Abstract
RORγt+ TH17 cells are a proinflammatory CD4+ T-cell population associated with autoimmune tissue injury. In mice, priming of TH17 requires TGF-β, which alone directs the priming of FOXP3+ regulatory T cells (Treg), in association with inflammatory cytokines. Priming of human TH17 cells from conventional naive CD4+ T cells under similar conditions, however, has proved difficult to achieve. Here, we report that differentiation of human TH17 cells preferentially occurs from FOXP3+ naive Treg (NTreg) in the presence of IL-2 and IL-1β and is increased by IL-23 and TGF-β. IL-1β–mediated differentiation correlated with IL-1RI expression in stimulated NTreg and was accompanied by induction of RORγt along with down-regulation of FOXP3. IL-17–secreting cells in NTreg cultures cosecreted TNF-α and IL-2 and contained distinct subpopulations cosecreting or not cosecreting IFN-γ and other TH17-associated cytokines. Polarized NTreg contained significant subpopulations of CCR6-expressing cells that were highly enriched in IL-17–secreting cells. Finally, analysis of CCR6 expression with respect to that of IL-1RI identified distinct IL-17–secreting subpopulations that had maintained or lost their suppressive functions. Together our results support the concept that priming of human TH17 from naive CD4+ T cells preferentially takes place from FOXP3+ Treg precursors in the presence of lineage-specific polarizing factors.
Keywords: IL-1, IL-17, IL-23, TGF-β, CD4 T cells
Recent studies have identified T helper 17 (TH17) cells as a distinct lineage of CD4+ effector T cells producing the proinflammatory cytokine IL-17A (hereafter IL-17), leading to chemokine production and recruitment of neutrophils to inflamed tissues (1). In mice, TH17 cells have been shown to be involved in the pathogenesis of experimental autoimmune diseases previously attributed to unchecked TH1 responses (1–4). In addition, assessment of patients with autoimmune diseases has suggested an involvement of TH17 cells in some human autoimmune disorders (5, 6). In humans, CD4+ T cells producing IL-17 ex vivo can be detected in circulating lymphocytes from healthy individuals, among memory CD4+ T cells, mainly in the CCR6+ fraction (6, 7) and contain two subpopulations cosecreting or not cosecreting IFN-γ. Both in mice and in humans, the retinoid-related orphan nuclear receptor RORγt has been identified as a lineage-specific transcription factor for TH17 cells (6–8).
In mice, differentiation of TH17 cells has been shown to take place in the presence of TFG-β and IL-6 (9), with IL-23 and IL-21 amplifying their expansion and/or stabilizing their phenotype (10–12). Differentiation of human TH17 cells from naive precursors, however, has proved difficult to achieve (13), and several discrepant findings have been reported. Differentiation has been initially reported to require IL-1β and IL-6, and to be inhibited by TFG-β and IL-2 (14). Subsequent studies, however, have shown TGF-β and IL-2 to be instead required (15, 16). Yang et al. have reported differentiation of TH17 cells from human naive conventional CD4+ T cells in the presence of TGF-β and IL-21 but not TGF-β and IL-6 (17). Another study however, has suggested that several inflammatory cytokines including IL-1β, IL-6 and IL-23 are all required and act synergistically (18). Overall, these discrepancies have been attributed to the difficulty to ensure a truly naive population of human CD4+ T cells (19). Finally, a recent study has proposed that human TH17 cells originate from CD161+ precursors present in thymus and cord blood but not in peripheral blood from adult individuals (20).
Opposite to TH17 cells, a distinct subset of CD4+ T cells, called T regulatory cells (Treg), actively control autoimmunity and tissue homeostasis. CD4+ Treg populations in the periphery of adult individuals include thymically derived natural Treg, in charge of maintaining self-tolerance, and induced Treg that are generated in the periphery to limit exuberant immune responses to microbial or tissue antigens. We have previously shown that, in addition to memory Treg, human Treg include a naive (CD45RA+/RO−CCR7+) CD25+FOXP3+ population, that we have named natural naive Treg (NTreg) present in cord blood and circulating lymphocytes (21). Similar to memory Treg, CD25+FOXP3+NTreg are anergic and suppressive ex vivo (21). So far, NTreg are the only identified subpopulation of naive T cells that constitutively express a lineage-specific transcription factor and are therefore precommitted to differentiation into a specific lineage. Several recent studies, including studies from our group, have documented a close relationship between FOXP3+Treg and TH17 lineages (22–25). We have shown that stimulation of human FOXP3+Treg in the presence of LPS-activated monocytes and IL-2 converts them into TH17 cells (22) and have recently identified a subpopulation of memory Treg that coexpress RORγt and secrete high levels of IL-17 ex vivo (24). Together, these findings suggested that human TH17 cells could preferentially originate from NTreg rather than from conventional naive CD4+ T-cell precursors. In this study, we show that differentiation of TH17 cells from human circulating naive CD4+ T cells is indeed predominantly obtained from NTreg, and optimally occurs following stimulation in the presence of IL-2 and of lineage-specific differentiation factors.
