Abstract
Female fertility requires estrogen to specifically stimulate estrogen receptor α (ERα)-dependent growth of the uterine epithelium in adult mice, while immature females show proliferation in both stroma and epithelium. To address the relative roles of ERα in mediating estrogen action in uterine epithelium versus stroma, a uterine epithelial-specific ERα knockout (UtEpiαERKO) mouse line was generated by crossing Esr mice with Wnt7a-Cre mice. Expression of Wnt7a directed Cre activity generated selective deletion of ERα in uterine epithelium, and female UtEpiαERKO are infertile. Herein, we demonstrate that 17β-estradiol (E2)-induced uterine epithelial proliferation was independent of uterine epithelial ERα because DNA synthesis and up-regulation of mitogenic mediators were sustained in UtEpiαERKO uteri after E2 treatment. IGF-1 treatment resulted in ligand-independent ER activation in both wild-type (WT) and UtEpiαERKO and mimicked the E2 stimulatory effect on DNA synthesis in uterine epithelium. Uterine epithelial ERα was necessary to induce lactoferrin, an E2-regulated secretory protein selectively synthesized in the uterine epithelium. However, loss of uterine epithelial ERα did not alter the E2-dependent progesterone receptor (PR) down-regulation in epithelium. Strikingly, the uterine epithelium of UtEpiαERKO had robust evidence of apoptosis after 3 d of E2 treatment. Therefore, we surmise that estrogen induced uterine hyperplasia involves a dispensable role for uterine epithelial ERα in the proliferative response, but ERα is required subsequent to proliferation to prevent uterine epithelial apoptosis assuring the full uterine epithelial response, illustrating the differential cellular roles for ERα in uterine tissue and its contribution during pregnancy.
Keywords: conditional knockout, paracrine regulation
Estrogens exert their actions through estrogen receptor (ER) proteins. ER alpha and beta (α and β) are distributed throughout diverse tissues of the body. ERα is predominantly expressed in the uterus, mammary glands, pituitary, hypothalamus, and ovarian theca cells, whereas ERβ is primarily in ovarian granulosa cells, lung, and prostate (1). The uterus is a major target tissue for estrogen, and significant levels of ERα are expressed throughout all the major uterine compartments (luminal and glandular epithelium, stroma, and the myometrium), and the expression level of ERα varies during the estrous cycle and peaks during proestrous in accordance with the elevation of circulating 17β-estradiol (E2) to elicit a proliferative response (2). Uterine proliferation is shown from a number of studies to be regulated by E2 in an ERα-dependent manner, as demonstrated by the lack of uterine stimulation and mitotic growth responses in αERKO uteri (3). In immature rodents, E2 stimulates the proliferation of both uterine luminal and glandular epithelium as well as stroma, whereas in adult mice, E2 stimulates the proliferation of only the luminal and glandular epithelium (4). A prior series of studies have proposed that estrogen acting on stromal cells initiates responses to mediate epithelial proliferation. Such evidence was generated from neonatal tissue recombination studies in which E2-activated uterine epithelial DNA synthesis was stromal ERα-dependent via induction of autocrine or paracrine mitogenic factors in the stroma that stimulated the epithelial proliferation (5). Evaluating an epithelial-specific role for ERα in tissue proliferation was recently reported in the mammary gland. Use of a MMTV-Cre crossed with floxed ERα mice demonstrated that mammary gland growth and ductal epithelial cell proliferation required the mammary epithelial ERα (6), which contrasted with previous mesenchymal-epithelial tissue recombination studies that showed mammary epithelial ERα was dispensable for mammary epithelial cell proliferation (7). Therefore, we evaluated whether a similar discrepancy would occur in another estrogen proliferating tissue or whether uterine tissue would follow a similar pattern as the mammary gland regarding an indispensable role of ERα in uterine epithelial proliferation.
Several studies demonstrated that a number of growth factors, such as IGF-1, EGF, or transforming growth factor α (TGFα) (8–10) and their receptors were induced and activated, respectively, in the uterus under the influence of E2 treatment (8–12). IGF-1 was shown experimentally to be a key growth factor that stimulates growth of the nearby epithelial cells as a result of their estrogen-dependent expression in the uterus (13). Lack of IGF-1 stimulation in αERKO mice demonstrated that ERα was required for mediating the uterine IGF-1 action (14–16). The transcriptional factor CCAAT enhancer binding protein beta (C/EBPβ) is another estrogen-regulated gene in female reproductive tissues that is essential for uterine functions (17) and regulation of mammary epithelial cell proliferation and differentiation (18).
