Abstract
Cellular Fcγ receptors are essential for IgG-dependent effector functions in vivo. There is convincing evidence that selective activating Fcγ receptors are responsible for the activity of individual IgG subclasses. Thus, IgG1 activity is absent in FcγRIII-deficient mice, and several studies suggest that the activity of the most potent IgG subclasses, IgG2a and IgG2b, might be dependent on either individual or a combination of activating FcγRs. To study the role of individual activating FcγRs for IgG subclass activity, we generated an FcγRIV-deficient mouse and showed that a variety of IgG2a- and IgG2b-dependent effector functions are impaired in the absence of this activating Fc receptor in models of autoimmunity and antibody-dependent cellular cytotoxicity.
Keywords: autoimmune disease, immunoglobulin G, inflammation
Cellular receptors that recognize the constant fragment of IgG molecules (Fcγ receptors, or FcγRs) are essential for IgG-dependent effector functions including cell activation, degranulation, phagocytosis, and antibody-dependent cellular cytotoxicity (ADCC) (1–3). Mice deficient in the common FcR γ-chain, but not in the central complement component C3, were resistant to IgG-induced inflammation in a variety of active and passive models of autoimmune disease and in therapeutic models of ADCC in vivo (3–5). These results were confirmed in primate models of IgG-dependent protection against HIV infection and in tumor patients showing enhanced therapeutic responses to anti-tumor antibodies if they carried alleles of the human low-affinity Fc receptors FcγRIIA (CD32A) and FcγRIIIA (CD16A), which showed enhanced binding to the therapeutic antibody (6–9). Further studies demonstrated that the different IgG subclasses selectively interact with certain activating FcγRs in vitro and in vivo. Thus, the activity of IgG1 was abrogated in mice deficient in activating FcγRIII, consistent with the selective binding to this receptor determined by surface plasmon resonance analysis in vitro (10–14). With respect to IgG2a and IgG2b, the most potent IgG subclasses, the picture was more complex because these subclasses can bind either to all three (IgG2a binds to FcγRI, III, and IV) or to two (IgG2b binds to FcγRIII and IV) activating FcγRs (15). Despite the capacity of FcγRI to bind to IgG2a with high affinity, the activity of this IgG subclass remained largely intact in FcγRI and FcγRI/FcγRIII double-deficient animals in many model systems, suggesting that the high-affinity FcγR participates in IgG2a-dependent reactions but does not dominate them (16, 17). Similar results were obtained for the role of FcγRIII for the activity of IgG2a and IgG2b, which remained intact in several model systems of autoimmune disease and ADCC (11–15). An explanation for these observations was afforded by the identification of the mouse activating FcγRIV, which can bind to IgG2a and IgG2b with intermediate affinity (15). On the basis of protein sequence, genomic localization, and functional studies, mouse FcγRIV seems to be the ortholog to human CD16A, although it has some unique features, such as the capacity to bind to IgE with low affinity, and varies in its cellular expression pattern compared to its human counterpart (14, 15, 18, 19). Using blocking antibodies, it was demonstrated that FcγRIV was essentially involved in IgG2a- and IgG2b-dependent killing of B cells, melanoma metastasis in the lung, and phagocytosis of platelets and red blood cells (11, 14, 15, 20–22). Moreover, blocking of this receptor protected animals from development of nephrotoxic nephritis and glomerular infiltration induced by IgG2a and IgG2b switch variants (23, 24). Interestingly, blocking FcγRIV also afforded protection in model systems where FcγRIII knockout animals showed abrogation of IgG2a or IgG2b activity (20, 22, 23). A similar observation was made in models of antibody-mediated killing of melanoma cells, where blocking FcγRIV interfered with the activity of the therapeutic IgG2a antibody most severely on an FcγRI knockout background (14, 21). Further studies in this direction are hampered by the fact that the available blocking antibody originates from hamster and becomes immunogenic after prolonged administration. Therefore, we generated an FcγRIV-deficient mouse on the C57BL/6 background and showed that IgG2a- and IgG2b-dependent effector functions are severely impaired in this strain in a variety of in vivo model systems. Thus, the FcγRIV-deficient mouse completes the set of mouse strains deficient for individual FcγRs and will be an important tool in delineating the role of the individual activating Fc receptors for the different IgG subclasses in vivo.
