Abstract
Thymic stromal lymphopoietin (TSLP) is a type I cytokine that plays essential roles in allergic/inflammatory skin and airway disorders, in helminth infections, and in regulating intestinal immunity. TSLP signals via IL-7Rα and a specific TSLPR subunit that is highly related to the common cytokine receptor γ chain, γc. Although TSLP has effects on a broad range of hematopoetic cells and can induce STAT5 phosphorylation, TSLP was reported to not signal via JAK kinases, and the mechanism by which TSLP regulates STAT5 phosphorylation has been unclear. We now demonstrate the role of JAK1 and JAK2 in TSLP-mediated STAT5 phosphorylation in mouse and human primary CD4+ T cells, in contrast to the known activation of JAK1 and JAK3 by the related cytokine, IL-7. We also show that just as JAK1 interacts with IL-7Rα, JAK2 is associated with TSLPR protein. Moreover, we demonstrate the importance of STAT5 activation for TSLP-mediated survival and proliferation of CD4+ T cells. These findings clarify the basis for TSLP-mediated signaling and provide an example wherein a cytokine uses JAK1 and JAK2 to mediate the activation of STAT5.
Thymic stromal lymphopoietin (TSLP) is a cytokine produced by stromal cells, epithelial cells, fibroblasts, keratinocytes, and basophils (1–3). Increased TSLP levels are associated with airway inflammatory disease and atopic dermatitis in humans and mice (1, 3–5). In addition, TSLP regulates intestinal immunity and inflammation (6) and is important in helminth infections (6–8). TSLP is closely related to IL-7, another stromal factor. IL-7 signals via IL-7Rα and the common cytokine receptor γ chain, γc (9, 10), a protein that is also a critical component of the receptors for IL-2, -4, -9, -15, and -21 (11) and is mutated in humans with X-linked severe combined immunodeficiency (12). In contrast, TSLP signals via IL-7Rα and a specific subunit, TSLPR, that is highly related to γc (13, 14). IL-7 is known to critically control the development, expansion, and survival of naive and memory T cells, thereby regulating the number of mature T cells and maintaining lymphoid homeostasis (11). TSLP can directly act on both mouse and human CD4+ and CD8+ T cells (15–18) and contributes to T-cell lymphopoiesis and homeostasis (15, 18, 19). However, whereas IL-7 induces proliferation and survival of mouse naive T cells, TSLP preferentially promotes survival, with less of an effect on the proliferation of these cells (15, 18). Consistent with this, bone marrow-derived IxN/2B cells, which express TSLPR and IL-7Rα and respond to both TSLP and IL-7 to induce STAT5 phosphorylation, potently proliferate in response to IL-7 but not TSLP (20).
Although both IL-7 and TSLP are essential in the mouse for normal B-cell lymphopoesis in vivo (15, 19), IL-7 plays a greater role, as evidenced by the profound B-cell lymphopenia in the absence of IL-7 (21). IL-7 preferentially promotes the generation of B220+/IgM− pre-B cells from fetal liver lymphocyte precursors, whereas TSLP mediates production of B220+/IgM+ immature B cells (22, 23). Interestingly, neither cytokine is required for human B cell development, as revealed by the normal B-cell numbers in humans with XSCID (IL2RG mutations) (12), JAK3-deficient SCID (24, 25), and IL7R-deficient SCID (26).
TSLP, as well as IL-7, activates STAT5 proteins (27, 28). However, the upstream activators of STAT5 in TSLP-induced signaling have not been determined. Although it is well known that JAK kinases phosphorylate and thereby activate STAT proteins (29), it was unexpectedly reported that TSLP did not activate any of the JAK kinases in the NAG8/7 pre-B cell line (22) and that overexpression of dominant-negative forms of JAK1 and JAK2 did not affect TSLP-mediated STAT5 activation (28). Nevertheless, mutational analysis of TSLPR and IL-7Rα demonstrated that the presence in both chains of conserved residues typical of Box1 motifs, which can mediate association of type I cytokine receptor chains with JAK kinases (30), was essential for TSLP-induced STAT5 activation (20). We now demonstrate that TSLP activates JAK1 and JAK2 but not JAK3 or TYK2 in primary T cells. Correspondingly, the absence of either JAK1 or JAK2, but not of JAK3 or TYK2, prevents TSLP-mediated STAT5 activation. We clarify the molecular basis for this activation by demonstrating that TSLPR associates with JAK2, just as IL-7Rα is known to associate with JAK1 (10, 31). These findings indicate a key molecular mechanism for signaling by TSLP, resolving a long-standing dilemma on the mode of signaling by this cytokine.
Results
Role of STAT5 Activation in TSLP-Induced Signaling.