Results
Priming of IL-17–Producing Cells from Adult Naive Circulating CD4+ T Cells Predominantly Occurs from NTreg.
We initially stimulated total naive CD45RA+CD4+ T cells isolated from circulating lymphocytes of adult subjects with anti-CD2/CD3/CD28–coated microbeads in the presence of several previously described TH17 polarizing cytokines, in various combinations. Under these conditions, we observed very low proportions (generally <1%) of cells secreting IL-17A (IL-17 hereafter, unless otherwise specified). To assess whether TH17 cells could specifically differentiate from NTreg (which represent ∼5% of total naive circulating CD4+ T cells), we purified naive conventional (CD45RA+CD25−CD127high) and NTreg (CD45RA+CD25+CD127low) populations by flow cytometry cell sorting (Fig. 1A). Staining of the sorted populations with FOXP3-specific mAb confirmed the isolation of highly pure conventional (FOXP3−) and regulatory (FOXP3+) populations (Fig. 1A). We stimulated the sorted populations with anti-CD2/CD3/CD28–coated microbeads in the presence of IL-2 alone or with TGF-β, IL-1β, IL-23, IL-6, or IL-21, separately or in various combinations and, 12 d later, assessed the proportion of IL-17–secreting cells in the cultures by intracellular staining. Whereas stimulation of conventional naive CD4+ T cells under these various conditions resulted in the induction of very low proportions of IL-17–producing cells, stimulation of NTreg with some of the cytokine combinations resulted in the induction of a significant fraction of IL-17–producing cells (Fig. 1 B and C). In particular, we obtained optimal induction in the presence of IL-2, IL-1β, IL-23, and TGF-β. Low proportions of IL-17–producing cells were induced from NTreg in the absence of IL-2, even in the presence of the most effective cytokine combination. Thus, priming of TH17 cells from adult circulating naive CD4+ T cells consistently occurred, and predominantly took place from NTreg in the presence of IL-2 and of lineage specific polarizing factors.
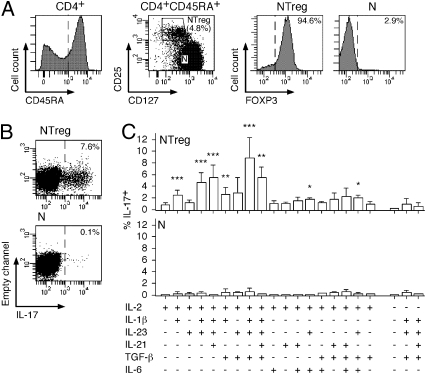
Fig. 1.
In vitro differentiation of NTreg into IL-17–producing cells. (A) Enriched CD4+ T cells were stained with anti-CD25, -CD45RA, and -CD127 mAb and CD45RA+ cells (left histogram) were sorted by flow cytometry into naive conventional CD4+ T cells (N, CD25−CD127high) and naive Treg (NTreg, CD25+CD127low) (dot plot). A fraction of sorted populations was stained with anti-FOXP3 mAb and analyzed by flow cytometry (right histograms). (B and C) Sorted N and NTreg were stimulated in vitro with anti-CD2/CD3/CD28 microbeads in the presence of the indicated cytokines and cultured for 12 d in the absence or presence of IL-2, as indicated. IL-17 production was assessed by intracellular cytokine staining and flow cytometry analysis following stimulation with PMA/ionomycin. Dot plots for N and NTreg from one donor stimulated in the presence of IL-2, IL-1β, IL-23, and TGF-β are shown in B. Results for all conditions and donors are shown in C (mean ± SD, n = 6). Statistical analyses were performed using two-tailed t test. *P < 0.05; **P < 0.01; ***P < 0.001.
Expression of IL-1RI Is Selectively Up-Regulated in NTreg Following Stimulation and Is Increased by TGF-β.