Results and Discussion
Generation of the FcγRIV Knockout Mouse.
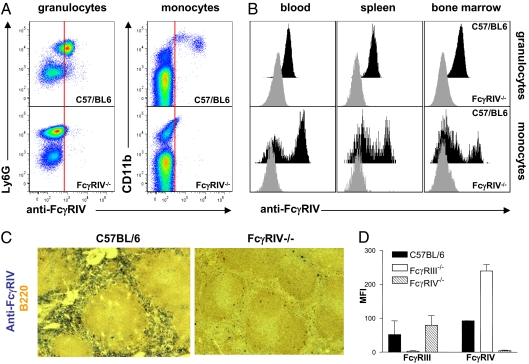
To generate an FcγRIV-deficient mouse, a targeting vector was constructed with loxP sites flanking the first three exons of the fcgr4 gene (Fig. S1). Upon homologous recombination in embryonic stem cells derived from C57BL/6 mice and generation of an FcγRIV-floxed mouse, ubiquitous deletion of the floxed fcgr4 gene was achieved by crossing this mouse to the CAG-cre mouse strain, which results in a ubiquitous deletion of the floxed first three exons of the fcgr4 gene. PCR was used to detect FcγRIV knockout animals (Fig. S1). During the steady state, FcγRIV is expressed mainly on neutrophils, monocytes, and macrophages (14, 15, 18, 19). To demonstrate deletion of FcγRIV on the protein level, we analyzed FcγRIV expression on neutrophils (expressing Ly6G) and monocytes (Ly6G negative, CD11b positive) in blood, spleen, and bone marrow. As shown in Fig. 1 A and B, FcγRIV expression was absent on these innate immune effector cells. Moreover, F4/80 positive macrophages in the red pulp of the spleen, which are highly positive for FcγRIV expression, were negative for this receptor in FcγRIV knockout animals, confirming the effective cre-mediated deletion (Fig. 1C).
Fig. 1.
Characterization of the FcγRIV knockout mouse. (A) Analysis of FcγRIV expression (anti-FcγRIV) on granulocytes (identified by Ly6G expression) and monocytes (identified by CD11b expression) in the blood. (B) Expression of FcγRIV on granulocytes and monocytes in the blood, spleen, and bone marrow of C57BL/6 and FcγRIV knockout mice. (C) Immunohistochemical analysis of FcγRIV expression on splenic macrophages in C57BL/6 and FcγRIV knockout animals. B-cells were identified by staining with B220. (D) The expression level of activating FcγRIII and FcγRIV in C57BL/6 (solid bars), FcγRIII (open bars), and FcγRIV (hatched bars) knockout mice on neutrophils in the blood.
Impact of FcγRIV Deletion on the Expression of Other Activating Fcγ Receptors.
In mice, the functionality of all activating Fcγ receptors is dependent on the fcer1g gene that codes for the common FcR γ-chain (2, 25). To investigate whether deletion of FcγRIV has an effect on the abundance of the other activating FcγRs, we studied the expression of these activating receptors on innate immune effector cell populations. As shown in Fig. 1D, deletion of FcγRIV resulted in a slight up-regulation of FcγRIII on neutrophils. To analyze whether this altered expression is also observed in other FcγR knockout mouse strains, we included FcγRIII-deficient animals in this analysis. Indeed, enhancement of FcγRIV expression was observed on neutrophils in FcγRIII-deficient animals (Fig. 1D). As FcγRIII and FcγRIV are coexpressed on neutrophils, monocytes, and macrophages, one possible explanation for this observation might be the competition of these two receptors for association with the common FcR γ-chain, which is essential for stable cell surface expression of the respective receptor α-chains. A similar observation was made in FcεRI knockout mice in which enhanced FcγRIII expression was observed on mast cells, resulting in an enhanced IgG-dependent degranulation via FcγRIII (26). Thus, when using knockout mice for single or multiple activating FcγRs, one should take into consideration that a partial loss of IgG subclass activity might be due to the compensatory up-regulation of the residual activating Fc receptor(s). In this scenario, the use of blocking antibodies might be an alternative or additional experiment to draw definitive conclusions.