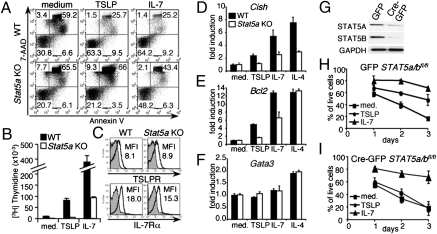
It is known that TSLP induces the activation of STAT5A and STAT5B proteins, but the importance of this signaling pathway on T-cell function is unclear. Previously, it was shown that TSLP increases proliferation and survival of T cells in vitro (15). We thus evaluated if TSLP-mediated STAT5 activation is involved in these processes. Because Stat5a/Stat5b double KO mice exhibit perinatal lethality (32), we used T cells from Stat5a single KO mice. These cells can respond to TSLP and IL-7 because the presence of STAT5B and perhaps other signaling pathways, but freshly isolated (Fig. 1A) or preactivated (Fig. S1) Stat5a KO CD4+ T cells exhibited markedly lower TSLP-mediated survival than WT cells, as well as markedly diminished TSLP-induced proliferation (Fig. 1B). The attenuated effects of TSLP and IL-7 in Stat5a KO cells were not a result of lower receptor expression, as TSLPR and IL-7Rα expression on WT and Stat5a KO CD4+ T cells were similar (Fig. 1C).
Fig. 1.
The role of STAT5 in TSLP mediated signaling. (A) Freshly isolated mouse WT or Stat5a KO CD4+ T cells were not stimulated or stimulated with 100 ng/mL TSLP or 10 ng/mL IL-7 for 5 d. The apoptotic rate of these cells was determined by flow cytometry. The experiment was performed three times. (B) WT or Stat5a KO mouse CD4+ T cells were preactivated with anti-CD3+anti-CD28, rested, and then not stimulated or stimulated with TSLP or IL-7 for 48 h, and pulsed with thymidine for the last 10 h of culture. The experiment was performed five times. (C) Cell surface expression of TSLPR and IL-7Rα on mouse WT and Stat5a KO CD4+ T cells was measured by flow cytometry. (D–F) Freshly isolated mouse WT or Stat5a KO CD4+ T cells were cultured in medium with or without TSLP, IL-7, or IL-4 for 4 h. The mRNA expression for indicated markers was normalized to Rpl7 mRNA levels and fold-induction calculated relative to untreated samples. Shown is a representative experiment from four independent experiments. (G) Purified Stat5a/bfl/fl CD4+ T cells were preactivated and infected with retroviruses expressing GFP or Cre-GFP. GFP+ cells were then sorted by FACS, cultured 24 h in medium, and evaluated for expression of STAT5A and STAT5B by Western blotting. (H–I) Stat5a/bfl/fl CD4+ T cells were infected and sorted as mentioned above. GFP+ cells were not stimulated or stimulated with TSLP or IL-7 for the indicated time points. The experiment in H and I was performed twice, with two to three mice per each.
We next assessed whether the absence of STAT5A affects expression of TSLP- and IL-7-induced genes. TSLP and IL-7 both can induce expression of the Cish and Bcl2 genes (18, 28), each of which is regulated by STAT5 (33, 34). The induction of Cish by TSLP, IL-7, and IL-4 was lower in Stat5a KO than in WT CD4+ T cells (Fig. 1D), whereas Bcl2 expression was lower in Stat5a KO cells in response to TSLP and IL-7 stimulation but not IL-4 (Fig. 1E). The diminished Bcl2 mRNA induction in Stat5a KO CD4+ T cells after TSLP and IL-7 stimulation might explain the higher apoptotic rate of these cells (Fig. 1A). As expected, induction of the Gata3 gene, which is STAT6-dependent, was observed only after IL-4 stimulation (Fig. 1F).
To extend these results from Stat5a KO cells, we used Cre-mediated deletion of Stat5a and Stat5b genes in mature preactivated CD4+ T cells from Stat5a/Stat5bfl/fl mice (32), which greatly lowered STAT5A and STAT5B expression (Fig. 1G). This process resulted in markedly lower TSLP-mediated survival than in cells transduced with a control GFP (Fig. 1 I vs. H). Interestingly, although IL-7 is a stronger survival factor than TSLP for preactivated CD4+ T cells (Fig. 1H and Fig. S1), Stat5a/b deletion (Fig. 1I) or Stat5a- deficiency (Fig. S1) did not significantly change IL-7–mediated survival of these cells, which might be explained by the role of other signaling pathways, including PI 3-kinase, which are involved in IL-7–induced survival (35). Taken together, these data show the importance of STAT5 in TSLP's actions on CD4+ T cells.
JAK1 Is Essential for TSLP-Induced STAT5 Phosphorylation.
To investigate the basis for signaling by TSLP and IL-7, primary mouse CD4+ T cells were stimulated with TSLP or IL-7 and lysates Western blotted with antibodies to phospho-STAT5 or STAT5 (Fig. 2A, Upper). As expected, both cytokines induced phosphorylation of STAT5, with IL-7 being more potent (Fig. 2A). We next immunoprecipitated lysates with anti-JAK1 (Fig. 2 A, Lower, and B) or anti-phosphotyrosine (4G10) (Fig. 2C) followed by immunoblotting for phospho-JAK1 (Fig. 2A), phosphotyrosine (Fig. 2B), or JAK1 (Fig. 2C, Upper). TSLP induced phosphorylation of JAK1, albeit less potently than did IL-7 (Fig. 2 A–C), correlating with less phosphorylation of STAT5 induced by TSLP (Fig. 2A, Upper).
Fig. 2.