Whereas IL-23 was not active alone but increased the differentiation of TH17 cells from NTreg in the presence of IL-1β, the latter was indispensable for differentiation to occur. To gain further insight into the differential role of these cytokines during TH17 polarization from NTreg, we assessed the expression of their specific receptors in conventional naive CD4+ T cells and NTreg. We detected no significant expression of IL-1RI ex vivo in naive CD4+ T cells, either conventional or Treg (Fig. 2A). Following in vitro stimulation, however, IL-1RI expression was induced in a significant fraction of NTreg but not in conventional naive CD4+ T cells. The proportion of IL-1RI–expressing cells induced in NTreg by stimulation in the presence of IL-2 was not significantly affected by the presence of other cytokines (including IL-1β) with the exception of TGF-β that significantly increased it. Ex vivo expression of IL-23R in naive CD4+ T cells was low and was induced by stimulation in a fraction of the cells (Fig. 2B). At variance with IL-1RI, however, IL-23R expression was induced in both conventional naive CD4+ T cells and NTreg. The presence of TGF-β, alone or in combination with inflammatory cytokines, further increased IL-23R expression in both populations. Thus, selective overexpression of IL-1RI in NTreg was in agreement with the important role of IL-1β in inducing their differentiation into TH17 cells, whereas TGF-β synergized with IL-1β and IL-23 by increasing the expression of the corresponding receptors.
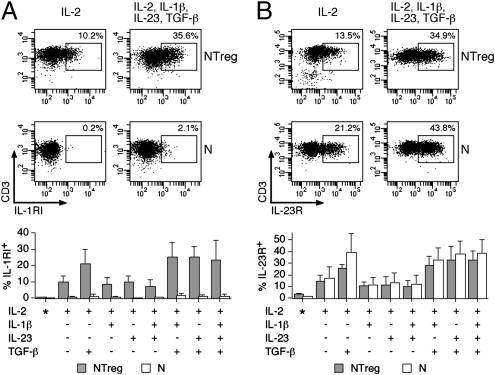
Fig. 2.
Expression of IL-1RI and IL-23R in stimulated N and NTreg. Ex vivo sorted N and NTreg were stimulated in vitro with anti-CD2/CD3/CD28 microbeads in the presence of the indicated cytokines and cultured in the presence of IL-2. Ex vivo sorted N and NTreg as well as day 5 cultures were stained with antibodies specific for IL-1RI (A) and IL-23R (B) and analyzed by flow cytometry. Examples of dot plots for one donor and data for all donors (mean ± SD, n = 5), ex vivo (*) or after culture are shown.
TH17 Cells Differentiating from NTreg Down-Regulate FOXP3 and Express RORγt.
Expression of lineage-specific transcription factors supports the differentiation of distinct T-cell subsets by shaping their phenotypic and functional profiles. It has been proposed that the balance between the TH17 lineage specific transcription factor RORγt, the expression of which is indispensable for IL-17 secretion, and the Treg-specific transcription factor FOXP3, which antagonizes RORγt activity, affects TH17 cell polarization. To follow the expression of FOXP3 and RORγt during TH17 priming from NTreg, we assessed cultures stimulated under nonpolarizing or polarizing conditions using RORγt, FOXP3, and IL-17–specific antibodies. Expression of FOXP3 in NTreg was maintained in a substantial proportion of cells after differentiation in all conditions (Fig. 3A). The large majority of IL-17–secreting cells derived from polarized NTreg (particularly in the absence of TGF-β), however, had lost or highly decreased FOXP3 expression (Fig. 3 B and C). Expression of RORγt, undetectable in naive CD4+ T cells ex vivo (24), was induced in a low proportion of conventional naive CD4+ T cells and NTreg after stimulation in the presence of IL-2 with or without inflammatory cytokines or with TGF-β alone. Following stimulation with IL-2 and inflammatory cytokines together with TGF-β, however, expression of RORγt was induced in more than half of total conventional naive and NTreg populations (Fig. 3A). Regardless of the total proportion of RORγt-expressing cells in the cultures, the large majority of IL-17–secreting cells expressed high levels of RORγt (Fig. 3 B and C). Thus, TH17 cells differentiating from NTreg were FOXP3− or FOXP3low and expressed high levels of RORγt.
Fig. 3.
