Abstract
Synucleins are a vertebrate-specific family of abundant neuronal proteins. They comprise three closely related members, α-, β-, and γ-synuclein. α-Synuclein has been the focus of intense attention since mutations in it were identified as a cause for familial Parkinson's disease. Despite their disease relevance, the normal physiological function of synucleins has remained elusive. To address this, we generated and characterized αβγ-synuclein knockout mice, which lack all members of this protein family. Deletion of synucleins causes alterations in synaptic structure and transmission, age-dependent neuronal dysfunction, as well as diminished survival. Abrogation of synuclein expression decreased excitatory synapse size by ∼30% both in vivo and in vitro, revealing that synucleins are important determinants of presynaptic terminal size. Young synuclein null mice show improved basic transmission, whereas older mice show a pronounced decrement. The late onset phenotypes in synuclein null mice were not due to a loss of synapses or neurons but rather reflect specific changes in synaptic protein composition and axonal structure. Our results demonstrate that synucleins contribute importantly to the long-term operation of the nervous system and that alterations in their physiological function could contribute to the development of Parkinson's disease.
Keywords: neurodegeneration, loss-of-function, Lewy bodies, ultrastructure, retina
Synucleins are a family of vertebrate-specific proteins with three closely related members, α-, β-, and γ-synuclein (1, 2). They are abundant neuronal proteins and are reported to account for 0.1% of total brain protein (3, 4). Synucleins have overlapping expression patterns and are enriched in presynaptic termini (5, 6). α-Synuclein has been the focus of intense attention since the identification of dominant mutations and gene multiplications that link it to familial Parkinson's disease (PD) (7). Recently, strong ties between the α-synuclein gene and sporadic PD have emerged in genomewide association studies, making α-synuclein the most broadly relevant PD gene (8, 9). Additionally, α-synuclein is the major component of Lewy bodies, the pathological hallmark of PD (10). The presynaptic localization and function of synucleins may also have a bearing on PD, as synapses are lost early in disease progression (11).
Analysis of α-, β-, and γ-synuclein sequences reveals a shared, highly conserved N-terminal domain (∼80% identical) with a less conserved acidic C terminus (2). The N-terminal domain has seven imperfect repeats of 11 residues with the consensus sequence XKTKEGVXXXX that binds acidic phospholipid surfaces. Upon lipid binding, synucleins undergo a dramatic change to an α-helical conformation (12–14). α-Synuclein adopts either a conformation consisting of two anti-parallel, amphipathic α-helices with an unfolded C terminus (13–15) or a single extended α-helical structure (16, 17). Human β- and γ-synuclein also adopt the two-helix conformation upon folding (18, 19). Together, the high sequence homology, structural conservation, and overlapping expression pattern of synucleins strongly suggest redundant synaptic functions.
To determine the physiological functions of synucleins, we generated synuclein null mice lacking α-, β-, and γ-synuclein. Using these mice, we show that synuclein deficiency leads to altered synapse structure and physiology, age-dependent neuronal dysfunction, and impaired survival. Our data suggest that the normal functions of synucleins influence the functional decline of the aging nervous system and impact the development of PD.
Results
Generation of Synuclein Null Mice.
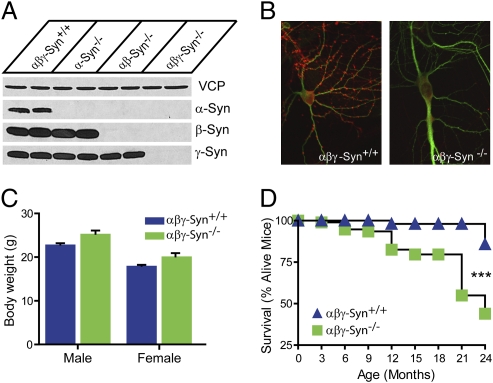
αβγ-Synuclein triple KO mice lack all murine synucleins and allow us to examine loss-of-synuclein phenotypes while avoiding complications of redundancy and compensation that have limited previous KO analyses of synucleins (5, 20–22). Synuclein null mice were generated by breeding αβ-synuclein double KO (21) to previously generated γ-synuclein KO mice (22). We maintain synuclein null mice as a congenic C57BL6/J homozygous KO line. Western blot and immunohistochemical analysis confirmed the deletion of synucleins (Fig. 1 A and B and Fig. S1D). Synuclein null mice are viable, fertile, and grossly normal with the expected body weight at 3 mo (Fig. 1C). Notably, deletion of synucleins results in a striking age-dependent survival deficit (Fig. 1D). Kaplan-Meier analysis of synuclein null mice revealed an increased mortality, with 12% dying by 12 mo as compared with <1% for C57BL6/J mice (23, 24) (The Jackson Laboratory, Mouse Phenome Project), demonstrating the importance of synucleins for long-term survival.