JAK1 is essential for TSLP-induced signaling. (A) Purified CD4+ T cells were not stimulated or stimulated for 5 or 15 min with TSLP (100 ng/mL) or IL-7 (10 ng/mL). Cell lysates were run on NuPAGE gels and Western blotted with anti-phospho-STAT5 or anti-STAT5 or immunoprecipitated with anti-JAK1 and analyzed by Western blotting with antibodies to phospho-JAK1 or JAK1. This experiment was performed more than five times. (B) CD4+ T cells were not treated or treated with TSLP, IL-7, or IL-2, lysed, immunoprecipitated with anti-JAK1, and analyzed by Western blotting with antiphosphotyrosine or anti-JAK1. This experiment was performed twice. (C) Lysates of CD4+ T cells treated as in Fig. 1A were immunoprecipitated with antiphosphotyrosine mAb 4G10 and Western blotted with antibodies to JAK1 or STAT5. This experiment was performed three times. (D) Levels of phospho-STAT5, STAT5, and JAK1 in WT or Jak1 KO MEFs or Jak1 KO MEFs reconstituted with JAK1 that had been transfected with cDNAs for TSLPR, IL-7Rα, and STAT5A and then not stimulated or stimulated with TSLP (100 ng/mL) for 20 min, followed by Western blotting. This experiment was performed five times.
To confirm the importance of JAK1 in TSLP-induced STAT5 phosphorylation, we transfected WT or Jak1 KO mouse embryonic fibroblasts (MEFs) (36) with expression vectors encoding TSLPR, IL-7Rα, and STAT5A. STAT5 phosphorylation was detected in the WT cells stimulated with TSLP (Fig. 2D, lane 2) but not in unstimulated cells (lane 1) or in the Jak1 KO MEFs (lanes 3 and 4). However, when JAK1-reconstituted Jak1 KO MEFs were used, TSLP induced the phosphorylation of STAT5 (Fig. 2D, lane 6 vs. 5). Thus, TSLP can rapidly activate JAK1 and that JAK1 is essential for phosphorylation of STAT5.
JAK3 and TYK2 Are Not Required for TSLP-Induced STAT5 Phosphorylation.
Most cytokines whose receptors are heterodimers activate more than one JAK kinase (29). Accordingly, we examined whether other JAKs are also involved in TSLP signaling. JAK3 associates with γc and is phosphorylated upon stimulation by γc cytokines (37–39). Because TSLPR shows 26% identity and 47% similarity at the protein level with murine γc (13), we analyzed whether JAK3 also was required for TSLP-mediated STAT5 phosphorylation. WT or Jak3 KO CD4+ T cells were either not stimulated or stimulated with TSLP or IL-7, and tyrosine phosphorylation of STAT5 was evaluated (Fig. 3A). TSLP signaling was unaffected in Jak3 KO CD4+ T cells (Fig. 3A, lane 2), whereas IL-7–induced STAT5 phosphorylation was abrogated (Fig. 3A, lane 3). Both cytokines induced STAT5 phosphorylation in WT cells (Fig. 3A, lanes 5 and 6). Similarly, TSLP and GM-CSF but not IL-7 could induce STAT5 phosphorylation in Jak3 KO dendritic cells (DCs) (Fig. 3B, lanes 2–4 vs. 6–8). Moreover, we found that TSLP did not induce the TYK2 phosphorylation in CD4+ T cells (Fig. 3C, lane 2); as expected, IFN-β induced tyrosine phosphorylation of TYK2, whereas IL-2 did not (Fig. 3C, lanes 3 and 4). Correspondingly, TSLP-induced STAT5 phosphorylation was not diminished in Tyk2 KO CD4+ T cells (Fig. 3D, lane 2 vs. 5). Thus, neither JAK3 nor TYK2 is required for TSLP signaling.
Fig. 3.
TSLP-mediated STAT5 phosphorylation does not require JAK3 or TYK2. (A) CD4+ T cells from WT or Jak3-deficient mice were not stimulated or stimulated for 15 min with TSLP or IL-7, lysed, and Western blotted with antibodies to phospho-STAT5 or total STAT5. This experiment was done four times. (B) Bone marrow-derived DCs from WT or Jak3 KO mice were not stimulated or stimulated for 20 min with TSLP (100 ng/mL), IL-7 (20 ng/mL), or GM-CSF (10 ng/mL), lysed, and Western blotted, as in A. This experiment was performed twice. (C) Cell lysates from CD4+ T cells not stimulated or stimulated for 15 min with TSLP (100 ng/mL), IFN-β (1,000 U/mL), or IL-2 (100 U/mL) were immunoprecipitated with TYK2 Ab and Western blotted with antiphospho-TYK2 or anti-TYK2. This experiment was performed four times. (D) CD4+ T cells from Tyk2 KO and littermate control mice were not treated or treated for 15 min with TSLP or IL-7 and Western blotted with antibodies to phospho-STAT5, STAT5, and TYK2. This experiment was performed twice.
JAK2 Activation Is Required for TSLP-Induced STAT5 Phosphorylation.
