Abstract
The AMP-activated protein kinase (AMPK) is an αβγ heterotrimer that acts as a master metabolic regulator to maintain cellular energy balance following increased energy demand and increases in the AMP/ATP ratio. This regulation provides dynamic control of energy metabolism, matching energy supply with demand that is essential for the function and survival of organisms. AMPK is inactive unless phosphorylated on Thr172 in the α-catalytic subunit activation loop by upstream kinases (LKB1 or calcium-calmodulin-dependent protein kinase kinase β). How a rise in AMP levels triggers AMPK α-Thr172 phosphorylation and activation is incompletely understood. Here we demonstrate unequivocally that AMP directly stimulates α-Thr172 phosphorylation provided the AMPK β-subunit is myristoylated. Loss of the myristoyl group abolishes AMP activation and reduces the extent of α-Thr172 phosphorylation. Once AMPK is phosphorylated, AMP further activates allosterically but this activation does not require β-subunit myristoylation. AMP and glucose deprivation also promote membrane association of myristoylated AMPK, indicative of a myristoyl-switch mechanism. Our results show that AMP regulates AMPK activation at the initial phosphorylation step, and that β-subunit myristoylation is important for transducing the metabolic stress signal.
Keywords: myristome, signal transduction, adenylate charge, γ-subunit
The AMP-activated protein kinase (AMPK) is a key regulator of cellular and whole-body energy homeostasis that coordinates metabolic pathways in order to balance nutrient supply with energy demand. AMPK protects cells from physiological and pathological stresses (e.g., nutrient starvation, hypoxia/ischemia, and exercise) that lower cellular energy charge (increase AMP/ATP ratio) by directing metabolism toward ATP production and inhibiting anabolic pathways that utilize ATP/NADPH (1, 2). This regulation is achieved by acute phosphorylation of key enzymes in major branches of metabolism including fat synthesis, protein synthesis, and carbohydrate metabolism, as well as phosphorylation of transcription factors to have longer-term regulatory effects. AMPK also functions in regulating whole-body energy homeostasis, food intake, and body weight in response to a variety of hormones including leptin, adiponectin, and ghrelin. These properties have made AMPK a promising drug target to treat the growing incidence of metabolic diseases including obesity, type 2 diabetes, cancer growth and metastasis, and cardiovascular disease (1, 2).
AMPK is an αβγ heterotrimer consisting of an α catalytic subunit and β and γ regulatory subunits, with corresponding homologues in all eukaryotes. Multiple isoforms exist for each subunit in mammals (α1, α2, β1, β2, γ1, γ2, and γ3). The α-subunits consist of an N-terminal kinase catalytic domain followed by an autoinhibitory sequence and a C-terminal β-subunit binding domain (3). The β-subunits contain an internal carbohydrate-binding module and a conserved C-terminal sequence that functions to tether α- and γ-subunits (4, 5). The AMPK γ-subunit binds AMP and ATP (5, 6) via four putative nucleotide-binding sites, but in the crystal structure of mammalian AMPK only three of these (sites 1, 3, and 4) are occupied (7). Sites 1 and 3 bind AMP and ATP exchangeably whereas AMP at site 4 does not exchange (7). These occupied nucleotide-binding sites each contain a conserved Asp residue that forms bidentate hydrogen bonds with the nucleotide ribose hydroxyls (Fig. S1), whereas the unoccupied site 2 in mammalian γ has an Arg in place of this Asp. In all structures solved to date, minimal conformational rearrangement of the γ-subunit occurs upon the exchange of ATP for AMP, providing few clues as to the molecular mechanisms of how AMP regulates AMPK.