Expression of FOXP3 and RORγt in TH17 cells differentiating from NTreg. Ex vivo sorted N and NTreg were stimulated as in Fig. 2. Day 12 cultures were stimulated with PMA/ionomycin, stained with mAb specific for IL-17, FOXP3, and RORγt and analyzed by flow cytometry. Data corresponding to the percentage of IL-17–producing and FOXP3- and RORγt-expressing cells in the cultures are shown in A (mean ± SD, n = 3). Dot plots showing FOXP3 and RORγt expression among IL-17–producing cells in polarized cultures are shown for one donor in B, and data for all donors (mean ± SD, n = 3) are shown in C.
NTreg-Derived TH17 Cells Contain Distinct Subpopulations Cosecreting or Not IFN-γ, Cosecrete Other TH17-Associated Cytokines, and Secrete IL-2.
To further characterize NTreg-derived TH17 populations, we assessed the secretion of other cytokines that have been reported as associated or not associated with the lineage. We selected cultures polarized in the presence of IL-1β and IL-23 in either the absence or presence of TGF-β (Fig. 4). NTreg-derived TH17 cells contained both IL-17 single-secreting and IL-17/IFN-γ double-secreting populations, corresponding to the two distinct populations described in circulating human memory TH17 cells (6). Whereas single-secreting and double-secreting cells were present in roughly similar proportions following polarization in the absence of TFG-β, IL-17 single-secreting cells were prevalent in the presence of TFG-β. Most IL-17–secreting cells cosecreted TNF-α, and about half of them cosecreted IL-22, regardless of the presence of TGF-β. In addition, ∼30% of IL-17–secreting cells cosecreted IL-21 in the presence of TFG-β. No significant cosecretion of IL-4 or IL-10 was detected. Importantly, whereas NTreg fail to secrete IL-2 ex vivo (21), TH17 polarized NTreg populations contained significant proportions of IL-2–secreting cells that were particularly enriched in the IL-17–secreting fraction. A subpopulation of IL-17A–secreting cells in the cultures cosecreted IL-17F, another proinflammatory cytokine similar to IL-17A (26). Of note, no IL-17F single-positive cells were detected in the cultures.
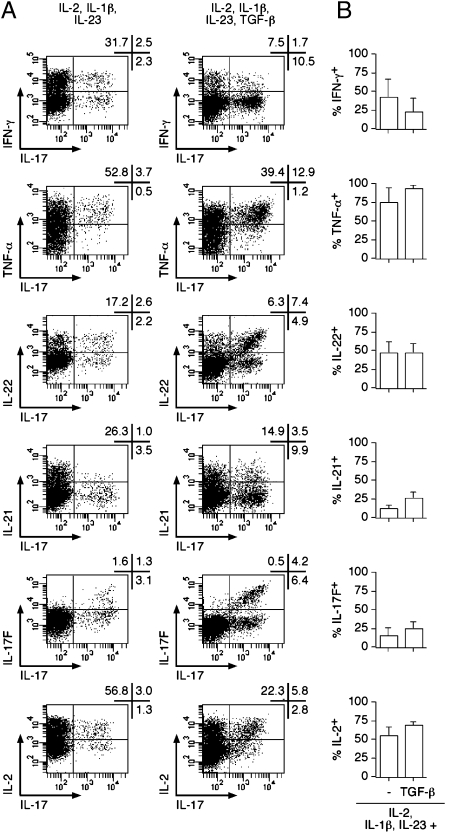
Fig. 4.
Cytokine production by TH17 cells differentiated from NTreg. Ex vivo sorted NTreg were stimulated in the presence of IL-1β and IL-23 in the absence or presence of TGF-β and cultured in the presence of IL-2 for 12 d. Cytokine production was determined by intracellular staining with specific mAb following stimulation with PMA/ionomycin. Dot plots for one donor are shown in A. Proportions of TH17 cells coproducing the indicated cytokines are summarized in B for all donors (mean ± SD, n = 3).
IL-17–Secreting Cells in TH17 Polarized NTreg Cultures Are Highly Enriched in CCR6+ Expressing Cells.
Expression of the chemokine receptor CCR6 has been described as associated with the TH17 lineage but also to the Treg lineage (6, 7, 27, 28). Expression of CCR6 in naive CD4+ T cells (both conventional and NTreg) was undetectable ex vivo (24). However, CCR6 expression was induced in a small fraction of NTreg, but not in conventional naive CD4+ T cells, following stimulation in the presence of IL-2 with or without IL-1β and IL-23 (Fig. 5A). In contrast, the presence of TFG-β slightly inhibited expression of CCR6. To assess whether TH17 cells in polarized NTreg were enriched within the CCR6-expressing fraction, we isolated CCR6+ and CCR6− populations by cell sorting and assessed secretion of IL-17 together with expression of RORγt in the isolated fractions. As shown in Fig. 5B, most IL-17–secreting cells that were, as expected, homogenously RORγt+ were contained in the CCR6+ fraction of the cultures. In addition, regardless of their capacity to secrete IL-17, most cells in the CCR6+ fraction expressed RORγt.