The TSLPR cytoplasmic domain, which contains a Box1 region typical of type I cytokine receptors, can activate JAK2 in the context of an artificial chimeric receptor system (14, 40), and FLAG-tagged human TSLPR was suggested to associate with JAK2 in Ba/F3 transfected cells (41); however, TSLP was reported to not induce JAK2 activation in cell lines (22, 28). To resolve these ostensibly conflicting findings as to whether TSLP signals via JAK2, we stimulated primary mouse CD4+ T cells with TSLP, IFN-γ (a known activator of JAK2), and IL-7 or IL-2 (which do not activate JAK2) (Fig. 4 A and B). TSLP, IL-7, and IL-2, but not IFN-γ, induced STAT5 phosphorylation (Fig. 4A, third blot). TSLP stimulation also increased STAT1 phosphorylation, but more weakly than other cytokines tested (Fig. 4A, Upper). We found TSLP could induce JAK2 phosphorylation within 5 min in CD4+ T cells (Fig. 4B, lanes 2–4 vs. 1); as expected, IFN-γ also induced JAK2 phosphorylation (Fig. 4B, lanes 5 and 6), whereas IL-7 and IL-2 did not (Fig. 4B, lanes 7 and 8, respectively). Moreover, treating CD4+ T cells with JAK2 specific inhibitor II, which suppresses activation of JAK2 but not of other kinases tested, including JAK1 (42), blocked TSLP–induced STAT5 phosphorylation (Fig. 4C, lane 2 vs. 6) and IFN-γ–induced STAT1 phosphorylation (Fig. 4C, lane 4 vs. 8) but did not affect STAT5 or STAT1 phosphorylation induced by IL-7 (Fig. 4C, lane 3 vs. 7). Furthermore, Cre-mediated in vitro deletion of Jak2 from Jak2fl/fl CD4+ T cells prevented activation of STAT5 and STAT1 in response to TSLP and IFN-γ, relatively (Fig. 4D, lanes 6 and 8), but did not prevent activation of STAT5 in response to IL-7 (Fig. 4D, lane 7).
Fig. 4.
JAK2 is essential for TSLP-mediated STAT5 activation. (A) CD4+ T cells were not treated or treated as indicated with TSLP (100 ng/mL), IFNγ (100 ng/mL), IL-7 (10 ng/mL), or IL-2 (100 U/mL). Cell lysates were Western blotted with antiphospho-STAT1, anti-STAT1, anti-phospho-STAT5, or anti-STAT5. This experiment was performed more than five times. (B) Cell lysates from A were immunoprecipitated with anti-JAK2 and Western blotted with antiphospho-JAK2 or anti-JAK2. This experiment was performed more than five times. (C) CD4+ T cells were treated with DMSO or JAK2 inhibitor II for 15 h (see Materials and Methods), then not stimulated or stimulated with TSLP, IL-7, or IFN-γ for 15 min, lysed, and Western blotted with antibodies to phospho-STAT5, total STAT5, phospho-STAT1, and total STAT1. This experiment was performed five times. (D) Purified Jak2fl/fl CD4+ T cells were preactivated and infected with retroviruses expressing GFP or Cre-GFP. After 1 d of rest, GFP+ cells were sorted by FACS and rested for 3 h in medium alone. The cells were then not stimulated or stimulated with TSLP, IL-7, or IFN-γ for 15 min. The phosphorylation of STAT5 and STAT1 and total expression of these proteins and JAK2 were evaluated by Western blotting. The experiment was repeated three times. (E) A WT MEF cell line was cotransfected with cDNAs encoding TSLPR, IL-7Rα, IL-2Rγ, and STAT5, as well as with control (lanes 1–3) or JAK2 (lanes 4–6) siRNA. Cells were not stimulated or stimulated with TSLP or IL-7 for 20 min, lysed, and Western blotted with Abs to phospho-STAT5, total STAT5 and JAK2. This experiment was performed four times. (F) Primary WT or Jak2 KO MEFs were transfected with cDNAs encoding TSLPR, IL-7Rα, IL-2Rγ, and STAT5A, and either not treated or treated with TSLP or IL-7 for 20 min, lysed, and Western blotted as in (E). The experiment was repeated three times.
We additionally performed experiments with WT MEFs that were cotransfected with TSLPR, IL-7Rα, IL-2Rγ, and STAT5A plasmids, as well as with a control siRNA or a pool of JAK2-specific siRNAs (Fig. 4E). The JAK2 siRNAs reduced STAT5 phosphorylation in response to TSLP (Fig. 4E, lane 5 vs. 2) but not to IL-7 (Fig. 4E, lane 6 vs. 3). We also cotransfected Jak2 KO primary MEFs with appropriate DNA plasmids, as mentioned above, and found that TSLP no longer could activate STAT5 (Fig. 4F). Thus, JAK2 and JAK1 are the critical kinases in TSLP-induced STAT5 phosphorylation.
TSLP Induces Phosphorylation of JAK1, JAK2, and STAT5 in Human T Cells.
Our laboratory previously showed that TSLP acts directly on mouse CD4+ T cells (15) and, more recently, that TSLP, in addition to its known action on human DCs (1), also directly acts on human CD4+ (17) and CD8+ T cells (18), inducing activation of STAT5 and increasing cellular responsiveness to other stimuli, such as TCR and IL-2 (17). Consistent with these reports, we did not observe phosphorylation of STAT3, STAT4, or STAT6 in response to TSLP, and only very weak phosphorylation of STAT1 (Fig. 5A, lane 2 vs. 1) in human CD4+ T cells. Because of suggested differences in TSLP action on human and mouse T cells (1, 43), we sought to clarify whether TSLP uses the same signaling mechanism in both species. As shown in Fig. 5B, treatment of human CD4+ T cells with TSLP, IL-7, or IL-4 for 15 min induced phosphorylation of JAK1, whereas IL-12 did not (Fig. 5B, lanes 2, 3, and 5 vs. lane 4) and TSLP and IL-12 promoted phosphorylation of JAK2, whereas IL-7 and IL-4 did not (Fig. 5B, lanes 2 and 4 vs. 3 and 5). As expected, TSLP did not induce the phosphorylation of either TYK2 or JAK3 (Fig. 5B), consistent with the lack of TSLP signaling in Jak3 (Fig. 3A) and Tyk2 (Fig. 3D) KO T cells.