Role of FcγRIV for the Activity of Anti-Tumor Antibodies.
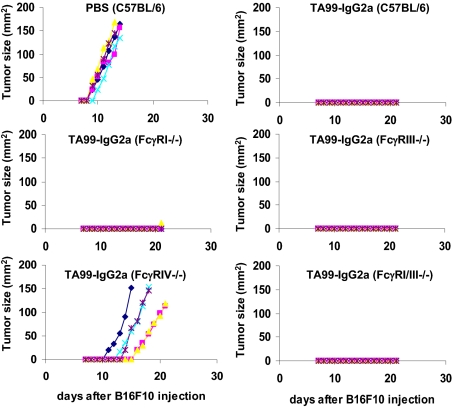
Several studies using blocking antibodies for FcγRIV indicated that this activating FcγR might play an important role for the activity of anti-tumor antibodies of the IgG2a and IgG2b subclass. Thus, in models of lung and liver metastases of melanoma cells and anti-CD20 antibody-dependent depletion of B cells, inhibition of FcγRIV activity resulted in the loss of the protective effects conferred by the therapeutic antibody (11, 14, 21). To gain further insight into the role of this receptor for the activity of anti-tumor antibodies of the IgG2a subclass, we turned to a s.c. melanoma model. To achieve local tumor growth, syngeneic B16F10 melanoma cells were injected subcutaneously into the flanks of C57BL/6 mice or mice deficient in various activating FcγRs (Fig. 2). The mice were then split into two groups receiving either PBS or the therapeutic anti-tumor antibody TA99-IgG2a, recognizing gp75 expressed on melanoma cells, and then followed for the development of tumors over the next 3 wk. As shown in Fig. 2, the TA99-IgG2a antibody lost its therapeutic activity selectively in FcγRIV-deficient animals. Neither the individual absence of FcγRI or FcγRIII nor the combined deletion of FcγRI and FcγRIII resulted in a loss of antibody activity. Thus, these findings further strengthen the results obtained by previous studies and point toward a major role for this receptor in antibody-dependent cellular cytotoxicity. This is also consistent with the important role of the human ortholog of FcγRIV, CD16A, for the activity of human IgG1 anti-tumor antibodies as determined by epidemiologic studies in which patients with the high-affinity alleles of this receptor showed an improved survival compared with patients with the low-affinity variants of CD16A (7–9).
Fig. 2.
Role of FcγRIV in IgG2a-mediated killing of s.c. melanoma cells. C57BL/6, FcγRI−/−, FcγRIII−/−, FcγRIV, and FcγRI/III−/− mice (n = 5) were injected subcutaneously with B16F10 melanoma cells followed by treatment with the therapeutic TA99-IgG2a antibody specific for mouse gp75 (TA99-IgG2a) or PBS as a control. The tumor size over time in the indicated mouse strains is graphed.
Involvement of FcγRIV in the Arthus Reaction and Passive Cutaneous Anaphylaxis.