In addition to mitogenic effects, estrogen also plays a role in controlling apoptosis (programmed cell death) in several cell types (19). In the uterus, steroid hormones regulate apoptosis. Circulating levels of E2 are inversely correlated with apoptosis in uterine luminal and glandular epithelium during the estrous cycle, with the highest level of apoptosis in the epithelium at metestrous as E2 levels decline (20). Estrogen was shown to suppress apoptosis in the neonatal mouse uterine epithelium via an ERα-dependent induction of apoptosis inhibitory proteins (AIPs) including Birc1a (Baculoviral inhibitors of apoptosis repeat-containing 1) and the inhibition of the activation of the apoptosis executioner, cysteine-aspartic protease-3 (caspase-3) (21). Herein, we evaluated the effect of E2 on adult uterine epithelial apoptosis when the ERα was selectively deleted in the uterine epithelium. The Wnt7a-Cre recombinase and Esr1-loxP expression system was utilized in our study to address the effect of E2 on uterine epithelial responsiveness following selective loss of ERα.
Results
Generation of Uterine Epithelial-Specific ERα Knockout Mice.
A uterine epithelial-specific ERα knockout (UtEpiαERKO) mouse model was generated by crossing a transgenic mouse line expressing Wnt7a-Cre recombinase with our new line of floxed ERα mice containing loxP sites flanking exon 3 of the mouse Esr1 gene (22). The cell type specific expression of Wnt7a-Cre resulted in complete deletion of ERα specifically in uterine luminal and glandular epithelial cells. ERα protein expression was evaluated by immunohistochemistry (IHC) and was robustly expressed throughout WT uteri (epithelium, stroma, and myometrium) (Fig. 1, Left). ERα protein was detected in stromal and myometrial tissues but absent in the uterine luminal and glandular epithelium of UtEpiαERKO mice (Fig. 1, Right), demonstrating that the Cre-specific deletion of ERα was effective. Test matings of UtEpiαERKO females indicated that they are infertile (Table S1). Additional analysis indicates the females exhibit regular estrous cycles, and ovarian histology shows all stages of follicular development and indication of ovulation based on the presence of corpus lutea (Fig. S1). However, implantation sites were observed in 6 of 9 WT but only 0 of 6 in UtEpiαERKO after natural mating or embryo transfer into pseudopregnant females, indicating that UtEpiαERKO mice have a compromised uterine embryo receptivity. Accordingly, the estrogen-regulated transcripts Lif (leukemia inhibitory factor) and Ihh (Indian hedgehog), which are essential for embryo attachment (23, 24), remained unchanged in UtEpiαERKO but were up-regulated in WT after E2 treatment (Fig. S2).
Fig. 1.
ERα protein expression by IHC analysis in WT (Left) and UtEpiαERKO (Right) uteri with longitudinal sections (Upper; 40× magnification) and crossections (Lower; 200× magnification).
Proliferative Effect of E2 on Uterine Epithelium Is Stromal ERα Dependent.
E2 significantly increased the uterine wet weight in ovariectomized (OVEX) WT mice 24 h after treatment (p < 0.001) but not in UtEpiαERKO mice (Fig. 2A). Uterine weight was significantly increased by E2 in UtEpiαERKO after a 3-d bioassay; however, the increase was significantly lower than WT (Fig. 2B, p < 0.05), and the uterine proliferative response induced by E2 was fully attenuated by ICI 182,780 (ICI) in both WT and UtEpiαERKO mice, indicating the increase is an ER-dependent response. Incorporation of the deoxy-thymidine analog EdU (5-ethynyl-2′-deoxyuridine) demonstrated that E2 induced DNA synthesis in luminal epithelium (Fig. 2C) at 24 h in both WT and UtEpiαERKO uteri and decreased in both genotypes after 3 d.
Fig. 2.