Fig. 1.
Generation of synuclein null mice. (A) Western blots of spinal homogenates from wild-type, α-, αβ- and αβγ-synuclein KO mice using isoform-specific antibodies. Valosin-containing protein (VCP) is a loading control. Samples are loaded in duplicate. (B) Immunostaining of cultured wild-type and synuclein null hippocampal neurons for α-synuclein (red) and the dendrite marker MAP2 (green). (C) Body weight of wild-type (αβγ-Syn+/+ blue bar) and synuclein null (αβγ-Syn−/− green bar) mice at 8 wk of age. (D) Survival curves for wild-type (blue triangle) and synuclein null mice (green square). ***P < 0.001.
Brain Architecture of Synuclein Null Mice.
We next performed a morphological analysis of αβγ-synuclein KO brains. As these mice show a late-onset survival deficit, we analyzed mice at three ages—3, 12, and 24 mo. We examined the brains of wild-type and αβγ-synuclein KO mice after Nissl staining. The overall brain architecture of synuclein null mice is normal, even at 24 mo of age (Fig. S2). To determine whether subtle neuronal loss occurs, we stained sections with the neuronal nuclei marker NeuN and quantified neuronal density in the CA3 and CA1 subfields of the hippocampus. Again, we observed no difference between synuclein null mice and age-matched controls at 3, 12, and 24 mo (Fig. S3 A–C). We also stained young and aged synuclein null brains for tyrosine hydroxylase, a marker for dopaminergic neurons, and saw a mild decrement in staining with age (Fig. S3 E and F). Finally, we stained wild-type and synuclein null brain sections for glial fibrillary acidic protein and activated caspase 3, markers for gliosis and apoptosis, respectively. We saw no evidence for gliosis (Fig. S3D) or apoptosis in synuclein null mice. Overall, these data strongly suggest that deletion of synucleins does not alter brain architecture or cause neuronal loss. These findings are consistent with the fact that aging in itself is not associated with any significant neuronal loss (25, 26).
Synapse Density and Structure in Synuclein Null Brains.
Previous immunoelectron microscopic analysis showed that synucleins are localized to the presynaptic terminal (1). We confirmed this synaptic localization by performing immunocytochemistry on wild-type and synuclein null neuronal cultures (Fig. 1B). A fraction of α-synuclein is also localized to axons. We crossed αβγ-synuclein triple KOs to Thy-1 driven mouse or human wild-type α-synuclein transgenic mice and determined the localization of α-synuclein (Fig. S1 and ref. 27). Transgenically expressed α-synuclein is also colocalized with presynaptic markers (Fig. S1 B and C).
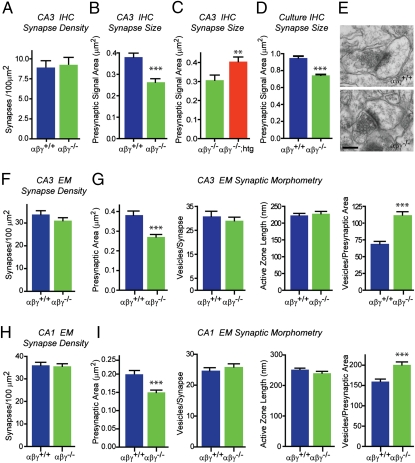
Synucleins have been implicated in both synapse structure and maintenance, but the precise role they play is not known (20, 27). To address this in our triple KO mice, we evaluated synapse density in the hippocampus as a function of age. First, we confirmed that synucleins are expressed in the synaptic CA3 and CA1 layers of the hippocampus (Fig. S1D). Then, we quantified synapse density in the CA3 region of young (3 mo) wild-type and synuclein null mice by quantitative immunohistochemistry using presynaptic markers (Fig. S1E) as well as electron microscopy (Fig. 2E). Both methods revealed that synapse density in young synuclein null mice was similar to wild-type controls (Fig. 2 A and F, and Fig. S4 A and B). Older synuclein null mice also had normal synapse density at 12 and 24 mo (Fig. S4 A and B), even though synuclein null mice show a survival deficit at 12 mo. Expanding our analysis, we determined excitatory synapse density in the CA1 stratum radiatum of 3- and 12-mo-old control and synuclein null mice by electron microscopy (Fig. 2H and Fig. S4C). The CA1 excitatory synapse density in 3-mo-old mice was 36 ± 2 synapses/100 μm2 (wild-type) and 36 ± 1 synapses/100 μm2 (synuclein nulls), identical to previously published data (28, 29). In addition, we found no decrease in synapse density in 12-mo-old synuclein null mice (Fig. S4C; 12 mo αβγ-Syn+/+ = 38 ± 2 versus αβγ-Syn−/− = 40 ± 2). These results suggest that synucleins do not contribute significantly to controlling synapse number, but may be important for maintaining synapse function.