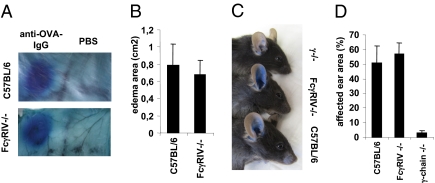
The Arthus reaction is initiated by crosslinking of activating FcγRs on mast cells by IgG immune complexes, which induces the release of vasoactive substances, resulting in edema formation and the subsequent recruitment of neutrophils and monocytes (3, 12, 25). As mast cells selectively express FcγRIII and not FcγRIV, the Arthus reaction should not be impaired in FcγRIV-deficient animals if mast cells are indeed the major cell type involved in this reaction (18). Consistent with this notion, the size and severity of edema formation was indistinguishable between wild-type and FcγRIV-deficient animals (Fig. 3 A and B). In contrast, mice lacking the common γ-chain were completely protected from IgG-dependent edema formation as observed previously (not shown) (25). These findings support earlier functional studies showing that the Arthus reaction is abrogated in FcγRIII-deficient animals (12, 15, 18, 27, 28). In addition to the capacity of FcγRIV to bind to IgG2a and IgG2b, it was shown that this activating receptor can also bind IgE with medium affinity (18, 19). Therefore, we performed an IgE-dependent passive cutaneous anaphylaxis (PCA) reaction by injection of IgE anti-dinitrophenyl (anti-DNP) antibodies into the ears of C57BL/6-, FcγRIV-, and γ-chain–deficient animals followed by systemic administration of DNP coupled to human serum albumin 12 h later. As shown in Fig. 3 C and D, edema development was normal in FcγRIV-deficient mice compared with the wild-type control. Consistent with previous results, no edema formation was observed in γ-chain–deficient mice (25). These results suggest that at least under these experimental conditions mast cells and FcεRI are essential for PCA and that IgE binding to FcγRIV expressed on neutrophils and monocytes is not essential for edema development. A further possible reason for these results may be the more than 100-fold lower affinity of FcγRIV compared with FcεRI for IgE. Further studies will be necessary to study the relevance of IgE binding to FcγRIV in vivo.
Fig. 3.
Role of FcγRIV in the Arthus and PCA reaction. C57BL/6 and FcγRIV knockout mice were injected intradermally with rabbit anti-OVA IgG or PBS followed by i.v. administration of ovalbumin in Evans blue. Shown is edema formation at the sites of IgG deposition (A) and the area (B) of dye influx into the skin in the indicated mouse strains. (C) The indicated mouse strains received an injection of mouse IgE anti-DNP intradermally into the ear followed by systemic administration of DNP-human serum albumin (HSA) in Evans blue 12 h later. Shown is the edema formation 60 min after DNP-HSA injection in one representative of four animals per group. (D) Quantification of edema formation in C57BL/6-, FcγRIV-, and FcR γ-chain–deficient mice by calculating the percentage of the ear area affected by the edema.
Involvement of FcγRIV in the Development of Rheumatoid Arthritis.
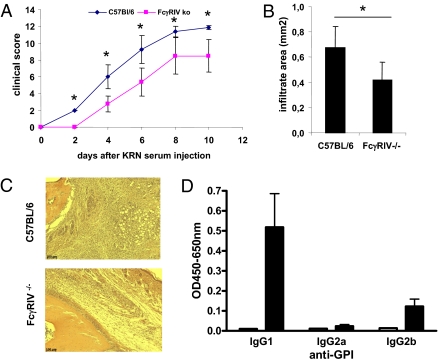
In addition to the therapeutic use of monoclonal antibodies in tumor therapy, antibodies reactive for self-proteins (autoantibodies) substantially contribute to the pathology that can be observed during autoimmune disease (4). Therefore, we investigated the role of FcγRIV in a model of rheumatoid arthritis. Rheumatoid arthritis was induced by transfer of serum from arthritogenic KBxN mice as described previously (29). As shown in Fig. 4 A–C, a significant reduction in clinical signs of arthritis with a reduced level of innate immune effector cell recruitment was observed in FcγRIV-deficient animals. This was surprising as previous studies demonstrated that arthritis development was largely driven by IgG1 antibodies specific for glucose 6-phosphate isomerase (anti-GPI