Uterine proliferative response to E2 and IGF-1 in WT and UtEpiαERKO mice. The uterine wet weight increase after (A) 24 h or (B) 3-d (three consecutive days) treatment of vehicle, E2 in the presence or absence of ICI treatment, and (C) the fluorescent signal of Hoescht 33342 and EdU incorporation/Alexafluor488 staining in the uterine sections from mice treated with vehicle, E2 (24 h or 3 d), or IGF-1 (24 h). (Scale bar: 20 μm.) Results are mean ± SEM (n = 7). *, and ***p < 0.05 and 0.001, significantly different from vehicle control of the corresponding genotype, respectively. +, p < 0.05 significantly different from E2-treated WT mice.
C/EBPβ and IGF-1: The Postulated Mediators of E2-Induced Proliferation in Uterine Epithelium.
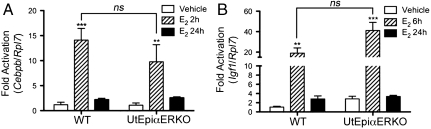
Based on previous studies (5, 8, 9, 17), we evaluated whether Cebpb and Igf1 could be mediators of E2-induced uterine epithelial proliferation. E2 significantly up-regulated Cebpb at 2 h (Fig. 3A) and Igf1 at 6 h (Fig. 3B) in both WT and UtEpiαERKO uteri, and the expression in both genotypes declined to the basal level at 24 h. The expression levels of Cebpb and Igf1 after E2 treatment in WT and UtEpiαERKO were not significantly different. Therefore, the uterine epithelial ERα was not required for the estrogen induction of the uterine Cebpb and Igf1.
Fig. 3.
E2-regulated genes in WT and UtEpiαERKO uteri. (A) Cebpb mRNA at 2 h and 24 h, and (B) Igf1 mRNA at 6 h and 24 h after vehicle or E2 treatment (n = 4). Results are mean ± SEM. *, **, and ***p < 0.05, 0.01, and 0.001, significantly different from vehicle control of the corresponding genotype, respectively. ns; not significantly different in E2 treated for 2 h (A) and 6 h (B) in WT and UtEpiαERKO, analyzed by nonparametric Mann–Whitney test.
We further investigated the effects of growth factor alone on uterine epithelial proliferation. Growth factor, such as IGF-1, was known to be the mitogenic factor that stimulated rodent uterine epithelium proliferation (9). To bypass the effect of E2, we treated the animals with IGF-1 (50 μg/mouse) in the absence of E2. IGF-1 induced uterine luminal epithelial DNA synthesis to a lesser extent than with E2 treatment as illustrated by EdU incorporation at 24 h in both WT and UtEpiαERKO (Fig. 2C). Therefore, IGF-1 appears to be a possible downstream effector of E2 action in the stroma that stimulates uterine epithelial proliferation.
Evaluation of E2-Specific Responses in Uterine Epithelium and Stroma.
Lactoferrin (Ltf) is a protein regulated by E2 in mammalian uterine epithelium (3, 25). After 3 d, E2 significantly induced Ltf mRNA in WT uteri (Fig. 4A, p < 0.001) and also increased Ltf protein in the uterine luminal and glandular epithelium (Fig. 4B, arrowheads), and these increases were inhibited by ICI (Fig. 4A and B). Loss of ERα in UtEpiαERKO uteri resulted in loss of the E2-stimulated lactoferrin expression (Fig. 4A and B). Furthermore, the progesterone receptor (PR) is essential for establishment and maintenance of pregnancy (26) and is also regulated by E2 in the uterus (27). Therefore, we evaluated the PR protein expression, which showed that PR was down-regulated in the epithelium and increased in the stroma following E2 treatment in WT mice. This PR response was not mediated via the uterine epithelial ERα since the response was sustained in UtEpiαERKO (Fig. 4C). Moreover, the inhibitory effect of ICI in both genotypes further indicated that stromal ERα appeared responsible for the E2 down-regulation of PR in the uterine epithelium (Fig. 4C). Other examples of uterine estrogen responsive transcripts were evaluated as well (Fig. S3) and indicate loss of some responses, such as Aqp8 (aquaporin 8) and Cdkn1a (cyclin dependent kinase inhibitor A or p21), and retention of others, such as Dhcr24 (24-dehydrocholesterol reductase) and Ube2c (ubiquitin-conjugating enzyme E2C).
Fig. 4.