Fig. 5.
TSLP activates JAK1 and JAK2 in human CD4+ T cells. (A) Human CD4+ T cells were purified from peripheral blood mononuclear cells (PBMCs), preactivated for 4 d with anti-CD3 + anti-CD28, and rested for 1 d in medium containing 10% FBS. Cells then were left unstimulated or were stimulated with TSLP (50 ng/mL), IL-7 (10 ng/mL), IL-12 (10 ng/mL), or IL-4 (10 ng/mL) for 15 min, and cell lysates Western blotted, as indicated. This experiment was performed more than five times. (B) Lysates from human CD4+ T cells treated as in A were immunoprecipitated with antibodies to JAK1, JAK2, JAK3, or TYK2, and phosphorylation and total expression of each JAK evaluated by Western blotting. This experiment was performed three times.
We next sought to verify the importance of JAK1 and JAK2 in TSLP-induced STAT5 phosphorylation in human cells. We transfected preactivated CD4+ T cells with JAK1 (Fig. 6A) or JAK2 (Fig. 6B), or control (Fig. 6 A and B) siRNAs. JAK1 down-regulation (Fig. 6A, fifth blot) resulted in an ≈80% decrease in STAT5 phosphorylation by TSLP and IL-7 (Fig. 6 A, Top blot, lanes 2 and 3, and C) but did not alter STAT4 phosphorylation mediated by IL-12 (Fig. 6 A and C), which activates JAK2 and TYK2 but not JAK1. JAK2 down-regulation (Fig. 6B) resulted in a 70% decrease in TSLP-induced STAT5 phosphorylation (Fig. 6B, lane 2 vs. 6) and a 50% decrease in IL-12-induced STAT4 phosphorylation (Fig. 6B, lane 4 vs. 8 and C), but had only a weak effect on IL-7–induced STAT5 phosphorylation. Given that IL-7 uses JAK1 and JAK3, it is possible that this weak effect was the result of a more general deleterious effect of diminished JAK2, which mediates signals by multiple cytokines (29) and, thus, is vital for multiple cellular processes. These results demonstrate that TSLP signals via the same JAK-STAT pathway in human as well as in mouse cells.
Fig. 6.
Lowering JAK1 and JAK2 expression decreased TSLP-mediated STAT5 phosphorylation. (A and B) Human CD4+ T cells were purified from PBMCs, preactivated for 4 d with anti-CD3 + anti-CD28, and transfected with JAK1 siRNA (A), JAK2 siRNA (B), or control siRNA. The next day, cells were not treated or treated with TSLP, IL-7, or IL-12 for 15 min. Cells lysates were Western blotted for phosphorylated STAT5 or STAT4, or for total expression of STAT5, STAT4, JAK1, or JAK2. The percent-decrease was estimated by comparing the signal in the experimental siRNA to control siRNA samples by densitometry. Each experiment was performed three times. (C) The intensity of each phosphorylated STAT was normalized to the total expression of the STAT protein on the same membrane, and the percent phosphorylated STAT5 (for TSLP and IL-7 stimulation) or STAT4 (for IL-12 stimulation) was determined. Shown is the average percent phosphorylation from three independent experiments of the types shown in A and B.
JAK2 Interacts with TSLPR.
It was shown that activation of STAT5 by TSLP stimulation requires the presence of Box1 domains of both TSLPR and IL-7Rα (20). Because IL-7Rα can bind JAK1 (31), we hypothesized that TSLPR associates with JAK2. To test this hypothesis, we first used NAG8/7 cells, which rapidly responded to TSLP, with phosphorylation of JAK1, JAK2, and STAT5, but not JAK3 (Fig. 7A), analogous to what we found (above) in primary CD4+ T cells. We prepared lysates from these cells and immunoprecipitated with antibodies to JAK1, JAK2, or STAT5, followed by immunoblotting with antibodies to TSLPR or IL-7Rα (Fig. 7B). The anti-TSLPR polyclonal antibody revealed a band of ≈45 kDa, which corresponds to the size of TSLPR (13) that was associated only with JAK2 (Fig. 7B, Top, lane 2). We confirmed that IL-7Rα associates with JAK1 (Fig. 7B, Middle, lane 1) and found that STAT5 binds to IL-7Rα but not to TSLPR in cells cultured with TSLP (Fig. 7B, Middle, lane 3). The association of STAT5 with IL-7Rα is consistent with prior studies that demonstrated recruitment of STAT5 to the phosphorylated Tyr449 of IL-7Rα following IL-7 stimulation (27, 31).
Fig. 7.