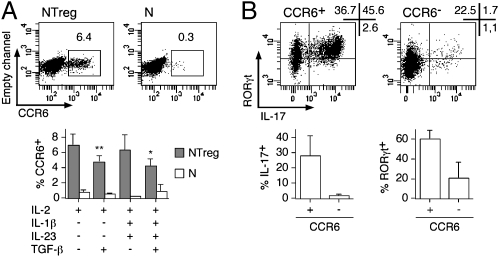
Fig. 5.
CCR6-expressing fraction within polarized NTreg is enriched in TH17 cells. (A) Ex vivo sorted N and NTreg were stimulated in vitro with anti-CD2/CD3/CD28 microbeads in the presence of the indicated cytokines and cultured in the presence of IL-2. Day 5 cultures were stained with anti-CCR6 mAb and analyzed by flow cytometry. Dot plots for one donor and data for all donors (mean ± SD, n = 5) are shown. *P < 0.05; **P < 0.01. (B) Day 12 cultures of NTreg, stimulated in the presence of IL-1β, IL-23, TGF-β, and IL-2, were stained with anti-CCR6 mAb and CCR6+ and CCR6− populations were sorted by flow cytometry. After stimulation with PMA/ionomycin, sorted cells were stained with IL-17 and RORγt-specific mAb and analyzed by flow cytometry. Dot plots for one donor and data for all donors (mean ± SD, n = 6) are shown.
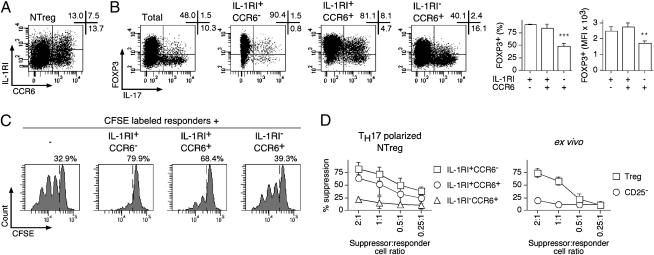
Expression of IL-1RI and CCR6 in TH17 Polarized NTreg Cultures Identifies Distinct Suppressive and Nonsuppressive Subpopulations.
A recent study has shown that expression of IL-1RI in in vitro cultured human memory Treg specifically identified populations of cells that have maintained suppressor functions (29). To clarify the relationship between expression of IL-1RI and CCR6, we costained polarized NTreg cultures with the corresponding specific mAb. This analysis identified several distinct populations (Fig. 6A). Namely, whereas a subpopulation expressing IL-1RI but not CCR6 was clearly identified, the CCR6-expressing population included both IL-1RI− and, to a lesser extent, IL-1RI+ cells. To further evaluate these subpopulations, we isolated them by cell sorting, stimulated them with PMA/ionomycin, and assessed FOXP3 expression and IL-17 production. In addition, we also assessed the capacity of the isolated populations to suppress the proliferation of responder CFSE-labeled CD4+ T cells in a functional suppression assay as described previously (21). As illustrated in Fig. 6B, IL-1RI–expressing populations, both CCR6− and CCR6+, contained significantly higher proportions of FOXP3+ cells, which expressed significantly higher levels of FOXP3, as compared with the IL-1RI− fraction. As expected, most IL-17–secreting cells were detected in the CCR6+ populations. However, it is noteworthy that, whereas most IL-17–secreting cells in the IL-1RI−CCR6+ fraction were FOXP3−, a large proportion of IL-17–secreting cells in the IL-1RI+CCR6+ population had maintained FOXP3 expression. In line with FOXP3 expression, the IL-1RI+ populations (both CCR6− and CCR6+) displayed suppressive functions, whereas the IL-1RI−CCR6+ failed to inhibit the growth of responder cells (Fig. 6 C and D). In conclusion, our analysis identified two distinct subpopulations of CCR6+ IL-17–secreting cells: one subpopulation, less abundant, expressed IL-1RI and maintained FOXP3 expression and suppressive functions, whereas the prominent subpopulation was IL-1RI− and had lost FOXP3 expression and suppressive functions.
Fig. 6.