Uterine epithelial-specific E2-regulated responses. Ltf expression by using real-time PCR analysis (A), IHC analysis of lactoferrin (B), and progesterone receptor (C) protein expression in WT (Left) and UtEpiαERKO (Right) uteri when treated with vehicle, E2 or E2 + ICI for three consecutive days (n = 7). (Scale bar: 40 μm.) Arrowheads indicate the positive staining of lactoferrin. Results are mean ± SEM. ***p < 0.001, significantly different from vehicle of the corresponding genotype.
Loss of ERα in Uterine Epithelium Enhances Uterine Apoptosis.
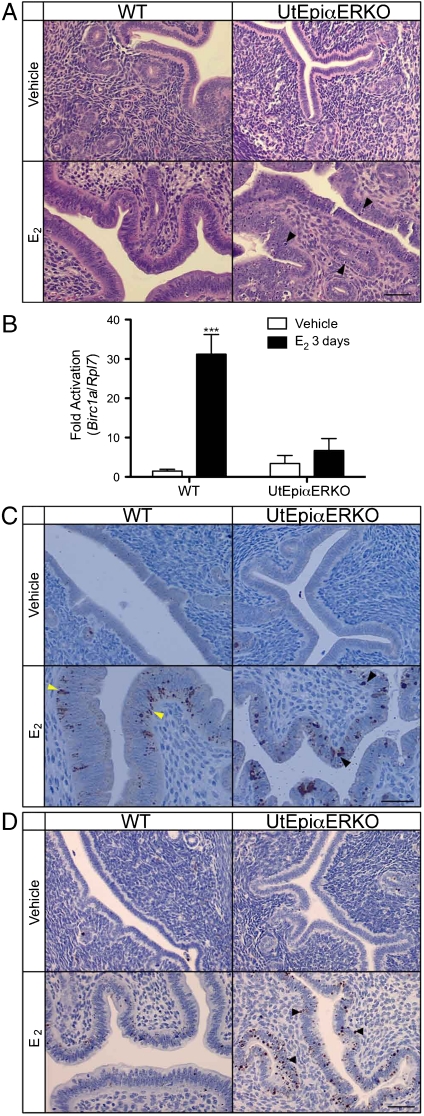
Loss of ERα in uterine epithelium in combination with the growth responses following 3 d of E2 treatment altered the appearance of the epithelia of UtEpiαERKO (Fig. 5A, arrowheads) in a manner suggesting increased apoptosis in UtEpiαERKO compared to WT. Consistent with this, E2 treatment significantly induced the apoptosis inhibitor Birc1a expression in WT (p < 0.001) but not in UtEpiαERKO uteri (Fig. 5B). Cleaved caspase-3 (the active form of caspase-3) was slightly increased by 3 d of treatment with E2 in WT (Fig. 5C, yellow arrowheads) but intensively detected in UtEpiαERKO luminal and glandular epithelium as dense, round spots on both basolateral and luminal sides of the epithelial cells (Fig. 5C, black arrowheads). However, increased levels of the DNA fragmentation marker, TUNEL (Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling), was found only in UtEpiαERKO uteri, which further suggested increased apoptosis following 3 d of E2 treatment (Fig. 5D, arrowheads). Therefore, loss of the uterine epithelial ERα correlated with increased uterine apoptosis subsequent to the uterine growth induced after E2 treatment for 3 d.
Fig. 5.
Apoptosis-related mediator expressions in WT and UtEpiαERKO uteri. After the treatment of vehicle or E2 for three consecutive days, H&E staining (A) indicated apoptotic appearance of epithelial cells in E2-treated UtEpiαERKO uteri (black arrowheads). Birc1a expression by using real-time PCR analysis (B) increased only in E2-treated WT. Cleaved Caspase-3 IHC (C) increased in both WT (yellow arrowheads) and UtEpiαERKO (black arrowheads) uteri, and TUNEL IHC (D) increased only in E2-treated UtEpiαERKO uteri (black arrowheads) (n = 7). (Scale bar: 20 μm.) Results are mean ± SEM. ***p < 0.001, significantly different from vehicle control of the corresponding genotype, respectively.
Discussion
Herein, we illustrated the physiological relevance of ERα to uterine epithelial proliferation and apoptosis under the influence of E2 regulation. We demonstrated that uterine epithelial ERα was dispensable for the E2-induced uterine epithelial proliferation in adult OVEX mice in contrast to what has been reported for the mammary gland epithelial proliferation (6). In the absence of uterine epithelial ERα, potential mitogenic mediators such as Cebpb and Igf1 were increased with E2 treatment, and the growth factor IGF-1 could bypass the effect of E2 on uterine epithelial proliferation. We confirmed the uterine epithelial E2-specific loss of response by showing that deletion of ERα in uterine epithelium caused the loss the lactoferrin production. However, the uterine epithelial down-regulation and stromal redistribution of PR by E2 remained intact in UtEpiαERKO mice. Surprisingly, lack of functional ERα in the uterine epithelium had a pronounced effect on apoptosis.
