Abstract
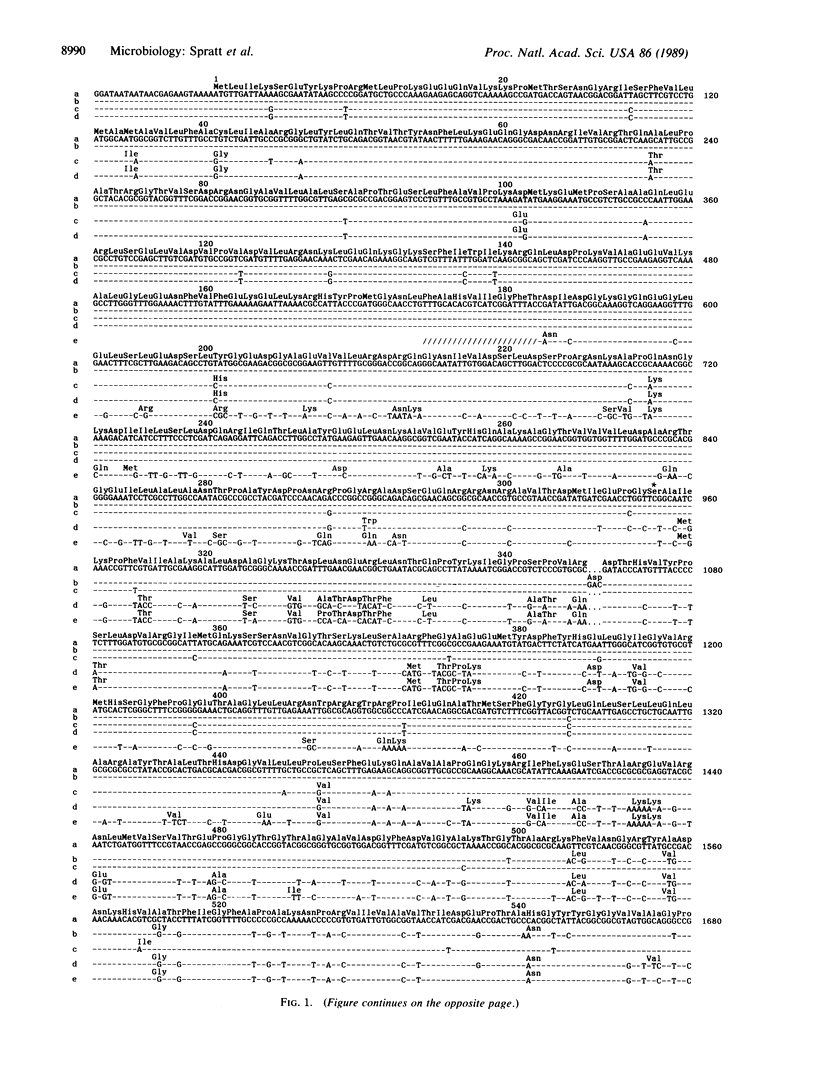
Non-beta-lactamase-producing, penicillin-resistant strains of Neisseria meningitidis produce altered forms of penicillin-binding protein 2 that have decreased affinity for penicillin. The sequence of the penicillin-binding protein 2 gene (penA) from a penicillin-resistant strain of N. meningitidis was compared to the sequence of the same gene from penicillin-sensitive strains and from penicillin-sensitive and penicillin-resistant strains of Neisseria gonorrhoeae. The penA genes from penicillin-sensitive strains of N. gonorrhoeae and N. meningitidis were 98% identical. The gene from the penicillin-resistant strain of N. meningitidis consisted of regions that were almost identical to the corresponding regions in the penicillin-sensitive strains (less than 0.2% divergence) and two regions that were very different from them (approximately 22% divergence). The two blocks of altered sequence have arisen by the replacement of meningococcal sequences with the corresponding regions from the penA gene of Neisseria flavescens and result in an altered form of penicillin-binding protein 2 that contains 44 amino acid substitutions and 1 amino acid insertion compared to penicillin-binding protein 2 of penicillin-sensitive strains of N. meningitidis. A similar introduction of part of the penA gene of N. flavescens, or a very similar commensal Neisseria species, appears to have occurred independently during the development of altered penA genes in non-beta-lactamase-producing penicillin-resistant strains of N. gonorrhoeae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botha P. Penicillin-resistant Neisseria meningitidis in southern Africa. Lancet. 1988 Jan 2;1(8575-6):54–54. doi: 10.1016/s0140-6736(88)91029-x. [DOI] [PubMed] [Google Scholar]
- Dillon J. R., Pauzé M., Yeung K. H. Spread of penicillinase-producing and transfer plasmids from the gonococcus to Neisseria meningitidis. Lancet. 1983 Apr 9;1(8328):779–781. doi: 10.1016/s0140-6736(83)91846-9. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Koller A. E., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Nov;18(5):730–737. doi: 10.1128/aac.18.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Brannigan J. A., George R. C., Hansman D., Liñares J., Tomasz A., Smith J. M., Spratt B. G. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8842–8846. doi: 10.1073/pnas.86.22.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Spratt B. G. Extensive re-modelling of the transpeptidase domain of penicillin-binding protein 2B of a penicillin-resistant South African isolate of Streptococcus pneumoniae. Mol Microbiol. 1989 Jan;3(1):95–102. doi: 10.1111/j.1365-2958.1989.tb00108.x. [DOI] [PubMed] [Google Scholar]