Fig. 2.
Morphometric analysis of synuclein null synapses. (A) Synapse density in CA3 stratum lucidum region of 3-mo-old wild-type αβγ+/+ (blue bar) and synuclein null αβγ−/− mice (green bar) as determined by immunohistochemistry (IHC) with the presynaptic marker synapsin. (B) Presynaptic bouton area of CA3 synapses in wild-type αβγ+/+ and synuclein nulls αβγ−/−. (C) Presynaptic bouton area of CA3 synapses of synuclein nulls αβγ−/− and rescued littermates expressing human wild-type α-synuclein [αβγ−/−; human transgene (htg), red bar]. (D) Presynaptic area of synapses in dissociated hippocampal cultures from wild-type αβγ+/+ and synuclein nulls αβγ−/− mice. (E–I) Analysis of excitatory synapses by electron microscopy (EM). (E) Representative electron micrographs of CA1 synapses from αβγ+/+ and αβγ−/− brains (Scale bar, 250 nm.) (F) Excitatory synapse density of CA3 synapses in wild-type and synuclein null brains. (G) Ultrastructural analysis of CA3 synapses of 3-mo-old wild-type and synuclein null mice. (H) Synapse density in CA1 stratum radiatum of wild-type and synuclein null brains. (I) Ultrastructural analysis of CA1 synapses of wild-type and synuclein null mice. Student's t test. **P < 0.001, ***P < 0.0001.
To determine whether deletion of synucleins alters the structure of presynaptic termini, we examined synapse size and ultrastructure as a function of age. We first examined CA3 hippocampal synapses of 3-mo-old wild-type and synuclein null mice by immunohistochemistry using presynaptic markers (Fig. S1E). Interestingly, synaptic puncta are ∼30% smaller in synuclein null brains compared with age-matched wild-type controls (αβγ-Syn+/+ = 0.38 ± 0.02 μm2 versus αβγ-Syn−/− = 0.26 ± 0.02 μm2; Fig. 2B). We then performed this analysis on 12- and 24-mo-old mice and determined that the size reduction of CA3 termini in synuclein null mice persists with age (Fig. S4D). Next, we established that this decrease was indeed due to abrogation of synuclein function by evaluating synaptic puncta size in synuclein null mice that express a human wild-type α-synuclein transgene, i.e., “rescued” mice (Fig. S1 A–C). Overexpression of α-synuclein alone was sufficient to rescue the deficit in terminal size of synuclein null neurons (Fig. 2C and Fig S1F), confirming that synapse size is synuclein dependent and that synucleins are functionally redundant. Lastly, we determined that dissociated hippocampal neurons of synuclein null mice also have smaller synapses (Fig. 2D).
We extended our confocal imaging results by quantitative ultrastructural analysis of CA3 synapses (Fig. 2 E–G). Consistent with our light microscopic analysis, the presynaptic terminal area of CA3 excitatory synapses is diminished by 28% in synuclein null mice (Fig. 2G; αβγ-Syn+/+ = 0.38 ± 0.02 μm2; αβγ-Syn−/− = 0.27 ± 0.012 μm2). This change in area was not accompanied by other alterations in synaptic ultrastructure, such as active zone length or number of synaptic vesicles (Fig. 2G). Due to the decrease in terminal size, the packing density of synaptic vesicles was increased (Fig. 2G, rightmost graph). To explore whether these synaptic changes were pervasive in synuclein null brains, we examined the ultrastructure of Schaffer collateral excitatory synapses in the CA1 subfield of the hippocampus (Fig. 2I). Again, we observed a decrease in the presynaptic terminal area (αβγ-Syn+/+ = 0.203 ± 0.01 μm2; αβγ-Syn−/− = 0.153 ± 0.01 μm2), with all other presynaptic ultrastructural parameters unchanged (Fig. 2I). These in vivo findings strongly suggest that smaller excitatory synapses are seen throughout the brain of synuclein null mice.
Electrophysiological Properties of Synuclein Null Synapses.