TH17 polarized NTreg cultures contain distinct suppressive and nonsuppressive subpopulations. (A) Ex vivo sorted NTreg were stimulated in vitro with anti-CD2/CD3/CD28 microbeads in the presence of IL-1β, IL-23, TGF-β, and IL-2. Day 12 cultures were stained with mAb specific for IL-1RI and CCR6 and analyzed by flow cytometry. (B) NTreg were stimulated and stained as in A, and IL-1RI+CCR6−, IL-1RI+CCR6+, and IL-1RI−CCR6+ populations were sorted by flow cytometry. Cells from the total unsorted culture as well as sorted populations were stimulated in the presence of PMA/ionomycin, stained with mAb specific for FOXP3 and IL-17, and analyzed by flow cytometry. Dot plots for one donor and data for all donors (% FOXP3+ cells and mean fluorescence intensity (MFI) of FOXP3 staining in the FOXP3+ populations, mean ± SD, n = 4) are shown. **P < 0.01; ***P < 0.001. (C and D) CFSE-labeled conventional CD4+ T cells were stimulated with PHA in the absence or presence of IL-1RI+CCR6−, IL-1RI+CCR6+, and IL-1RI−CCR6+ populations sorted from TH17 polarized NTreg cultures as in B or in the presence of ex vivo sorted Treg and conventional CD25− cells. Growth of responder CD4+ T cells was assessed by flow cytometry analysis of CFSE dilution. Examples of CFSE dilution histograms, at 1:1 suppressor-to-responder cell ratio, are shown in C where numbers correspond to the proportion of undivided cells. Percent suppression is shown in D for all tested suppressor-to-responder cell ratios, populations, and donors (mean ± SD, n = 3).
Discussion
In this study, we have unambiguously shown that priming of human TH17 cells consistently takes place from adult naive circulating CD4+ T cells and occurs mainly from NTreg rather than conventional naive CD4+CD25− T cells. Together, our results reconcile previous reports of heterogeneous and discrepant findings likely due to the use of nonoptimally defined starting populations containing variable proportions of naive conventional CD4+ T cells and NTreg populations.
Priming of TH17 cells from NTreg was optimally achieved in the presence of IL-2, IL-1β, IL-23, and TGF-β. Based on the enhancement of TH17 generation following genetic deletion or antibody blockade in murine models, IL-2 has been initially proposed to inhibit TH17 differentiation (30). The exact underlying mechanism, however, has not been elucidated; and it has been suggested that IL-2, through binding of STAT5 to the IL-17 promoter, might attenuate IL-17 production in differentiated TH17 cells rather than inhibiting TH17 differentiation (16). In line with this hypothesis, it was later shown that IL-2 is instead required for in vitro differentiation of human TH17 cells (15). Similarly, in our system, the presence of IL-2 highly increased differentiation of TH17 cells from NTreg in all conditions.
TGF-β is crucial for maintenance of Treg in the periphery, although it may be dispensable for their generation in the thymus (31). TGF-β was initially reported to inhibit the in vitro differentiation of human TH17 cells (14), but was proved later as required (15, 16). In this study, the presence of TGF-β was not indispensable, but increased the proportion of TH17 cells differentiating from NTreg. It is noteworthy that NTreg express endogenous TGF-β (21), which likely has an impact on their differentiation into TH17 cells even in the absence of exogenous sources.
We and others have recently shown that TCR stimulation in the presence of IL-1β (or IL-1α) and IL-2 or IL-15 induces the conversion of human memory Treg into TH17 cells (22, 23). In this study, IL-1β was indispensable for NTreg differentiation into TH17 cells. IL-1β signals through receptor type I (IL-1RI), homologous to Toll, the conserved region being the Toll/IL-1 receptor (TIR) domain, which defines the IL-1R/TLR superfamily (32). Interestingly, at odds with previous findings suggesting a marginal role of IL-1β in the differentiation of murine TH17 cells in vitro, IL-1RI has recently been shown to be necessary for early differentiation of murine TH17 cells in vivo (33). We found selective expression of IL-1R1 in stimulated NTreg in good agreement with their increased proficiency, with respect to conventional naive CD4+ T-cell precursors, to differentiate into TH17 cells. Interestingly, IL-1RI expression in NTreg was not affected by IL-1β or IL-23 but was significantly enhanced by TGF-β. Consistent with the role of TGF-β in inducing IL-1RI expression, the latter correlated with that of FOXP3 and identified suppressive populations in the cultures.