JAK2 associates with TSLPR. (A) NAG8/7 cells were cultured for 1 d without cytokines in medium with 10% FBS. Cells then were left unstimulated or were stimulated with TSLP (100 ng/mL) or IL-7 (10 ng/mL) as indicated. Cell lysates were run on gels or immunoprecipitated with affinity-purified rabbit IgG polyclonal antibodies to JAK1, JAK2, or JAK3, followed by Western blotting for phosphorylated or total STAT5 and JAKs. This experiment was performed twice. (B) Lysates from NAG8/7 cells grown in TSLP were immunoprecipitated with polyclonal antibodies to JAK1 (lane 1), JAK2 (lane 2), or STAT5 (lane 3), and immunocomplexes were Western blotted with goat anti-mouse TSLPR, goat anti-mouse IL-7Rα, mouse anti-JAK1, rabbit anti-JAK2, or mouse anti-STAT5. This experiment was performed three times for IL-7R and four times for TSLPR. (C) Flow cytometric measurement of cell surface expression of TSLPR and IL-7Rα on NAG8/7 cells or on WT and Tslpr KO CD4+ T cells that were preactivated for 3 d and then rested for 2 d. This experiment was performed more than five times. (D) Lysates from NAG8/7 cells, WT and Tslpr KO CD4+ T cells were immunoprecipitated with polyclonal antibodies to JAK1 (lanes 1–3) or JAK2 (lanes 4–6) and then Western blotted with antibodies as in B. The experiment was performed twice.
To confirm these data from NAG/7 cells, we next evaluated the interaction of TSLPR and JAK2 in primary T cells. We used WT CD4+ T cells, which express TSLPR and IL-7Rα (Fig. 7C), and Tslpr KO cells, which express only IL-7Rα (Fig. 7C), and subjected them to lysis, followed by immunoprecipitation with JAK1 or JAK2 antibodies. Immunoblotting with anti-TSLPR revealed a band corresponding to TSLPR in lysates not only from NAG8/7 cells but also from WT CD4+ T cells immunoprecipitated with anti-JAK2, but not from Tslpr KO cells (Fig. 7D, Upper, lanes 4 and 5 vs. 6) or when anti-JAK1 was used (Fig. 7D, lanes 1–3). As expected, immunoblotting with anti-IL-7Rα showed a band only in JAK1 precipitates (Fig. 7D, second blot, lanes 1–3).
Discussion
The mode of signaling by TSLP has been controversial. Unlike other four α-helical bundle type 1 cytokines, TSLP was reported to not activate JAK kinases in TSLP-dependent NAG8/7 cells (22), and kinase-deficient JAK constructs were reported to not inhibit TSLP signaling in human hepatoma HepG2 cells transfected with components of the TSLP receptor and STAT5B (28). However, using transfected EPOR-TSLPR or MPL-TSLPR fusion protein constructs in cell lines and EPO- or TPO-induced homodimerization, JAK2 but not JAK1 phosphorylation was observed (14, 40). JAK1 phosphorylation was not detected; presumably because these studies used homodimeric constructs to which JAK1 could not be recruited. Our studies included experiments that evaluated TSLP-signaling in primary mouse and human cells. Using specific antibodies, siRNA technology, and cells derived from knockout mice, we now have established the critical roles of JAK1 and JAK2 for TSLP-mediated STAT5 activation, without involvement of JAK3 and TYK2. The noninvolvement of JAK3 was anticipated as JAK3 uniquely associates with γc, a component of the IL-7 receptor but not the TSLP receptor complex. The phosphorylation of JAK1 and JAK2 by TSLP correlates with the ability of these JAKs to associate with IL-7Rα and TSLPR, respectively, mediating the docking of STAT5 to IL-7Rα within the receptor complex and its phosphorylation (Fig. 8A). In contrast, IL-7 induces the activation of JAK1 and JAK3, also resulting in the phosphorylation of STAT5 (Fig. 8B). The differential use of ubiquitous and constitutive JAK2 versus lineage-restricted and inducible JAK3 may help to explain some of the differences in the actions of TSLP and IL-7. However, this distinction does not immediately explain differences in the biological actions of these cytokines, such as the greater effect of IL-7 than TSLP on T-cell development (15, 19) and maintenance (18, 43) versus the proinflammatory characteristics of TSLP in pathological conditions (1, 3, 4). When and where each cytokine is produced and each receptor complex is expressed and the relative affinities are major determinants of the actions of each cytokine.
Fig. 8.
Schematic model for JAK-STAT signaling by TSLP and IL-7 receptors. (A) TSLP induces heterodimerization of TSLPR with IL-7Rα leading to activation of JAK1, JAK2, and STAT5. (B) IL-7 signals via JAK1 and JAK3 to activate STAT5. Although STAT5 is the major STAT activated by IL-7, IL-7 also induces phosphorylation of STAT1 and STAT3.
Because of the suggestion that Tec family kinases might contribute to TSLP-mediated activation of STAT5 (28), we also examined TSLP signaling in T cells derived from Tec-, Itk-, and Rlk-deficient mice; however, we found no defects in TSLP-dependent STAT5 activation in any of these cells (Fig. S2), confirming that JAK1 and JAK2 uniquely are required for TSLP-induced STAT5 activation.