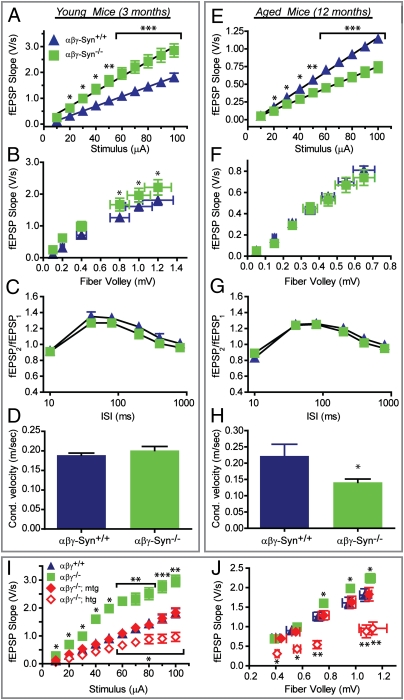
Because deletion of synucleins has a dramatic effect on synapse structure, we next examined the electrophysiological properties of these synapses. We performed hippocampal slice electrophysiology experiments on synuclein null mice at 3 and 12 mo of age. The hippocampus was selected due to the abundant expression of synucleins in the synaptic layers (Fig. S1D) and its well-characterized electrophysiology. Additionally, the effect of aging on neurotransmission has been well documented, with amplitude of the field excitatory postsynaptic potentials (fEPSPs) decreasing with age (25). fEPSPs were used to assess basal synaptic transmission at the Schaffer collateral–CA1 synapses. Young synuclein null mice show a larger input (stimulus strength)–output (fEPSP slope) relationship (I/O) compared with age-matched wild-type controls (Fig. 3A; slope for αβγ-Syn+/+ = 0.0188; slope for αβγ-Syn−/− = 0.0289). In sharp contrast to young mice, aged synuclein null mice show diminished I/O compared with wild-type controls (Fig. 3E; αβγ-Syn+/+ slope = 0.0122; αβγ-Syn−/− slope = 0.00773). When the I/O was plotted as a function of fiber volley amplitude, which represents the action potential firing of the Schaffer collaterals, younger synuclein null mice still showed larger I/O (Fig. 3B), whereas there was no difference between the two genotypes in older animals (Fig. 3F). These results raise the possibility that in young synuclein null mice, transmitter release could be increased, whereas in older animals this enhancement is diminished or compounded by changes in action potential conduction and/or axonal excitability.
Fig. 3.
Hippocampal physiology of young and aged synuclein null mice. (A and E) I/O curves of Schaffer collateral synapses in 3 mo (A, αβγ-Syn+/+, blue triangle; αβγ-Syn−/−, green square) and 12-mo-old mice (E). (B and F) Data in A and E plotted as a function of fiber volley. (C and G) Paired-pulse facilitation in Schaffer collateral synapses in the hippocampus of 3- (C) and 12-mo-old mice (G). (D and H) Action potential conduction velocity of Schaffer collaterals in 3- (D) and 12-mo-old mice (H). (I and J) I/O curves of Schaffer collateral synapses as a function of stimulus intensity (I) and fiber volley (J) in 3-mo-old mice. [Wild-type αβγ-Syn+/+, blue triangle; synuclein null αβγ-Syn−/−, green square; synuclein null rescued by mouse α-synuclein transgene, αβγ-Syn−/−; mtg, red filled diamond; synuclein null rescued by human α-synuclein transgene αβγ-Syn−/−; htg, red unfilled diamond.] Student's t test *P < 0.05, **P < 0.001, ***P < 0.0001.
To test the above premise, we repeated these slice recordings in 3-mo-old synuclein nulls and littermates that express a mouse or human wild-type α-synuclein transgene. As seen in Fig. 3 I and J, α-synuclein expression can completely restore the synuclein null I/O curve to that of wild-type control, confirming that deletion of synucleins improves basic synaptic transmission in young mice. Intriguingly, elevated expression of the human α-synuclein transgene diminishes the I/O ratio below that of wild type (Fig. 3 I and J), suggesting overexpression of α-synuclein produces a gain of its normal function and decreases input–output ratios similar to aging (Fig. 3 E and I). Since multiplications of the human α-synuclein gene cause PD (7), alterations in the normal activity of α-synuclein may play a role in PD.
To determine whether the altered I/O curves in the synuclein null mice are due to a change in the probability of neurotransmitter release, we measured paired-pulse facilitation, which is inversely correlated with release probability. The paired-pulse ratio remains unchanged in young, old, and rescued synuclein null mice (Fig. 3 C and G and Fig. S1G), suggesting that alterations in release probability are not contributing to changes in I/O curves.