IL-23 is a heterodimeric cytokine composed of a p40 chain, common to IL-12, disulfide-linked to a specific p19 chain (34, 35). Studies using p19−/− murine models of autoimmunity identified IL-23 as the major factor involved in the induction of TH17 cells in vivo (36–38). However, researchers have subsequently failed to demonstrate efficient induction of TH17 cells from naive CD4+ T cells by IL-23 in vitro (39, 40), and have concluded that the role of IL-23 was not to induce but rather to stabilize the TH17 phenotype. In line with this hypothesis, in this study, IL-23 alone was not able to differentiate NTreg into TH17 cells but synergized with other polarizing factors.
TH17 cells derived from NTreg exhibited a cytokine secretion pattern similar to those described thus far for human TH17 populations, as they contained both cells cosecreting IL-17 and IFN-γ (double-secreting) and cells secreting IL-17 in the absence of IFN-γ secretion (single-secreting) (6). Interestingly, whereas double-secreting cells were prevalent following polarization in the absence of TGF-β, polarization in the presence of TGF-β prevalently induced single-secreting cells. This suggests that both single- and double-secreting TH17 cells can originate from NTreg depending on the in vivo differentiation conditions. All IL-17–secreting cells derived from NTreg cosecreted TNF-α, also highly proinflammatory. Some of them cosecreted IL-21 and IL-22, two cytokines that have been described as TH17 associated, although not exclusively secreted by TH17 cells (41–44). IL-17F is a proinflammatory cytokine with functions initially reported as similar to those of IL-17A and sharing the same receptor (45). Recent studies have revealed that IL-17A and IL-17F can be expressed as homo- or heterodimers that may bind IL-17 receptors with various affinities and may play not completely redundant functions (26, 46, 47). We found expression of IL-17F in only a fraction of IL-17A–secreting cells from NTreg, indicating the existence of distinct subpopulations that differentially express these cytokines and may be functionally distinct, an observation that warrants further investigation.
Differentiation of TH17 cells from NTreg was accompanied by induction of RORγt expression, down-regulation of FOXP3 expression and acquisition of IL-2 secretion, particularly in the IL-17–secreting fraction, suggesting that NTreg-derived TH17 cells may have mostly lost their suppressor functions and may have instead acquired auxiliary functions. Expression of CCR6 has been reported as associated with the TH17 but also with the Treg lineage (6, 7, 27, 28). We found increased expression of CCR6 in stimulated NTreg as compared with conventional naive CD4+ T cells. In addition, by isolating CCR6-expressing cells from polarized cultures, we demonstrated that most IL-17–secreting cells in NTreg-derived TH17 cell populations were contained in the CCR6+ fraction. Interestingly, assessment of CCR6 expression in relation to that of IL-1RI revealed the existence of two distinct subpopulations of CCR6+ IL-17–secreting cells: the first, less abundant, coexpressed IL-1RI and FOXP3 and retained suppressive functions, whereas the most prominent one was IL-1RI− and FOXP3− and had lost the capacity to suppress. The identification of these subpopulations is in line with our recent finding that human IL-17 secreting cells in human peripheral blood (that are all memory cells) include a population of cells that coexpress FOXP3 and exert suppressor functions (24).
Overall, the findings reported here strongly suggest that at least part of human TH17 cells may differentiate in vivo from NTreg precursors in the presence of lineage-specific differentiation factors. NTreg are generated in the thymus as a distinct lineage of CD4+ T cells that are anergic but are specific for self-antigens, and therefore potentially auto-reactive if stimulated under conditions that break their anergy (48). The finding that TH17 cells mainly differentiate from NTreg therefore raises the possibility that some auto-reactive effectors in autoimmune pathologies associated with TH17 might be generated through this differentiation pathway. Because some tumor-associated antigens are nonmutated self-antigens also expressed in cells of normal tissues that undergo malignant transformation (e.g., melanocyte differentiation antigens), differentiation of NTreg specific for these antigens into TH17 would result in the generation of anti-tumor effectors. Interestingly, recent data have indeed shown a particular efficacy of TH17 effectors in anti-tumor responses (49).
A preferential differentiation of TH17 cells from NTreg, however, does not imply that all TH17 effectors are specific for self-antigens or autoreactive. First, because part of Treg in adults could also be generated in the periphery through conversion of conventional CD4+ T cells (50), some NTreg could represent an intermediate differentiation stage along a differentiation pathway leading from nonautoreactive, naive, conventional CD4+ T cells to memory TH17 cells. In addition, some TH17 cells could be generated in the periphery through conversion of induced MTreg, differentiated from CD4+ CD25−FOXP3− T cells, under inflammatory conditions (22, 23).