E2 is known to be a robust mitogenic ovarian hormone in uterine tissues that induces a pronounced hyperplastic tissue response, consistent with endometrial development during the estrous cycle (28, 29). Staged cell labeling studies showed an effect of E2 on recruiting uterine epithelial cells from a resting state (G0) into the cell cycle and shortened both G1 and S-phases of the cell cycle to elicit proliferation (4, 30). Administration of E2 induces the proliferation in both uterine epithelium and stroma of immature mice but only stimulates the proliferation in uterine epithelium in adult mice, and the cause of this lack of stromal cell responsiveness has been linked to progesterone activity (4). Previous work using neonatal tissue xenograph models and extrapolation of such a model to adult tissue (5) indicate that the proliferative effect of E2 on uterine epithelium in adult mice is independent of ERα expression in the luminal epithelium, suggesting the stromal ERα expression is crucial for the mitotic response of E2. To evaluate and extend those observations in a natural adult tissue system in vivo, our UtEpiαERKO mouse model was generated with the specific deletion of ERα in uterine epithelium. Adult UtEpiαERKO mice had a complete deletion of ERα protein in the uterine luminal and glandular epithelium throughout the uterine horn. The classical uterine bioassay was utilized to illustrate the estrogenic effect of E2 on uterine growth responses at both 24 h and 3 d. Absence of ERα in the uterine epithelium compromised the uterine weight increase induced by E2 after 24 h or 3 d, which suggests the epithelial ERα contributes to a full uterine growth response. Our results suggest that the initial (24 h) proliferation response is similar but that the luminal and glandular epithelial cells do not subsequently develop properly into multilayered tissue structures secreting lactoferrin and additionally show indications of apoptosis. These deficiencies and alterations most likely account for the hampered increase in uterine weight. However, the induction of DNA synthesis after 24 h and the decline after 3 d of E2 treatment was seen in both WT and UtEpiαERKO. Therefore, uterine epithelial ERα in vivo was dispensable for appropriate mitotic proliferative response from E2, consistent with earlier xenograph experiments (5), in contrast to the requirement for epithelial ERα in mammary gland development and proliferation (6).
Next, we evaluated the possible mitogenic mediators (C/EBPβ and IGF-1) downstream from E2. Previous studies showed that the transcription factor C/EBPβ is rapidly increased in both epithelial and stromal cells by E2 in the uterus (17). The essential role of C/EBPβ in uterine epithelial proliferation has been demonstrated by the decreased E2-dependent uterine epithelial proliferation as a result of impaired DNA replication in Cebpb knockout mice (17, 31). IGF-1 has been demonstrated to be a paracrine mitogenic effector secreted from uterine stroma to stimulate the proliferation of uterine epithelium (5). E2 increases production of growth factors such as IGF-1 and EGF, and these growth factors induce uterine DNA synthesis (8–10, 16). Igf1 knockout mice exhibited impaired uterine response to estrogen, which implicated the essential role of IGF-1 on uterine growth (32). Overexpression of IGF binding protein-1 (IGFBP-1) in the mouse uterus disrupted DNA synthesis, but the uterine wet weight increased in response to E2 (33). Therefore, the local production and availability of IGF-1 appears to play a role in uterine proliferation. Moreover, evidence also suggests the proliferative effect of E2 on IGF-1 production in the human epithelium was mediated in an autocrine fashion in human uterine epithelial cells (34). Further evidence came from the use of a growth factor inhibitor in combination with E2 administration, which partly inhibited the action of estrogen on the uterine weight increase (13). We previously showed that IGF-1 treatment stimulated the uterine epithelial proliferation in an ERα dependent manner (16). In this study, the complete deletion of ERα specifically in the epithelial target cell of the uterus did not alter DNA synthesis after 24 h of either E2 or IGF-1 treatment. Therefore, in the natural tissue environment, ERα residing in the stroma, not uterine epithelium, was the functional ERα that responded to E2 to produce the growth factor(s), which was released and acted as paracrine mediator(s) to promote DNA synthesis in the epithelium. As mentioned above, these findings in vivo are consistent with previous studies using neonatal tissue recombination experiments (5).