Interestingly, JAK1 and JAK2 cooperatively mediate the signaling in response to other cytokines in addition to TSLP. For example, IFN-γ and IL-6 also signal via JAK1 and JAK2, but in these cases, STAT1 and STAT3, respectively, are the major STAT proteins that are activated (29, 44), rather than STAT5 proteins. This difference in STAT protein utilization is consistent with differences in actions of these various cytokines. To our knowledge, TSLP is the only cytokine that uses the combination of JAK1 and JAK2 to principally activate STAT5 proteins. Our findings thus not only clarify the basis of TSLP signaling, resolving a dilemma in the literature, but together with the IFN-γ/STAT1 and IL-6/STAT3 systems underscore that multiple STAT proteins can be phosphorylated after JAK1/JAK2 activation, depending on the specific context. Moreover, we have elucidated a signaling difference between TSLP and IL-7, which has potential implications for differentially controlling the actions of these two cytokines, both of which share IL-7Rα as a critical receptor component.
Materials and Methods
Mice.
C57BL/6, BALB/c, Jak3 KO, Tyk2 KO, and C57BL/10SnJ (as control for Tyk2 KO) mice were from The Jackson Laboratory. Tslpr KO mice, Stat5a KO mice, and littermate controls were housed in National Institutes of Health animal facilities under specific pathogen-free conditions. Tec KO and Itk/Rlk double KO mice were provided by Pamela L. Schwartzberg, National Human Genome Research Institute, National Institutes of Health. Stat5a/bfl/fl (32) and Jak2fl/fl (45) mice have been described. Experiments with mice were performed in accord with a protocol approved by the National Heart, Lung, and Blood Institute Animal Use and Care Committee.
Cell Purification and Culture.
CD4+ T cells from mouse lymph nodes and spleens were purified by positive selection using Miltenyi Biotec or STEMCELL Tech. magnetic beads. Freshly isolated cells or cells preactivated for 2 to 3 d with anti-CD3 + anti-CD28 and then rested for 2 d were stimulated as indicated with TSLP (R&D Systems), IL-7 (PeproTech), IL-2 (Roche), IFN-γ, or IFN-β (R&D Systems). DCs were derived from bone-marrow precursors of Jak3 KO or WT mice and grown for 9 d in complete RPMI medium supplemented with 20 ng/mL GM-CSF. Before stimulation, cells were cultured for 5 h without GM-CSF in medium containing 5% FBS and then left unstimulated or stimulated with TSLP, IL-7, or GM-CSF. NAG8/7 cells were grown in RPMI medium 1640 containing 10% FBS and supplemented with 10 ng/mL of recombinant TSLP. The cells were starved of cytokines for 1 d and then stimulated as indicated with TSLP or IL-7. Human CD4+ T cells were purified from PBMC by positive selection using a kit (STEMCELL Tech.) and preactivated with anti-CD3+anti-CD28 (BD Bioscience) for 4 d, rested 1 d, and stimulated with TSLP, IL-7 (R&D Systems), IL-12, or IL-4 (PeproTech).
RNA Isolation and Real-Time PCR.
RNA was extracted using TRIzol (Invitrogen) and RNeasy (Qiagen), reverse-transcribed using the iScript cDNA Synthesis kit (Bio-Rad), and mouse Cish, Bcl2, Gata3, and Rpl7 cDNAs identified by a fluorogenic 5′-nuclease PCR assay and an ABI Prism 7900HT Sequence Detection System (Perkin-Elmer) using TaqMan FAM-MGB primers for Bcl2 (Applied Biosystems) or TaqMan FAM-TAMRA primers for Cish, Gata3, and Rpl7 (Operon Biotech).
Cre-Mediated Deletion of Stat5a/b and Jak2 in vitro.
Purified CD4+ T cells from Stat5a/bfl/fl or Jak2fl/fl mice were preactivated with anti-CD3 + anti-CD28 mAbs and infected with medium containing Cre or control retroviruses (both expressed GFP) on the second and third days of activation. Infected cells were FACS-sorted according to GFP-expression.
JAK2 Inhibition.
Purified mouse CD4+ T cells were preactivated with anti-CD3 + anti-CD28 Abs for 2 d, rested for 1 d, and then treated with JAK2 inhibitor II (Calbiochem) as described (42). Briefly, cells were incubated for 13 h with 50 μM inhibitor or DMSO in RPMI with 10% FBS and for 2 h in 2% FBS in the presence of inhibitor or DMSO. CD4+ T cells then were stimulated with TSLP, IL-7, or IFN-γ.
MEF Transfection.
WT MEFs (clone A48), Jak1 KO MEFs (clone A49), and Jak1 KO MEFs reconstituted with JAK1 (clone A49.11.8) were provided by Robert D. Schreiber, Washington University School of Medicine, St. Louis, MO. Jak2 KO primary MEFs were derived from conditional Jak2 KO mice (45). Cells were grown in DMEM (Invitrogen) supplemented with sodium pyruvate, glutamine, and 10% FBS, and transiently transfected with expression vectors for TSLPR, IL-7Rα, IL-2Rγ, and STAT5A using Effectine Transfection Reagent (Qiagen) for immortalized Jak1 KO MEFs or AMAXA protocol (Lonza) for primary Jak2 KO MEFs. After overnight incubation, cells were washed and incubated in medium alone for 3 h, and then stimulated as indicated. To decrease JAK2 expression, immortalized WT MEFs were also cotransfected with On-TARGET plus SMART pool JAK2 siRNA (0.4 nmol) or control pool siRNA (0.4 nmol) (Dharmacon) using TransMessenger Transfection Reagent (Qiagen).