Although the involvement of TH17 cells in autoimmune pathology has been strongly suggested by studies in mice and in humans, little information is available on the antigens that they recognize (7), and their potential self-reactivity has not been thus far investigated. Based on the knowledge that TH17 cells can derive from precursors common to autoreactive Treg, insights into their antigen specificity, including reactivity to self-antigens, in human TH17-associated pathologies, will undoubtedly contribute to clarify their role in inflammation-induced tissue damage.
Materials and Methods
Samples, Cell Purification, and ex Vivo Cell Sorting.
Peripheral blood samples were obtained from the Etablissement Français du Sang (Nantes, France) upon informed consent and approval by institutional review board. Naive conventional CD4+ T cells and NTreg were isolated by flow cytometry cell sorting, as previously described (24) and detailed in SI Materials and Methods.
In Vitro Differentiation of CD4+ T-cell Populations and Phenotypic Assessment and Flow Cytometry Cell Sorting of Differentiated Cultures.
Ex vivo sorted N and NTreg (3 × 104) were stimulated with anti-CD2/CD3/CD28–coated microbeads (Miltenyi Biotec) in the absence or presence of IL-1β (10 ng/mL, R&D Systems), IL-6 (50 ng/mL, R&D Systems), IL-21 (50 ng/mL, R&D systems), IL-23 (100 ng/mL, R&D Systems), and TGF-β (10 ng/mL, PeproTech) alone or in combination, as specified. Cells were maintained in culture in complete IMDM (Invitrogen) in the absence or presence of IL-2 (100 IU/mL, Chiron), as indicated. Day 5 or 12 cultures were stained with IL-RI-, IL-23R- and/or CCR6-specific antibodies and were analyzed and/or sorted by flow cytometry, as detailed in SI Materials and Methods.
Assessment of Cytokine Production, RORγt and FOXP3 Expression, and Suppressor Function.
Cytokine production and expression of RORγt and FOXP3 by in vitro differentiated CD4+ T cells were assessed by intracellular staining and flow cytometry analysis following stimulation with PMA and ionomycin as detailed in SI Materials and Methods. Their ability to suppress the proliferation of responder CD4+CD25− T cells was assessed as previously described (21) and detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
The study was supported by the Ludwig Institute for Cancer Research, Cancer Research Institute, Institut National de la Santé et de la Recherche Médicale (France), Institut National du Cancer (France), Conseil Régional des Pays de la Loire (France), and European regional development fund.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008247107/-/DCSupplemental.
References
- 1.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Gran B, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: Evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 4.Calida DM, et al. Cutting edge: C3, a key component of complement activation, is not required for the development of myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis in mice. J Immunol. 2001;166:723–726. doi: 10.4049/jimmunol.166.2.723. [DOI] [PubMed] [Google Scholar]
- 5.Matusevicius D, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 6.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 10.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 13.Romagnani S, Maggi E, Liotta F, Cosmi L, Annunziato F. Properties and origin of human Th17 cells. Mol Immunol. 2009;47:3–7. doi: 10.1016/j.molimm.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 15.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stockinger B. Good for goose, but not for gander: IL-2 interferes with Th17 differentiation. Immunity. 2007;26:278–279. doi: 10.1016/j.immuni.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpe E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 19.Laurence A, O'Shea JJ. T(H)-17 differentiation: of Mice and men. Nat Immunol. 2007;8:903–905. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 20.Cosmi L, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin Immunol. 2009;131:298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Koenen HJ, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 24.Ayyoub M, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci USA. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright JF, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol. 2008;181:2799–2805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 27.Kleinewietfeld M, et al. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 28.Hirahara K, et al. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 29.Tran DQ, et al. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 32.O'Neill LA, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: Crucial receptors for inflammation and host defense. Immunol Today. 2000;21:206–209. doi: 10.1016/s0167-5699(00)01611-x. [DOI] [PubMed] [Google Scholar]
- 33.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 36.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 37.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 40.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 43.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 44.Monteleone G, Pallone F, MacDonald TT. Interleukin-21: A critical regulator of the balance between effector and regulatory T-cell responses. Trends Immunol. 2008;29:290–294. doi: 10.1016/j.it.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Toy D, et al. Cutting edge: Interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 46.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 47.Liang SC, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 48.Sakaguchi S, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 49.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.