Are the altered I/O curves in synuclein null mice instead due to changes in axonal function? To assess this question, we measured action potential conduction velocity at Schaffer collaterals. Interestingly, 12-mo-, but not 3-mo-old, synuclein null mice show slower action potential conduction velocity (Fig. 3 D and H and Fig. S1H). Consistent with a decrease in axonal excitability, these aged mice exhibit impaired fiber volley amplitudes in response to repetitive stimulation (Fig. S5). The reduced conduction velocity could explain in part the decreased I/O seen in aged synuclein null mice (Fig. 3E). To find a structural correlate for decreased conduction velocity in the aged synuclein null mice, we measured axon diameter and myelin thickness of Schaffer collaterals. A comparison of young wild-type and synuclein null sections found no significant differences in either axon diameter or myelin thickness (Fig. S6, Left). However, aged synuclein null mice show an increase in axon diameter (αβγ-Syn+/+ 1083 ± 27 versus αβγ-Syn−/−1224 ± 30 nm) accompanied by a decrease in myelin thickness (αβγ-Syn+/+ 130 ± 3 versus αβγ-Syn−/− 120 ± 3 nm) compared with age-matched controls (Fig. S6 A and B, Right). From these measurements, we calculated the ratio g [axon diameter: (axon diameter + myelin thickness)]. Theoretical modeling and experimental data suggest that increases in g (g > 0.6) are associated with a decrease in conduction velocity (30). Consistent with a decreased conduction velocity, aged synuclein null mice exhibit higher g values than their wild-type controls (Fig. S6C). Hence, structural alterations of axons may contribute to the decrease in action potential conduction observed in aged synuclein null mice.
Age-Dependent Retinal Dysfunction in Synuclein Null Mice.
How general are the roles of synucleins in the nervous system? To determine whether deletion of synuclein leads to age-dependent neuronal dysfunction in another brain region, we characterized retinal responses. The advantages of the retina are its accessibility, well-documented degenerative phenotypes, and role in PD. In addition, all synuclein isoforms are abundantly expressed in the retinal layers. We assessed retinal function by recording electroretinograms (ERG) (31) from 3- and 12-mo-old wild-type and synuclein null mice. In particular, we measured scotopic a-wave, b-wave, and oscillatory potential amplitudes as a function of increasing light intensity. The a-wave largely represents activation of photoreceptors, the b-wave reports the synaptic activity of rod bipolar cells and ON cone bipolar cells in the outer plexiform layer (OPL), and the oscillatory potentials monitor inner retinal activity. At 3 mo, synuclein null mice show no deficits in ERG recordings (Fig. 4A; b-wave at highest light intensity αβγ-Syn+/+ = 700 ± 34; αβγ-Syn−/− = 747 ± 75). In contrast, at 12 mo, synuclein null mice show diminished responses to even the highest intensity of light, suggesting that they are visually impaired in an age-dependent manner. The amplitude of the b-wave was severely decreased (Fig. 4B; b-wave αβγ-Syn+/+ = 409 ± 46; αβγ-Syn−/− = 138 ± 23), strongly suggesting a decrement in neuronal function in aged synuclein null retina.
Fig. 4.
Age-dependent retinal dysfunction in synuclein null mice. ERG analysis of retinal function in wild-type αβγ-Syn+/+ (blue triangle) and synuclein null mice αβγ-Syn−/− mice (green square) in (A) 3 mo and (B) 12 mo. From the ERG traces, the amplitude of the a-wave, b-wave, and the oscillatory potential (OP) was measured (Top, Middle, and Bottom, respectively). (C) Visual placement behavioral analysis of young (2–3 mo) and old (12–18 mo) wild-type (αβγ-Syn+/+; blue bar) and synuclein null (αβγ-Syn−/−; green bar) mice. (D) Inverted grid test analysis of vision and strength. Student's t test *P < 0.05, **P < 0.001, ***P < 0.0001.
To assess whether the observed retinal dysfunction (Fig. 4 A and B) leads to blindness, we used two behavioral tests. The visual placing test scores the ability of a suspended mouse to see an approaching rod and reach for it (32). Young mice of both genotypes could perform this test with ease (Fig. 4C). However, older αβγ-synuclein KO mice were severely impaired in their performance, consistent with being blind (Fig. 4C). The inverted grid test is a behavioral paradigm that requires both vision and muscle strength, in which mice are placed on an elevated wire mesh and then overturned. Young synuclein null mice were able to carry out this task facilely (Fig. 4D). However, aged synuclein null mice performed poorly in this test and on average could grip the mesh for only 11 ± 2 s (Fig. 4D), compared with 84 ± 15 s for age-matched wild-type controls. Collectively, these data demonstrate that deletion of synucleins leads to age-dependent retinal dysfunction and blindness.
Next, we identified morphological correlates for the severely reduced ERGs in old synuclein null mice, we examined the retina of the same mice from which we recorded ERGs. Remarkably, the retinal architecture is unaltered in aged αβγ-synuclein KO mice (Fig. S7). Quantification of synapse density in the OPL and nuclei in the nuclear layers revealed no differences between genotypes at both 3 and 12 mo. Furthermore, electron microscopic analysis of wild-type and αβγ-synuclein KO retina at 12 mo showed normal synaptic ultrastructure (Fig. S8). Hence, neuronal dysfunction in synuclein null retina is not a result of widespread neurodegeneration or synapse loss. These results mirror our findings in the hippocampus of aged synuclein null mice.