The primary event in AMPK activation is phosphorylation of Thr172 in the α-catalytic subunit activation loop by either upstream kinase LKB1 or calcium-calmodulin-dependent protein kinase kinase β (CaMKKβ), leading to > 50-fold increase in AMPK activity (8). Following α-Thr172 phosphorylation, AMP causes a further two- through fivefold allosteric activation and also serves to maintain the active form of the enzyme by inhibiting α-Thr172 dephosphorylation by protein phosphatases PP2c or PP2a (9). Because neither LKB1 nor CaMKKβ are AMP sensitive (10), and phosphorylation of recombinant (Escherichia coli expressed) AMPK is not stimulated by AMP (11–13), it has been proposed that only AMP inhibition of α-pThr172 dephosphorylation controls the accumulation of phosphorylated, active AMPK. Direct AMP promotion of α-Thr172 phosphorylation was reported in early studies employing crude preparations of AMPK and upstream kinase (9), but this regulatory mechanism has now been discounted and attributed to AMP inhibiting contaminating phosphatase PP2c present in the hepatic preparations used (11).
AMPK is modified cotranslationally by N-terminal myristoylation of Gly2 in the β1-subunit, and posttranslationally by extensive phosphorylation on α- and β-subunits (14, 15). In examining the roles of these modifications in regulating the key steps of AMPK activation, we found that myristoylation of the β-subunit is an essential requirement for the initiation of AMPK signaling in response to AMP. Moreover, AMP and glucose deprivation promote myristoyl-group-mediated association with synthetic liposomes and intracellular membranes, respectively, indicative of myristoyl-switch mechanisms found in several other proteins.
Results and Discussion
Myristoylation of the β-Subunit is Essential for AMP Activation of α-Thr172 Phosphorylation.
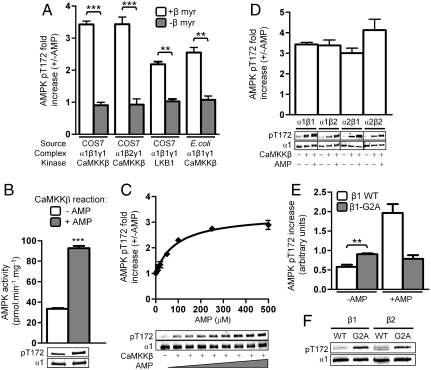
We confirmed previous reports using E.-coli-expressed AMPK (Table S1) that CaMKKβ-mediated α-Thr172 phosphorylation was not stimulated by AMP (11–13). However, we found AMP increases the rates of CaMKKβ- and LKB1-mediated α-Thr172 phosphorylation, approximately 3.4- and 2.3-fold, respectively, compared to basal controls, when AMPK is prepared from transiently transfected COS7 cells (Fig. 1A, open bars, and Fig. S2A). Increased α-Thr172 phosphorylation by CaMKKβ in the presence of AMP was also reflected by a corresponding increase in AMPK activity when measured by a linked peptide substrate assay (Fig. 1B). All protein preparations were devoid of contaminating phosphatase activity because phosphorylated AMPK was not dephosphorylated by upstream kinase preparations in incubations performed in the absence of ATP (Fig. S2B). AMP-activated phosphorylation was dose dependent and sensitive to ionic strength; at 50 mM NaCl, Ka0.5 for AMP was 33 ± 10 μM but at 120 mM NaCl Ka0.5 for AMP was increased to 73 ± 14 μM (Fig. 1C).
Fig. 1.