To substantiate the absence of ERα activity in the uterine epithelium, we evaluated gene regulation of Ltf as an ERα-dependent estrogen-regulated response in uterine epithelium (35). Expression of lactoferrin has been correlated with the circulating level of E2 (36). Our result showed that the up-regulation of Ltf mRNA and Ltf protein was a uterine epithelial-specific ERα-dependent action, which was consistent with the previous finding in a tissue recombinant study (37) and supports the absence of ERα activity in the epithelium. We found that E2 redistributed the PR expression from epithelium to stroma in UtEpiαERKO in a similar manner as in WT. These findings again concur with the previous studies using neonatal tissue recombination models (38, 39). Therefore, the estrogen-dependent responsiveness for redistribution of PR from uterine epithelium to stroma upon E2 treatment is intact and appears to be mediated by stromal ERα.
During the female reproductive cycle, estrogen stimulates the proliferative phase to facilitate implantation and pregnancy. In rodents, the proliferate epithelial layer is eliminated by inducing apoptosis in situ (40). The proliferation and subsequent elimination of uterine epithelial cells during the estrous cycle is regulated by the relative mitotic and apoptotic rates under the control of ovarian hormones, especially the circulating level of E2 (40). Withdrawal of E2 treatment from newborn mice results in a high apoptosis index in the uterus (41). AIPs have a protective effect against the activation of the essential apoptosis mediator caspase-3, which is a member of a class of cysteine-aspartyl proteases (42). Caspases are also present in normal healthy cells as inactivated enzymes and are activated by cleavage to initiate apoptosis and induce cell death (43). In our study, the increase in active caspase-3 in WT may result from the normal regulation between growth and apoptosis after E2-induced proliferation of the uterine epithelium. After diethylstilbestrol treatment, uteri of neonatal ERα knockout mice exhibited severe apoptosis, whereas WT mice sustained the apoptotic protection (21), thereby indicating that estrogen protection against uterine epithelial apoptosis is mediated via ERα. In this study, we showed that after the growth induced by E2 treatment, UtEpiαERKO epithelia exhibited severe apoptosis as shown by increases in active caspase-3 and TUNEL staining. Moreover, in the absence of uterine epithelial ERα, E2-mediated increase of the apoptotic inhibitor, Birc1a, is dramatically attenuated. As mentioned earlier, C/EBPβ is a critical mediator of E2-stimulated proliferative response in uterine epithelial cells. C/EBPβ knockout mice undergo uterine epithelial apoptosis as a result of G1-S-phase arrest (31), demonstrating a need for C/EBPβ in DNA replication and damage response. In our study the level of C/EBPβ after E2 treatment in UtEpiαERKO is comparable to that of in WT. However, we have not evaluated the relative levels of C/EBPβ protein in stromal and epithelial cells, and it is possible that there is a deficiency of this important factor intrinsic to the epithelial cells of UtEpiαERKO following uterine growth. Although the uterine epithelial apoptosis in UtEpiαERKO following E2 treatment may not be due to an altered C/EBPβ regulation, it may alternatively result from a defect in other mediators of DNA damage response. Therefore, we postulate that during the adult reproductive cycle following the E2 stimulated growth, the lack of ERα and E2 action in the epithelial cells impairs responses initiated by unknown E2-dependent secondary mediators resulting in apoptosis of the epithelial cells. Thus, epithelial apoptosis occurs due to the absence of epithelial ERα. Interestingly, normal regulation of apoptosis has been shown to be important for implantation (44). Therefore, alteration of growth and apoptosis as a result of the loss of uterine epithelial ERα leads to the infertility phenotype in UtEpiαERKO. More complete fertility studies will be conducted to address additional details regarding the roles of uterine epithelial ERα.
Overall, this model indicates that uterine epithelial proliferation is mediated by ERα-dependent mitotic proliferative signal secreted from the stroma but that ERα is needed following mitosis within epithelial cells to minimize apoptosis. Therefore, ERα is required in both tissue compartments for a complete uterine growth response. It is surprising that epithelial ERα is required for mammary gland proliferation but is dispensable in uterine response. Further studies will be required to determine how this differential cellular role of ER may change in diseases such as endometriosis, endometrial hyperplasia, or cancer.