Changes in Synaptic Composition in Synuclein Null Brains.
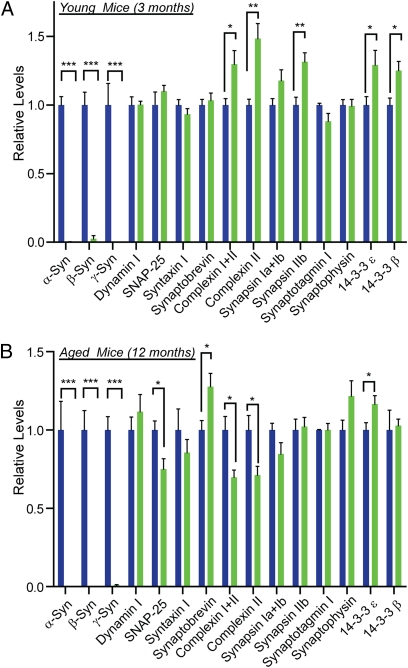
What molecular changes may underlie the phenotypes of synuclein loss? Previous findings have suggested that deletion of α-synuclein alters brain lipid metabolism (33). Therefore, we measured the levels of total brain lipid by HPLC, but found no significant changes (Fig. S9). As synucleins are abundantly expressed at synapses (Fig. 1B and Fig. S1D), we next measured the levels of candidate synaptic proteins in the brains of wild-type and synuclein null mice (Fig. 5). Intriguingly, young synuclein null mice exhibit increases in complexin II, synapsin IIb, and 14-3-3 β and ε isoforms (Fig. 5A and Table S1). This is consistent with the previously noted interaction between synucleins and synapsins (34) and 14-3-3 proteins (35). No changes in the other proteins analyzed were observed in young mice (Table S1). Upon aging we saw dramatic, yet select, changes in protein composition in the synuclein null mice. Most notably, the levels of proteins that were up-regulated in young mice were decreased to wild-type levels or even diminished (Fig. 5B). Aged synuclein null mice also show changes in the SNARE proteins SNAP-25 and synaptobrevin 2. These protein changes may contribute to the observed neuronal dysfunction in aged αβγ-synuclein KO mice.
Fig. 5.
Immunoblotting analysis of wild-type and synuclein null brains. Quantitation of synucleins and the indicated proteins in (A) 3-mo- and (B) 12-mo-old wild-type (blue bar) and αβγ-synuclein KO (green bar) brains. Student's t test *P < 0.05, **P < 0.001, ***P < 0.0001.
Discussion
This study demonstrates that synuclein null mice exhibit striking age-dependent neuronal dysfunction (Figs. 3 E and H and 4B) and decreased survival (Fig. 1D). Aged synuclein null mice show severe neuronal dysfunction in both regions of the CNS that we examined—the hippocampus and retina (Figs. 3 E and H and 4B). In the hippocampus, aged synuclein null mice have diminished I/O curves and conduction velocity compared with wild-type controls (Fig. 3 E and H). Searching for the causes of these pronounced phenotypes, we determined that they are not due to a loss of neurons (Figs. S2 and S3) or synapses (Fig. 2 A, F, and H and Fig. S4 A–C). Instead we see prominent age-dependent changes in synaptic protein composition (Fig. 5B) and axonal structure (Fig S6). Particularly, the “g” value (axon diameter:overall fiber diameter) for Schaffer collaterals is increased in aged synuclein null mice (Fig S6C) consistent with the observed decreases in conduction velocity and diminished excitability (Fig. 3H and Fig. S5). The reason why axonal morphology is altered in aged synuclein null mice is presently unclear but may reflect putative interactions of α-synuclein with microtubules (36, 37).
Deletion of synucleins has a dramatic effect on synapse structure and decreased synaptic terminal size (Fig. 2). This effect could be observed in both the CA1 and CA3 subfields of the hippocampus, as well as in dissociated hippocampal neurons. Interestingly, we found other synaptic parameters such as active zone length and number of synaptic vesicles to be unchanged. Smaller terminals indicate that synucleins possibly function in synaptic vesicle trafficking to control the flow of membrane either as positive regulators of exocytosis or as negative regulators of endocytosis. Earlier overexpression studies of wild-type α-synuclein largely support such roles (38–40). Future studies will aim to determine the exact contribution of synucleins to this phenotype to obtain molecular insight into their function.