Myristoylation of the β-subunit regulates AMP-activated phosphorylation of α-Thr172. In all panels, values are presented as mean ± SEM, n = 3–7. Immunoblots shown are single representative experiments or independent transfections, vertical lines indicate separate gels. (A) COS7- or E.-coli-expressed AMPK containing myristoylated (open bars) or nonmyristoylated (shaded bars) β-subunit was phosphorylated by CaMKKβ or LKB1 as indicated, in the presence or absence of AMP. The fold increase in pThr172 compared to basal, non-AMP treated controls is shown. **P < 0.01 and ***P < 0.001 compared to myristoylated AMPK. (B) COS7-expressed AMPK was phosphorylated by CaMKKβ in the presence or absence of 200 μM AMP, and AMPK activity of both was then measured in the presence of 200 μM AMP using the SAMS assay. ***P < 0.0001 compared to basally phosphorylated AMPK. (C) AMP dose response curve for the activation of CaMKKβ-mediated Thr172 phosphorylation at 120 mM NaCl. (D) AMP-activated Thr172 phosphorylation occurs with all α and β isoform combinations. (E) Myristoylation of the β-subunit inhibits basal Thr172 phosphorylation. Results shown detail CaMKKβ-mediated phosphorylation of α1β1γ1 and α1β1(G2A)γ1 from A, displayed as absolute increase in pThr172 compared to a nonphosphorylated AMPK control. **P < 0.01 compared to basally phosphorylated WT control. (F) Basal pThr172 levels of AMPK [α1β1γ1, α1β1(G2A)γ1, α1β2γ1, and α1β2(G2A)γ1] expressed in COS7 cells was measured by immunoblot after normalization for α1 expression.
Because β-subunit myristoylation has only been shown experimentally for β1 (14, 15), we confirmed that skeletal muscle AMPK β2-subunit is also myristoylated (Fig. S3). AMPK was then expressed in COS7 cells with either myristoylated WT (α1β1γ1, α1β2γ1, α2β1γ1, or α2β2γ1) or nonmyristoylated, G2A mutant β-isoforms [α1β1(G2A)γ1 or α1β2(G2A)γ1]. TOF MS confirmed that G2A mutation prevented β1- and β2-myristoylation (Fig. S4 A and B and Table S2). All four WT α/β-subunit combinations showed AMP regulation of α-Thr172 phosphorylation (Fig. 1D) with greatest activation shown by the most abundant complex in skeletal muscle, α2β2γ1 (4.1-fold).
AMP activation of α-Thr172 phosphorylation was lost with AMPK containing nonmyristoylated β-G2A mutant isoforms (Fig. 1A, shaded bars). We also noted Sf21 insect cell-expressed AMPK is ≈60% myristoylated, and these preparations show partial AMP activation of α-Thr172 phosphorylation (16) (Fig. S2C). The importance of myristoylation was corroborated by coexpressing AMPK with N-myristoyl transferase (NMT) in E. coli, which leads to almost stoichiometric β-myristoylation (Fig. S4C) and reconstitutes AMP stimulation of CaMKKβ-mediated α-Thr172 phosphorylation (Fig. 1A). These preparations were also partially phosphorylated on α- and β-subunits and immunoblotting using phosphospecific antibodies confirmed these were the known autophosphorylation sites α-Ser485 and β-Ser108 (Fig. S4D). Mutation of either site (Ala) in COS7 cell-expressed AMPK had no effect on AMP-activated α-Thr172 phosphorylation by CaMKKβ, eliminating the possibility that either α- or β-subunit phosphorylation was a corequirement for the AMP response (Fig. S4E).
In addition to being required for AMP-activated α-Thr172 phosphorylation, β-myristoylation is important for achieving the maximal extent of AMPK activation. Although loss of the myristoyl group causes a modest 1.5-fold increase in basal α-Thr172 phosphorylation by CaMKKβ, this increase represents only 23% of the maximum response attainable in the presence of AMP with WT AMPK (Fig. 1E). Increased basal α-Thr172 phosphorylation of AMPK β-G2A mutants is also seen in COS7 cells (Fig. 1F). These results show that the myristoyl group is required for maintaining AMPK in an inactive state, but equally importantly allows maximum AMPK phosphorylation and activation in response to a metabolic stress signal from AMP.
