Cells at the 40%:80% interface were collected, stained for 30 min at 4 °C with fluorochrome-conjugated antibodies (eBioscience), and analyzed with a FACSCalibur flow cytometer. For intracellular cytokine staining, cells were incubated in complete RPMI medium 1640 (with 2% FBS; HyClone) containing PMA (50 ng/mL; Sigma), ionomycin (1 μg/mL; Sigma), and brefeldin (3 μg/mL; eBioscience). After 5 h of culture, cells were stained for CD4 and IL-4, IFNγ, or IL-17 according to eBioscience instructions.
Immunohistochemistry.
Brains and spinal cords were removed after perfusion with PBS solution and 4% PFA, postfixed overnight, and then transferred into 20% sucrose in PBS solution. Tissues were embedded in optimal cutting temperature compound and 15-μm sections were incubated with anti-CD4 (BD Pharmingen) and anti-laminin (Sigma) antibodies in PBS solution containing 1% BSA and 0.3% Triton X-100 at 4 °C overnight. Then, sections were washed and incubated with appropriate Alexa 594- and FITC-conjugated secondary antibodies for 40 min at room temperature. Slides were mounted using VectaShield with DAPI (Vector Labs).
Quantitative RT-PCR.
Real-time RT-PCR analyses were performed using SybrGreen (Bio-Rad) as described in SI Materials and Methods, with primers listed in Table S3.
Cell Cultures.
Cells (1 × 106/mL) were cultured in 96-well plates in complete medium with ovalbumin (Sigma) or MOG35–55 (10 μg/mL) at 37 °C in 5% CO2. For proliferation studies, cells were incubated for 3 d, and 1 μCi/well of [3H]TdR (Amersham Biosciences) was added in culture for the last 18 h. Then, cells were harvested, and [3H]TdR incorporation per well was measured after DNA precipitation using a β-scintillation counter. For cytokine assessment, 48 h supernatants were harvested and analyzed for IL-6, TNFα, IL-17, IL-10, and IFNγ by sandwich ELISA (Peprotech), according to the manufacturer's recommended procedures. For adoptive transfer, draining lymph node and spleen cells were cultured in complete RPMI medium with 30 μg/mL of MOG35–55 at 1.5 × 106 cells/mL or 3 × 106 cells/mL, respectively. After 3 d of culture, cells were harvested and washed, and 5 × 106 splenocytes and 5 × 106 lymph node cells were transferred i.v. to WT or VIP KO mice.
Statistical Analysis.
Differences between groups were evaluated using Student t tests, except for the incidence curve, for which the log-rank test was used.
Supplementary Material
Acknowledgments
This work was supported by National Multiple Sclerosis Society Grants PP1233 and RG3928 and National Institutes of Health Grants HD06576 and HD04612.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007622107/-/DCSupplemental.
References
- 1.Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- 2.Vaudry D, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- 3.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135(2 suppl):755s–765s. [PubMed] [Google Scholar]
- 4.Gomariz RP, Leceta J, Garrido E, Garrido T, Delgado M. Vasoactive intestinal peptide (VIP) mRNA expression in rat T and B lymphocytes. Regul Pept. 1994;50:177–184. doi: 10.1016/0167-0115(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 5.Martinez C, et al. Regulation of VIP production and secretion by murine lymphocytes. J Neuroimmunol. 1999;93:126–138. doi: 10.1016/s0165-5728(98)00216-1. [DOI] [PubMed] [Google Scholar]
- 6.Voice JK, et al. Immunoeffector and immunoregulatory activities of vasoactive intestinal peptide. Regul Pept. 2002;109:199–208. doi: 10.1016/s0167-0115(02)00182-9. [DOI] [PubMed] [Google Scholar]
- 7.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 8.Delgado M, et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-alpha and IL-6. J Immunol. 1999;162:1200–1205. [PubMed] [Google Scholar]
- 9.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 10.Abad C, et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- 11.Conlin VS, et al. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G735–G750. doi: 10.1152/ajpgi.90551.2008. [DOI] [PubMed] [Google Scholar]
- 12.Andersen O, Fahrenkrug J, Wikkelsø C, Johansson BB. VIP in cerebrospinal fluid of patients with multiple sclerosis. Peptides. 1984;5:435–437. doi: 10.1016/0196-9781(84)90249-3. [DOI] [PubMed] [Google Scholar]
- 13.Sun W, Hong J, Zang YC, Liu X, Zhang JZ. Altered expression of vasoactive intestinal peptide receptors in T lymphocytes and aberrant Th1 immunity in multiple sclerosis. Int Immunol. 2006;18:1691–1700. doi: 10.1093/intimm/dxl103. [DOI] [PubMed] [Google Scholar]