In the hippocampus, young synuclein null mice have augmented electrophysiological responses (Fig. 3 A, B, I, and J), as seen in the I/O curves of CA1 synapses. Importantly, we could rescue this phenotype with both mouse and human α-synuclein transgenes, confirming that synucleins affect basal neurotransmission (Fig. 3 I and J). As phenotypes in young null mice are likely to reflect immediate effects of loss of synuclein, we investigated the basis of the enhanced I/O ratios. We determined that it was unlikely due to changes in release probability or conduction velocity (Fig. 3 C and D). However, we found that young synuclein null mice exhibit increases in key proteins that regulate synaptic vesicle exo- and endocytosis, including SNAREs, complexins, and synapsins (Fig. 5). Therefore, synucleins may participate in steps regulated by these proteins. In line with this possibility is our previous work showing that α-synuclein cooperates with the presynaptic chaperone CSPα to maintain neuronal integrity, possibly at a step regulated by SNAREs (27), and a new study showing that α-synuclein can regulate SNARE complex levels (41).
Presently, it is unclear whether increased α-synuclein levels cause PD exclusively by gain of a toxic function or additionally due to alterations of its physiological function. Our data supports the latter premise. Modest overexpression of human α-synuclein in young mice caused a decrement in neurotransmission similar to aged synuclein null mice (Fig. 3 E and I), indicating that overexpression disrupts the normal functions of α-synuclein. Intriguingly, a recent study characterizing human α-synuclein transgenics showed that overexpression of α-synuclein decreases I/O curves and synaptic vesicle density (40), consistent with our results. Therefore, increased synuclein levels, seen in patients with α-synuclein gene multiplications, are likely to impact synaptic transmission. Also noteworthy are the retinal deficits observed in PD patients, which again suggests a role for the normal function of synucleins in disease physiology (42). Together, our findings support that the levels of α-synuclein are a critical risk factor for PD.
In conclusion, our data suggest that the physiological function of α-synuclein is important for the long-term vigor of the nervous system and the development of PD.
Materials and Methods
Detailed methods and numbers are provided in SI Text.
Mice.
αβγ-Synuclein triple KO mice were maintained on a C57BL6/J background and compared with wild-type C57BL6/J mice purchased from The Jackson Laboratory. αβγ-Synuclein triple KOs were crossed to human or mouse wild-type α-synuclein–expressing transgenics (27) to generate synuclein null and rescued littermate mice.
Quantitative Immunohistochemistry.
Immunofluorescence of brain sections was performed as described (43). Blinded confocal images were quantified using ImageJ software.
Electron Microscopy.
Hippocampal sections from 3- or 12-mo-old male mice were analyzed on a Tecnai Biotwin 12 microscope. Electron micrographs were analyzed blind to genotype using iTEM software. Only asymmetric synapses were included for these analyses.
Hippocampal Electrophysiology.
Hippocampal slices from 3- and 12-mo-old mice were used for field recordings as described (23). Frequency-following experiments were performed as described (44, 45).
ERG Recordings.
ERG recordings were performed on anesthetized mice as described (31).
Behavior.
Behavioral tests for vision were performed with age-matched cohorts, blind to genotype, as described (27).
Quantitative Immunoblotting.
Protein levels were quantified from Western blots using a Li-COR Odyssey infrared imaging system.
Supplementary Material
Acknowledgments
We thank Wayne Fenton, Thomas Biederer, Mike Henderson, Chriss Westphal, and Yongquan Zhang for critical reading of the manuscript; Morven Graham and Christina Horensavitz for technical help with electron microscopy; and Yongquan Zhang and Amar Srivastava for light and electron microscopic studies. This work was supported by the American Parkinson's Disease Association (S.S.C.), the William N. and Bernice E. Bumpus Foundation (S.S.C.), an anonymous foundation (S.S.C.), National Institutes of Health (R01 NS064963 to S.S.C.), and the Wellcome Trust (V.L.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005005107/-/DCSupplemental.