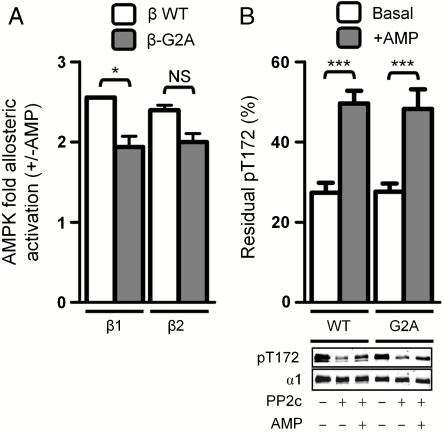
Two other AMP-mediated regulatory functions occur independently of β-myristoylation: AMP allosterically activates peptide substrate phosphorylation to a similar extent with WT or nonmyristoylated AMPK (Fig. 2A) and, similarly, neither basal nor AMP-inhibited rates of PP2c-mediated α-pThr172 dephosphorylation are altered by β2-myristoylation (Fig. 2B). As α-Thr172 phosphorylation represents the key initiating step in AMPK activation by upstream kinases in response to rising AMP levels, our results indicate that AMP-mediated inhibition of dephosphorylation is secondary in achieving net AMPK activation.
Fig. 2.
Effect of AMPK β-subunit myristoylation on AMP-mediated allosteric activation and phosphatase protection. In both panels, values are presented as mean ± SEM, n = 3–7. (A) AMP-mediated allosteric activation of purified AMPK [α1β1γ1 and α1β2γ1, open bars; α1β1(G2A)γ1 and α1β2(G2A)γ1, shaded bars]. *P < 0.05; NS, not significant. (B) AMP-mediated protection against dephosphorylation of purified AMPK [α1β2γ1 and α1β2(G2A)γ1] by PP2c. Graph displays percent residual pThr172 after PP2c treatment compared to a nonphosphatase treated AMPK control. ***P < 0.001 compared to basal dephosphorylation. Immunoblot shown is a single representative experiment.
It is likely that the regulatory role of β-myristoylation involves interaction between the myristoyl group and an intramolecular binding pocket. At present, there are no AMPK crystal structures containing a resolved β-subunit N terminus, however two distinct myristoyl binding sites have been identified in crystal structures of other myristoylated protein kinases, Abl [Protein Data Bank (PDB) ID code 1OPJ] and PKA (PDB ID code 1CMK) (17, 18) that may provide a precedent. In both cases, the myristoyl group binds the large lobe of the kinase catalytic core but in different positions; binding of the β-myristoyl group to either corresponding site in the AMPK α kinase large lobe would place the β N terminus in close proximity to the α-subunit autoregulatory sequence (19) (Fig. S5), potentially positioning it to modulate the autoinhibited conformation.
The γ-Subunit AMP Binding Sites Mediate AMP Regulation of α-Thr172 Phosphorylation.
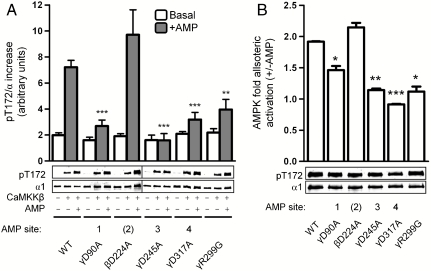
We tested whether specific AMP binding sites on the γ-subunit differentially regulated α-Thr172 phosphorylation. The AMPK γ-subunit contains four potential nucleotide-binding sites, of which three are occupied in crystal structures. Sites 1 and 3 exchange between AMP and ATP, whereas site 4 binds AMP nonexchangeably (Fig. S1). We disrupted each site by individually mutating the conserved Asp residues (Ala) required for hydrogen bonding with the AMP ribose hydroxyls (7). The unoccupied site 2 of the mammalian γ-subunit contains Arg171 in place of an Asp, however, in the crystal structure of Schizosaccharomyces pombe Snf1 γ-subunit ADP can occupy this site, with the nearby β-Asp224 residue contributing to the ribose hydrogen bonding (20). We therefore also generated the corresponding β1-D224A mutant.