Materials and Methods
Detailed methods appear in SI Materials and Methods. These include generation of a Wnt7a-Cre transgenic mouse line, generating uterine epithelial-specific ERα knockout mice, chemicals and compounds, uterine bioassay, RNA isolation, and real-time PCR analysis for gene expression, histological and IHC analysis of uteri, and statistical analysis.
Supplementary Material
Acknowledgments.
We thank James Clark, Page Myers, David Goulding from the National Institute of Environmental Health Sciences (NIEHS) Animal Surgery Group of Comparative Medicine branch for performing the animal surgeries and embryo transfer analysis, David Monroy for animal care, Geoffrey Hurlburt and Dave Olsen (NIEHS Immunohistochemistry core) for cleaved caspase-3 and TUNEL staining, Casey Reed for mouse genotyping and Drs. April Binder and Diane Klotz for the critical reading of the manuscript and helpful suggestions. This research was supported by the National Institute of Environmental Health Sciences, Division of Intramural Research (funding to W.W., S.C.H., and K.S.K.) project Z01ES70065 as well as NIH HD30284 and CA098258 (SPORE in Uterine Cancer) to R.R.B. G.D.O. was supported by the National Cancer Institute CA09299 Training Program in the Molecular Genetics of Cancer.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013226107/-/DCSupplemental.
References
- 1.Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Eriksson H, Sahlin L. Estrogen receptors alpha and beta in the female reproductive tract of the rat during the estrous cycle. Biol Reprod. 2000;63:1331–1340. doi: 10.1095/biolreprod63.5.1331. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt SC, et al. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 4.Quarmby VE, Korach KS. The influence of 17 beta-estradiol on patterns of cell division in the uterus. Endocrinology. 1984;114:694–702. doi: 10.1210/endo-114-3-694. [DOI] [PubMed] [Google Scholar]
- 5.Cooke PS, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci USA. 2007;104:14718–14723. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha GR, et al. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J Mammary Gland Biol. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- 8.DiAugustine RP, et al. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology. 1988;122:2355–2363. doi: 10.1210/endo-122-6-2355. [DOI] [PubMed] [Google Scholar]
- 9.Murphy LJ, Ghahary A. Uterine insulin-like growth factor-1: Regulation of expression and its role in estrogen-induced uterine proliferation. Endocr Rev. 1990;11:443–453. doi: 10.1210/edrv-11-3-443. [DOI] [PubMed] [Google Scholar]
- 10.Nelson KG, et al. Transforming growth factor-alpha is a potential mediator of estrogen action in the mouse uterus. Endocrinology. 1992;131:1657–1664. doi: 10.1210/endo.131.4.1396310. [DOI] [PubMed] [Google Scholar]
- 11.Kahlert S, et al. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 12.Hom YK, et al. Uterine and vaginal organ growth requires epidermal growth factor receptor signaling from stroma. Endocrinology. 1998;139:913–921. doi: 10.1210/endo.139.3.5817. [DOI] [PubMed] [Google Scholar]
- 13.Zhu L, Pollard JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci USA. 2007;104:15847–15851. doi: 10.1073/pnas.0705749104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitt SC, Collins J, Grissom S, Deroo B, Korach KS. Global uterine genomics in vivo: Microarray evaluation of the estrogen receptor alpha-growth factor cross-talk mechanism. Mol Endocrinol. 2005;19:657–668. doi: 10.1210/me.2004-0142. [DOI] [PubMed] [Google Scholar]
- 15.Curtis SW, et al. Physiological coupling of growth factor and steroid receptor signaling pathways: Estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA. 1996;93:12626–12630. doi: 10.1073/pnas.93.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klotz DM, et al. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem. 2002;277:8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- 17.Mantena SR, et al. C/EBPbeta is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci USA. 2006;103:1870–1875. doi: 10.1073/pnas.0507261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson GW, Johnson PF, Hennighausen L, Sterneck E. The C/EBPbeta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 1998;12:1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song RX, Santen RJ. Apoptotic action of estrogen. Apoptosis. 2003;8:55–60. doi: 10.1023/a:1021649019025. [DOI] [PubMed] [Google Scholar]