References
- 1.Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:120–129. [PubMed] [Google Scholar]
- 2.Chandra S. Synucleins. In: Squire L, editor. The New Encyclopedia of Neuroscience. Oxford: Academic; 2009. pp. 833–837. [Google Scholar]
- 3.Iwai A, et al. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 4.Petersen K, Olesen OF, Mikkelsen JD. Developmental expression of alpha-synuclein in rat hippocampus and cerebral cortex. Neuroscience. 1999;91:651–659. doi: 10.1016/s0306-4522(98)00596-x. [DOI] [PubMed] [Google Scholar]
- 5.Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 6.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasser T. Molecular pathogenesis of Parkinson disease: Insights from genetic studies. Expert Rev Mol Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- 8.Satake W, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 9.Simón-Sánchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 11.Nikolaus S, Antke C, Müller HW. In vivo imaging of synaptic function in the central nervous system: I. Movement disorders and dementia. Behav Brain Res. 2009;204:1–31. doi: 10.1016/j.bbr.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 13.Chandra S, Chen X, Rizo J, Jahn R, Südhof TC. A broken alpha -helix in folded alpha -Synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 14.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 15.Borbat P, Ramlall TF, Freed JH, Eliezer D. Inter-helix distances in lysophospholipid micelle-bound alpha-synuclein from pulsed ESR measurements. J Am Chem Soc. 2006;128:10004–10005. doi: 10.1021/ja063122l. [DOI] [PubMed] [Google Scholar]
- 16.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci USA. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreon AC, Gambin Y, Lemke EA, Deniz AA. Interplay of alpha-synuclein binding and conformational switching probed by single-molecule fluorescence. Proc Natl Acad Sci USA. 2009;106:5645–5650. doi: 10.1073/pnas.0809232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivers RC, et al. Molecular determinants of the aggregation behavior of alpha- and beta-synuclein. Protein Sci. 2008;17:887–898. doi: 10.1110/ps.073181508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung YH, Eliezer D. Secondary structure and dynamics of micelle bound beta- and gamma-synuclein. Protein Sci. 2006;15:1162–1174. doi: 10.1110/ps.051803606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabin DE, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra S, et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ninkina N, et al. Neurons expressing the highest levels of gamma-synuclein are unaffected by targeted inactivation of the gene. Mol Cell Biol. 2003;23:8233–8245. doi: 10.1128/MCB.23.22.8233-8245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Rowlatt C, Chesterman FC, Sheriff MU. Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab Anim. 1976;10:419–442. doi: 10.1258/002367776780956917. [DOI] [PubMed] [Google Scholar]
- 25.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 26.Dickstein DL, et al. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. α-Synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Kovalenko T, et al. Ischemia-induced modifications in hippocampal CA1 stratum radiatum excitatory synapses. Hippocampus. 2006;16:814–825. doi: 10.1002/hipo.20211. [DOI] [PubMed] [Google Scholar]
- 29.Geinisman Y, et al. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol Aging. 2004;25:407–416. doi: 10.1016/j.neurobiolaging.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- 31.Vistamehr S, Tian N. Light deprivation suppresses the light response of inner retina in both young and adult mouse. Vis Neurosci. 2004;21:23–37. doi: 10.1017/s0952523804041033. [DOI] [PubMed] [Google Scholar]
- 32.Crawley JN. What's Wrong with My Mouse? 2nd Ed. New York: Wiley; 2007. pp. 86–110. [Google Scholar]
- 33.Barceló-Coblijn G, Golovko MY, Weinhofer I, Berger J, Murphy EJ. Brain neutral lipids mass is increased in alpha-synuclein gene-ablated mice. J Neurochem. 2007;101:132–141. doi: 10.1111/j.1471-4159.2006.04348.x. [DOI] [PubMed] [Google Scholar]
- 34.Woods WS, et al. Conformation-specific binding of alpha-synuclein to novel protein partners detected by phage display and NMR spectroscopy. J Biol Chem. 2007;282:34555–34567. doi: 10.1074/jbc.M705283200. [DOI] [PubMed] [Google Scholar]
- 35.Ostrerova N, et al. Alpha-synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alim MA, et al. Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein. J Alzheimers Dis. 2004;6:435–442. doi: 10.3233/jad-2004-6412. discussion 443–449. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Khoshaghideh F, Lee S, Lee SJ. Impairment of microtubule-dependent trafficking by overexpression of alpha-synuclein. Eur J Neurosci. 2006;24:3153–3162. doi: 10.1111/j.1460-9568.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 38.Larsen KE, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben Gedalya T, et al. Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic. 2009;10:218–234. doi: 10.1111/j.1600-0854.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nemani VM, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burré J, et al. α-Synuclein Promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diederich NJ, Raman R, Leurgans S, Goetz CG. Progressive worsening of spatial and chromatic processing deficits in Parkinson disease. Arch Neurol. 2002;59:1249–1252. doi: 10.1001/archneur.59.8.1249. [DOI] [PubMed] [Google Scholar]
- 43.Ho A, et al. Genetic analysis of Mint/X11 proteins: Essential presynaptic functions of a neuronal adaptor protein family. J Neurosci. 2006;26:13089–13101. doi: 10.1523/JNEUROSCI.2855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malenka RC, Kocsis JD, Ransom BR, Waxman SG. Modulation of parallel fiber excitability by postsynaptically mediated changes in extracellular potassium. Science. 1981;214:339–341. doi: 10.1126/science.7280695. [DOI] [PubMed] [Google Scholar]
- 45.Poolos NP, Mauk MD, Kocsis JD. Activity-evoked increases in extracellular potassium modulate presynaptic excitability in the CA1 region of the hippocampus. J Neurophysiol. 1987;58:404–416. doi: 10.1152/jn.1987.58.2.404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.