Basal rates of CaMKKβ-mediated α-Thr172 phosphorylation were unaffected by all mutations (Fig. 3A, open bars), but individual disruption of sites 1, 3, or 4 significantly reduced AMP-enhanced α-Thr172 phosphorylation. Only mutation of site 3 completely abolished the AMP response. Similarly, allosteric activation of equally phosphorylated AMPK preparations was significantly reduced with mutation of either site 1, 3, or 4 (Fig. 3B). Allosteric activation is lost in the γ1-R299G mutant (6) and this mutation also causes a partial loss of AMP-activated α-Thr172 phosphorylation (Fig. 3A). Our results indicate that neither AMP-enhanced α-Thr172 phosphorylation nor allosteric activation is entirely dependent on any particular nucleotide-binding site, but rather depends on all three. We found that site 2 β1-D224A mutation did not affect AMP-activated α-Thr172 phosphorylation or AMP allosteric regulation (Fig. 3 A and B), arguing that this site has no detectable AMP-mediated regulatory function in mammalian AMPK.
Fig. 3.
Contribution of individual γ1 AMP binding sites to AMPK regulation by AMP. In both panels, values are presented as mean ± SEM, n = 3–7. (A) WT AMPK and indicated β1/γ1 mutants were phosphorylated by CaMKKβ in the presence or absence of AMP. Graph shows the absolute increase in pThr172 compared to nonphosphorylated AMPK controls. **P < 0.01 and ***P < 0.001 compared to AMP-treated WT control. Immunoblot shown is a single representative experiment, vertical line indicates separate gels. (B) AMP-mediated allosteric activation of WT AMPK and indicated β1/γ1 mutants following CaMKKβ activation. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to WT. Immunoblot shows pThr172 level of each sample assayed.
AMP and Nutrient Stress Promote Myristoyl-Group-Mediated Membrane Association of AMPK.
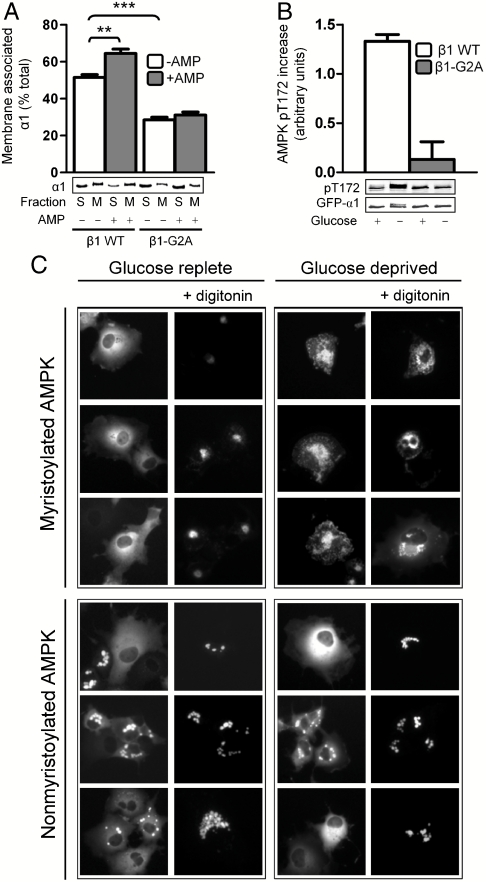
Previously we reported that the myristoyl group facilitates AMPK association with cellular membranes because β1-G2A mutation results in a diffuse cytosolic distribution of AMPK in transfected HEK-293 cells (21). The corresponding mutation in the yeast β-subunit ortholog Sip2 also results in a change in distribution from the plasma membrane to the cytoplasm and nucleus and is associated with reduced lifespan (22). A number of AMPK substrates are themselves membrane associated; acetyl-CoA carboxylase, endothelial NO synthase, and 3-hydroxy-3-methyl-glutaryl-CoA reductase hydroxymethylglutaryl-CoA reductase are associated with mitochondrial, plasma, and endoplasmic reticulum membranes, respectively (2), therefore we tested whether AMP altered membrane association of β1-AMPK. Using liposomes prepared from palmitoyl-oleoyl phosphatidylserine (POPS) and palmitoyl-oleoyl phosphatidylcholine (POPC) mixtures we find that substantial AMPK binding occurred independently of AMP (Fig. 4A and Fig. S6). Nevertheless, depending on the liposomal composition, addition of AMP resulted in up to a 56% increase in membrane partitioning above that conferred by myristoylation alone. Basal membrane association of AMPK decreases significantly with either removal of the myristoyl group or reduction in the liposomal POPS component.