Human Primary CD4+ T-Cell Transfection.
Human CD4+ T cells were purified from PBMC and preactivated with anti-CD3 + anti-CD28 for 4 d. Cells were then transfected with On-TARGET plus SMART pool human JAK1 siRNA, JAK2 siRNA, or control pool siRNA (Dharmacon) using AMAXA protocol (Lonza).
Methods for proliferation assays, immunoprecipitation and immunoblotting, and FACS analysis are detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Robert D. Schreiber (Washington University, St. Louis, MO) for Jak1 KO MEFs; Drs. Bingmei Zhu and Lothar Hennighausen (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health) for valuable discussions; Dr. Pamela L. Schwartzberg (National Human Genome Research Institute) for Tec, Itk, and Rlk knockout mice; Ms. Leigh Samsel (Flow Cytometry Core, National Heart, Lung, and Blood Institute) for helping with FACS sorting; and Drs. Rosanne Spolski and Jian-Xin Lin (National Heart, Lung, and Blood Institute) for critical comments. This work was supported by the Divisions of Intramural Research, National Heart, Lung, and Blood Institute, and National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Footnotes
Conflict of interest: W.J.L. is an inventor on patents and patent applications related to thymic stromal lymphopoietin.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008271107/-/DCSupplemental.
References
- 1.Liu YJ, et al. TSLP: An epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 2.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rochman Y, Leonard WJ. Thymic stromal lymphopoietin: A new cytokine in asthma. Curr Opin Pharmacol. 2008;8:249–254. doi: 10.1016/j.coph.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–714. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 5.Ying S, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 6.Taylor BC, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massacand JC, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci USA. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramalingam TR, et al. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J Immunol. 2009;182:6452–6459. doi: 10.4049/jimmunol.0900181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo M, et al. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science. 1994;263:1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi M, et al. Interleukin-2 receptor gamma chain: A functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 11.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noguchi M, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 13.Pandey A, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 14.Fujio K, et al. Molecular cloning of a novel type 1 cytokine receptor similar to the common gamma chain. Blood. 2000;95:2204–2210. [PubMed] [Google Scholar]
- 15.Al-Shami A, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 17.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: Daction of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 18.Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. 2008;181:7699–7705. doi: 10.4049/jimmunol.181.11.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110:3862–3870. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- 20.Isaksen DE, et al. Uncoupling of proliferation and Stat5 activation in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 2002;168:3288–3294. doi: 10.4049/jimmunol.168.7.3288. [DOI] [PubMed] [Google Scholar]
- 21.von Freeden-Jeffry U, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin SD, et al. Thymic stromal lymphopoietin: A cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 23.Friend SL, et al. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 24.Macchi P, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 25.Russell SM, et al. Mutation of Jak3 in a patient with SCID: Essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 26.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 27.Lin JX, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 28.Isaksen DE, et al. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163:5971–5977. [PubMed] [Google Scholar]
- 29.Leonard WJ, O'Shea JJ. Jaks and STATs: Biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 30.Tanner JW, Chen W, Young RL, Longmore GD, Shaw AS. The conserved box 1 motif of cytokine receptors is required for association with JAK kinases. J Biol Chem. 1995;270:6523–6530. doi: 10.1074/jbc.270.12.6523. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Q, et al. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–6513. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Y, et al. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto A, et al. CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood. 1997;89:3148–3154. [PubMed] [Google Scholar]
- 34.Li G, et al. STAT5 requires the N-domain for suppression of miR15/16, induction of bcl-2, and survival signaling in myeloproliferative disease. Blood. 2010;115:1416–1424. doi: 10.1182/blood-2009-07-234963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer MJ, et al. Interleukin-7 receptor signaling network: An integrated systems perspective. Cell Mol Immunol. 2008;5:79–89. doi: 10.1038/cmi.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodig SJ, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 37.Boussiotis VA, et al. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 38.Miyazaki T, et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 39.Russell SM, et al. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: Implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 40.Carpino N, et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004;24:2584–2592. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiroyama T, et al. Molecular cloning and characterization of CRLM-2, a novel type I cytokine receptor preferentially expressed in hematopoietic cells. Biochem Biophys Res Commun. 2000;272:224–229. doi: 10.1006/bbrc.2000.2764. [DOI] [PubMed] [Google Scholar]
- 42.Sandberg EM, et al. Identification of 1,2,3,4,5,6-hexabromocyclohexane as a small molecule inhibitor of jak2 tyrosine kinase autophosphorylation [correction of autophophorylation] J Med Chem. 2005;48:2526–2533. doi: 10.1021/jm049470k. [DOI] [PubMed] [Google Scholar]
- 43.Lu N, et al. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med. 2009;206:2111–2119. doi: 10.1084/jem.20090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schindler CW. Series introduction. JAK-STAT signaling in human disease. J Clin Invest. 2002;109:1133–1137. doi: 10.1172/JCI15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner KU, et al. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol. 2004;24:5510–5520. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.