- 20.Wood GA, Fata JE, Watson KL, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 2007;133:1035–1044. doi: 10.1530/REP-06-0302. [DOI] [PubMed] [Google Scholar]
- 21.Yin Y, et al. Estrogen suppresses uterine epithelial apoptosis by inducing birc1 expression. Mol Endocrinol. 2008;22:113–125. doi: 10.1210/me.2007-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 23.Stewart CL, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 24.Lee K, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–1209. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 25.Teng CT. Lactoferrin gene expression and regulation: An overview. Biochem Cell Biol. 2002;80:7–16. doi: 10.1139/o01-215. [DOI] [PubMed] [Google Scholar]
- 26.Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- 27.Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod. 1998;59:1143–1152. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- 28.Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: An autoradiographic study. J Endocrinol. 1973;56:133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 29.Martin L, Pollard JW, Fagg B. Oestriol, oestradiol-17beta and the proliferation and death of uterine cells. J Endocrinol. 1976;69:103–115. doi: 10.1677/joe.0.0690103. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland RL, Reddel RR, Green MD. Effects of oestrogens on cell proliferation and cell cycle kinetics. A hypothesis on the cell cycle effects of antioestrogens. Eur J Cancer Clin Oncol. 1983;19:307–318. doi: 10.1016/0277-5379(83)90127-x. [DOI] [PubMed] [Google Scholar]
- 31.Ramathal C, Bagchi IC, Bagchi MK. Lack of CCAAT enhancer binding protein beta (C/EBPbeta) in uterine epithelial cells impairs estrogen-induced DNA replication, induces DNA damage response pathways, and promotes apoptosis. Mol Cell Biol. 2010;30:1607–1619. doi: 10.1128/MCB.00872-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker J, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- 33.Rajkumar K, Dheen T, Krsek M, Murphy LJ. Impaired estrogen action in the uterus of insulin-like growth factor binding protein-1 transgenic mice. Endocrinology. 1996;137:1258–1264. doi: 10.1210/endo.137.4.8625897. [DOI] [PubMed] [Google Scholar]
- 34.Kashima H, et al. Autocrine stimulation of IGF1 in estrogen-induced growth of endometrial carcinoma cells: involvement of the mitogen-activated protein kinase pathway followed by up-regulation of cyclin D1 and cyclin E. Endocr-Relat Cancer. 2009;16:113–122. doi: 10.1677/ERC-08-0117. [DOI] [PubMed] [Google Scholar]
- 35.Jefferson WN, Padilla-Banks E, Newbold RR. Lactoferrin is an estrogen responsive protein in the uterus of mice and rats. Reprod Toxicol. 2000;14:103–110. doi: 10.1016/s0890-6238(00)00061-7. [DOI] [PubMed] [Google Scholar]
- 36.Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: Correlation with circulating estradiol and progesterone. Endocrinology. 1992;131:1458–1466. doi: 10.1210/endo.131.3.1505477. [DOI] [PubMed] [Google Scholar]
- 37.Buchanan DL, et al. Tissue compartment-specific estrogen receptor-alpha participation in the mouse uterine epithelial secretory response. Endocrinology. 1999;140:484–491. doi: 10.1210/endo.140.1.6448. [DOI] [PubMed] [Google Scholar]
- 38.Kurita T, Lee KJ, Cooke PS, Lydon JP, Cunha GR. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod. 2000;62:831–838. doi: 10.1095/biolreprod62.4.831. [DOI] [PubMed] [Google Scholar]
- 39.Kurita T, et al. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- 40.Sato T, et al. Apoptotic cell death during the estrous cycle in the rat uterus and vagina. Anat Rec. 1997;248:76–83. doi: 10.1002/(SICI)1097-0185(199705)248:1<76::AID-AR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 41.Jo T, Terada N, Saji F, Tanizawa O. Inhibitory effects of estrogen, progesterone, androgen and glucocorticoid on death of neonatal mouse uterine epithelial cells induced to proliferate by estrogen. J Steroid Biochem Mol Biol. 1993;46:25–32. doi: 10.1016/0960-0760(93)90205-b. [DOI] [PubMed] [Google Scholar]
- 42.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RC, Cullen SP, Martin SJ. Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 44.Pampfer S, Donnay I. Apoptosis at the time of embryo implantation in mouse and rat. Cell Death Differ. 1999;6:533–545. doi: 10.1038/sj.cdd.4400516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.