Fig. 4.
Effect of AMP and nutrient stress on myristoyl-regulated AMPK membrane association. In all panels, values are presented as mean ± SEM, n = 3–5. Immunoblots shown are single representative experiments. (A) Incubation of purified, COS7 cell-expressed AMPK [α1β1γ1 or α1β1(G2A)γ1] with liposomes (80∶20 (wt/wt) POPC∶POPS) in the presence or absence of AMP. The α1 content of soluble (S) and membrane (M) fractions were detected by immunoblot. **P < 0.01 and ***P < 0.001 compared to WT AMPK basal membrane association (see also Fig. S6). (B) Activation of AMPK in COS7 cells in response to glucose deprivation. COS7 cells expressing myristoylated or nonmyristoylated AMPK were incubated for 60 min in high or no glucose medium. Cells were harvested and pThr172 in lysates was measured by immunoblot after normalization for GFP-α1. (C) Subcellular localization of AMPK in COS7 cells in response to glucose deprivation. COS7 cells were transfected to express myristoylated (Top) or nonmyristoylated (Bottom) AMPK, and GFP-fusion α1-subunit was visualized by fluorescence microscopy under glucose replete conditions (Left) or after 60 min glucose deprivation (Right). Cells were also treated with digitonin to remove nonmembrane-bound AMPK. Images are representative of individual treatments.
We examined the role of β-myristoylation in nutrient-stress-mediated AMPK activation and localization using COS7 cells transfected to express AMPK containing GFP-tagged α1, γ1, and either WT or G2A mutant β1. After 60 min of glucose deprivation, pThr172 in myristoylated AMPK was significantly elevated above basal levels, whereas pThr172 in nonmyristoylated AMPK was unchanged (Fig. 4B). Using fluorescence microscopy we find that, under glucose-replete conditions, both myristoylated and nonmyristoylated AMPK adopted a diffuse, homogenous distribution throughout the cytosol (Fig. 4C, Left). Plasma membrane permeabilization and removal of the cytosolic compartment by digitonin treatment revealed a small amount of myristoylated AMPK was retained in a particulate cluster proximal to the nucleus. Also in the majority of cells expressing nonmyristoylated AMPK, GFP signal was evident in large, distinct, and mainly perinuclear structures which were retained after digitonin treatment. Under glucose-deprived conditions, myristoylated AMPK adopted a speckled distribution representative of association with intracellular membranes or had migrated to the nucleus (Fig. 4C, Right), with maximal translocation evident 60 min after glucose removal. In contrast, the distribution of nonmyristoylated AMPK was unaltered after the same period of glucose deprivation.
These findings are consistent with an AMP-regulated myristoyl-switching mechanism, similar to that of other proteins where myristoylation controls cytosol to membrane translocation in response to signaling cues. For example, the myristoyl moiety of recoverin (23, 24), a calcium sensor in retinal rod cells, is sequestered in a well-defined hydrophobic pocket within the nonactivated protein (25). Ca2+ binding triggers myristoyl group extrusion, allowing recoverin to interact with disc membranes and act as a rhodopsin kinase inhibitor. Myristoyl-switching is also initiated in HIV-1 Gag and ADP-ribosylation factor 1 by multimerization (26) and GTP binding (27, 28), respectively.