- 14.Delgado M, et al. Vasoactive intestinal peptide in the immune system: potential therapeutic role in inflammatory and autoimmune diseases. J Mol Med. 2002;80:16–24. doi: 10.1007/s00109-001-0291-5. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Zigmond RE. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J Neurochem. 1996;67:1751–1760. doi: 10.1046/j.1471-4159.1996.67041751.x. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong BD, et al. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- 17.Colwell CS, et al. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- 18.Lacombe A, et al. Lack of vasoactive intestinal peptide reduces testosterone levels and reproductive aging in mouse testis. J Endocrinol. 2007;194:153–160. doi: 10.1677/JOE-07-0102. [DOI] [PubMed] [Google Scholar]
- 19.Lelievre V, et al. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: A model for the study of intestinal ileus and Hirschsprung's disease. Peptides. 2007;28:1688–1699. doi: 10.1016/j.peptides.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szema AM, et al. Mice lacking the VIP gene show airway hyperresponsiveness and airway inflammation, partially reversible by VIP. Am J Physiol Lung Cell Mol Physiol. 2006;291:L880–L886. doi: 10.1152/ajplung.00499.2005. [DOI] [PubMed] [Google Scholar]
- 21.Hamidi SA, Prabhakar S, Said SI. Enhancement of pulmonary vascular remodelling and inflammatory genes with VIP gene deletion. Eur Respir J. 2008;31:135–139. doi: 10.1183/09031936.00105807. [DOI] [PubMed] [Google Scholar]
- 22.Said SI, et al. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation. 2007;115:1260–1268. doi: 10.1161/CIRCULATIONAHA.106.681718. [DOI] [PubMed] [Google Scholar]
- 23.Voice JK, et al. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J Immunol. 2003;170:308–314. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]
- 24.Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Rey E, et al. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Martin A, Gonzalez-Rey E, Chorny A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur J Immunol. 2006;36:318–326. doi: 10.1002/eji.200535430. [DOI] [PubMed] [Google Scholar]
- 27.Tan YV, et al. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2009;106:2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Abad C, Gomariz RP, Waschek JA. Neuropeptide mimetics and antagonists in the treatment of inflammatory disease: Focus on VIP and PACAP. Curr Top Med Chem. 2006;6:151–163. doi: 10.2174/156802606775270288. [DOI] [PubMed] [Google Scholar]
- 30.Siffrin V, Brandt AU, Herz J, Zipp F. New insights into adaptive immunity in chronic neuroinflammation. Adv Immunol. 2007;96:1–40. doi: 10.1016/S0065-2776(07)96001-0. [DOI] [PubMed] [Google Scholar]
- 31.Kivisäkk P, et al. Localizing central nervous system immune surveillance: Meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: Function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 33.Xia M, Gaufo GO, Wang Q, Sreedharan SP, Goetzl EJ. Transduction of specific inhibition of HuT 78 human T cell chemotaxis by type I vasoactive intestinal peptide receptors. J Immunol. 1996;157:1132–1138. [PubMed] [Google Scholar]
- 34.Jiang X, Jing H, Ganea D. VIP and PACAP down-regulate CXCL10 (IP-10) and up-regulate CCL22 (MDC) in spleen cells. J Neuroimmunol. 2002;133:81–94. doi: 10.1016/s0165-5728(02)00365-x. [DOI] [PubMed] [Google Scholar]
- 35.de la Fuente M, et al. Vasoactive intestinal peptide modulation of adherence and mobility in rat peritoneal lymphocytes and macrophages. Peptides. 1994;15:1157–1163. doi: 10.1016/0196-9781(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 36.De la Fuente M, et al. Stimulation by vasoactive intestinal peptide (VIP) of phagocytic function in rat macrophages. Protein kinase C involvement. Regul Pept. 1993;48:345–353. doi: 10.1016/0167-0115(93)90163-3. [DOI] [PubMed] [Google Scholar]
- 37.Xia M, et al. Stimulus specificity of matrix metalloproteinase dependence of human T cell migration through a model basement membrane. J Immunol. 1996;156:160–167. [PubMed] [Google Scholar]
- 38.Martinez C, et al. VIP and PACAP enhance IL-6 release and mRNA levels in resting peritoneal macrophages: in vitro and in vivo studies. J Neuroimmunol. 1998;85:155–167. doi: 10.1016/s0165-5728(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 39.Martínez C, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide modulate endotoxin-induced IL-6 production by murine peritoneal macrophages. J Leukoc Biol. 1998;63:591–601. doi: 10.1002/jlb.63.5.591. [DOI] [PubMed] [Google Scholar]
- 40.Yadav M, Rosenbaum J, Goetzl EJ. Cutting edge: Vasoactive intestinal peptide (VIP) induces differentiation of Th17 cells with a distinctive cytokine profile. J Immunol. 2008;180:2772–2776. doi: 10.4049/jimmunol.180.5.2772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.