- Dowson C. G., Jephcott A. E., Gough K. R., Spratt B. G. Penicillin-binding protein 2 genes of non-beta-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol Microbiol. 1989 Jan;3(1):35–41. doi: 10.1111/j.1365-2958.1989.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Faruki H., Sparling P. F. Genetics of resistance in a non-beta-lactamase-producing gonococcus with relatively high-level penicillin resistance. Antimicrob Agents Chemother. 1986 Dec;30(6):856–860. doi: 10.1128/aac.30.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanals D., Pineda V., Pons I., Rojo J. C. Penicillin-resistant beta-lactamase producing Neisseria meningitidis in Spain. Eur J Clin Microbiol Infect Dis. 1989 Jan;8(1):90–91. doi: 10.1007/BF01964130. [DOI] [PubMed] [Google Scholar]
- Hedge P. J., Spratt B. G. Resistance to beta-lactam antibiotics by re-modelling the active site of an E. coli penicillin-binding protein. Nature. 1985 Dec 5;318(6045):478–480. doi: 10.1038/318478a0. [DOI] [PubMed] [Google Scholar]
- Jephcott A. E. Epidemiology of resistance in Neisseria gonorrhoeae. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):199–205. doi: 10.1093/jac/18.supplement_c.199. [DOI] [PubMed] [Google Scholar]
- Mendelman P. M., Campos J., Chaffin D. O., Serfass D. A., Smith A. L., Sáez-Nieto J. A. Relative penicillin G resistance in Neisseria meningitidis and reduced affinity of penicillin-binding protein 3. Antimicrob Agents Chemother. 1988 May;32(5):706–709. doi: 10.1128/aac.32.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Caugant D. A., Kalaitzoglou G., Wedege E., Chaffin D. O., Campos J., Saez-Nieto J. A., Viñas M., Selander R. K. Genetic diversity of penicillin G-resistant Neisseria meningitidis from Spain. Infect Immun. 1989 Apr;57(4):1025–1029. doi: 10.1128/iai.57.4.1025-1029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature. 1988 Mar 10;332(6160):173–176. doi: 10.1038/332173a0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe E. M., Jones D. M., el-Sheikh S., Percival A. Penicillin-insensitive meningococci in the UK. Lancet. 1988 Mar 19;1(8586):657–658. doi: 10.1016/s0140-6736(88)91469-9. [DOI] [PubMed] [Google Scholar]
- Sáez-Nieto J. A., Fontanals D., Garcia de Jalon J., Martinez de Artola V., Peña P., Morera M. A., Verdaguer R., Sanfeliu I., Belio-Blasco C., Perez-Saenz J. L. Isolation of Neisseria meningitidis strains with increase of penicillin minimal inhibitory concentrations. Epidemiol Infect. 1987 Oct;99(2):463–469. doi: 10.1017/s0950268800067960. [DOI] [PMC free article] [PubMed] [Google Scholar]