Concluding Remarks
Our results show that AMPK β-myristoylation plays a gatekeeper role in signal initiation by allowing upstream kinases to fully phosphorylate and activate AMPK in response to an AMP metabolic stress signal (Fig. 5). Importantly, we have discovered a myristoylation-dependent regulatory function that is distinct from its classical role in membrane association, and may provide a precedent for reassessing the regulation of other members of the myristome that includes PKA and Abl kinases. The AMP-regulated myristoyl-switch may influence substrate selectivity and both the spatial and temporal properties of metabolic stress signaling via AMPK. Our results provide a clearer picture of how AMPK is maintained in an inactive form in the presence of constitutively active upstream kinase such as LKB1, until released by AMP. For phosphorylation by CaMKKβ, there are three levels of control: Both Ca2+ and calmodulin regulate the upstream kinase, and AMP the susceptibility of the target AMPK to phosphorylation and activation.
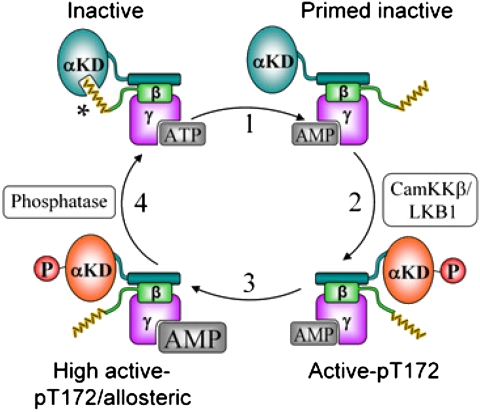
Fig. 5.
Illustration of the elements of AMPK regulation by AMP. When cellular ATP levels are replete myristoyl-group (yellow) sequestration suppresses Thr172 phosphorylation and maintains the inactive state (Upper Left). Increased AMP/ATP ratio triggers a myristoyl-switch, promoting AMPK membrane association when required (step 1) and Thr172 phosphorylation (step 2). AMPK can be allosterically activated and protected from pThr172 dephosphorylation by AMP (step 3). Restoration of intracellular ATP levels reverses the myristoyl-switch (step 4). αKD, α-subunit kinase domain; * putative myristoyl binding site on α.
Materials and Methods
Detailed descriptions of procedures and plasmids are provided in the SI Text.
AMPK Assays.
COS7 cell-expressed AMPK was used in all assays unless otherwise indicated. AMPK was purified from COS7 or Sf21 insect cells as described previously (16, 21). CaMKKβ and PP2c were purified from Sf21 insect cells, LKB1/MO25α/STRADα complex was purified from COS7 cells, and AMPK (alone or coexpressed with NMT, ref. 29) was purified from E. coli. AMPK bound to glutathione-agarose was dephosphorylated or phosphorylated on α-Thr172 by incubation with PP2c or CaMKKβ, respectively, prior to extensive washing and elution. For phosphorylation assays, dephosphorylated AMPK was incubated with CaMKKβ and MgATP for 10 min at 32 °C. For dephosphorylation assays, phosphorylated AMPK was incubated with PP2c for 10 min at 32 °C. In both cases, reactions were terminated by addition of SDS PAGE sample buffer, boiled, and immunoblotted simultaneously for α-pThr172 and total α. AMPK activity was determined by phosphorylation of the SAMS peptide substrate.
Liposome Partitioning Assays.
Small unilamellar vesicles were prepared from mixtures of POPS and POPC (Avanti Polar Lipids) and incubated with purified AMPK for 2 min at 25 °C. Following vesicle sedimentation, α1 content of soluble and pelleted membrane fractions was detected by immunoblotting.
Supplementary Material
Acknowledgments.
We thank F. Katsis for antibody preparation, J. Gordon (Washington University, St. Louis) for N-myristoyl transferase, N. Birnberg (Mercury Therapeutics, Inc.) for LKB1, and A. Means (Duke University Medical Center, NC) for partial CaMKKβ constructs. This work was supported by grants from the Australian Research Council and the National Health and Medical Research Council (NHMRC). B.E.K. is an NHMRC Fellow.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009705107/-/DCSupplemental.
References
- 1.Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 3.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 4.Iseli TJ, et al. AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186–270) J Biol Chem. 2005;280:13395–13400. doi: 10.1074/jbc.M412993200. [DOI] [PubMed] [Google Scholar]
- 5.Townley R, Shapiro L. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science. 2007;315:1726–1729. doi: 10.1126/science.1137503. [DOI] [PubMed] [Google Scholar]
- 6.Scott JW, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao B, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 8.Hawley SA, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 9.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 10.Fogarty S, Hardie DG. C-terminal phosphorylation of LKB1 is not required for regulation of AMP-activated protein kinase, BRSK1, BRSK2, or cell cycle arrest. J Biol Chem. 2009;284:77–84. doi: 10.1074/jbc.M806152200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods A, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Suter M, et al. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- 14.Mitchelhill KI, et al. Posttranslational modifications of the 5′-AMP-activated protein kinase beta1 subunit. J Biol Chem. 1997;272:24475–24479. doi: 10.1074/jbc.272.39.24475. [DOI] [PubMed] [Google Scholar]
- 15.Woods A, et al. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem. 2003;278:28434–28442. doi: 10.1074/jbc.M303946200. [DOI] [PubMed] [Google Scholar]
- 16.Iseli TJ, et al. AMP-activated protein kinase subunit interactions: beta1∶gamma1 association requires beta1 Thr-263 and Tyr-267. J Biol Chem. 2008;283:4799–4807. doi: 10.1074/jbc.M708298200. [DOI] [PubMed] [Google Scholar]
- 17.Nagar B, et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003;112:859–871. doi: 10.1016/s0092-8674(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J, et al. Crystal structures of the myristoylated catalytic subunit of cAMP-dependent protein kinase reveal open and closed conformations. Protein Sci. 1993;2:1559–1573. doi: 10.1002/pro.5560021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, et al. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature. 2009;459:1146–1149. doi: 10.1038/nature08075. [DOI] [PubMed] [Google Scholar]
- 20.Jin X, Townley R, Shapiro L. Structural insight into AMPK regulation: ADP comes into play. Structure. 2007;15:1285–1295. doi: 10.1016/j.str.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Warden SM, et al. Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem J. 2001;354:275–283. doi: 10.1042/0264-6021:3540275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SS, Manchester JK, Gordon JI. Sip2, an N-myristoylated beta subunit of Snf1 kinase, regulates aging in Saccharomyces cerevisiae by affecting cellular histone kinase activity, recombination at rDNA loci, and silencing. J Biol Chem. 2003;278:13390–13397. doi: 10.1074/jbc.M212818200. [DOI] [PubMed] [Google Scholar]
- 23.Zozulya S, Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci USA. 1992;89:11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dizhoor AM, et al. Role of the acylated amino terminus of recoverin in Ca2+-dependent membrane interaction. Science. 1993;259:829–832. doi: 10.1126/science.8430337. [DOI] [PubMed] [Google Scholar]
- 25.Ames JB, et al. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- 26.Tang C, et al. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc Natl Acad Sci USA. 2004;101:517–522. doi: 10.1073/pnas.0305665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helms JB, Palmer DJ, Rothman JE. Two distinct populations of ARF bound to Golgi membranes. J Cell Biol. 1993;121:751–760. doi: 10.1083/jcb.121.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanigawa G, et al. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1371. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duronio RJ, et al. Protein N-myristoylation in Escherichia coli: Reconstitution of a eukaryotic protein modification in bacteria. Proc Natl Acad Sci USA. 1990;87:1506–1510. doi: 10.1073/pnas.87.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.