antibodies) (29). As IgG1 cannot bind to FcγRIV, we investigated if other anti-GPI antibodies of other IgG subclasses are present in the arthritogenic serum obtained from KBxN mice (15). Indeed, autoantibodies of the IgG2b subclass, which can bind to FcγRIV with intermediate affinity, could be detected in the serum of these animals and contribute to tissue inflammation (Fig. 4D). These findings are in agreement with previous observations in this model system showing that the block of arthritis development is most severe in γ-chain–deficient animals lacking all activating FcγRs. In FcγRIII-deficient animals, however, a strong but incomplete block of tissue inflammation was observed (29). Thus, the FcγRIV knockout mouse revealed that even small amounts of IgG2b present in the serum transfer model of arthritis can substantially contribute to the clinical signs of arthritis via this activating FcγR. Notably, the presence of FcγRIII that can bind to IgG2b was not sufficient to induce the maximum level of inflammation. A possible explanation for this finding might be the saturation of FcγRIII-dependent cellular activation signals via binding to IgG1 immune complexes. In the presence of FcγRIII and FcγRIV, however, triggering of FcγRIII via IgG1 immune complexes and of FcγRIV via IgG2b immune complexes might allow a full activation of innate immune effector cells, resulting in a maximal level of tissue inflammation and recruitment of innate immune effector cells.
Fig. 4.
Role of FcγRIV in rheumatoid arthritis. (A) The development of clinical signs of arthritis in C57BL/6 and FcγRIV knockout mice upon injection of KBxN serum. (B) Measurement of the area of immigrated innate immune effector cells into the joints of C57BL/6 and FcγRIV knockout animals (n = 4). An asterisk in A and B indicates a significant difference with a p-value less than 0.05. (C) Histological analysis of inflamed joints of C57BL/6 and FcγRIV knockout animals. (D) Detection of IgG subclass anti-GPI autoantibodies in the serum of KBxN mice.
Involvement of FcγRIV in a Model of Nephrotoxic Nephritis.
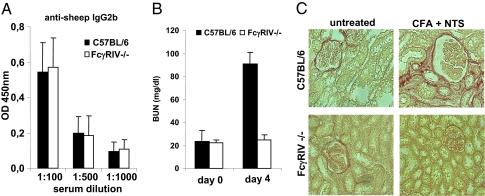
As a second model system, we used a model of IgG-dependent kidney inflammation. In this model, animals are preimmunized with sheep IgG before injection of nephrotoxic serum generated by immunizing sheep with preparations of mouse glomeruli. Upon transfer of this nephrotoxic serum into preimmunized animals, wild-type mice develop a rapid and often fatal glomerulonephritis characterized by elevated levels of blood urea nitrogen (24) (Fig. 5A). Kidney inflammation is critically dependent on the generation of mouse anti-sheep antibodies of the IgG2b subclass. As shown in Fig. 5A, FcγRIV mice immunized with sheep IgG showed a similar level of anti-sheep IgG2b response as wild-type animals. In contrast, FcγRIV-deficient animals were fully protected from IgG-dependent kidney inflammation and elevation of blood urea nitrogen levels (Fig. 5 B and C). These results are consistent with previous studies using FcγRIV-blocking antibodies and FcγRI, FcγRIII, or FcγRI/FcγRIII double-deficient mice, which showed no alteration in susceptibility to disease development.
Fig. 5.
Role of FcγRIV in a model of nephrotoxic nephritis. The indicated mouse strains were immunized with sheep IgG followed by injection of sheep nephrotoxic serum. (A) Detection of IgG2b mouse anti-sheep antibodies 2 wk after immunization of the indicated mouse strains with sheep IgG. (B) The blood urea nitrogen level (BUN) in C57BL/6- and FcγRIV-deficient mice before and 4 d after injection of nephrotoxic serum. (C) Detection of fibrotic tissue in the kidneys of C57BL/6- and FcγRIV-deficient mice 4 d after injection of nephrotoxic serum by staining with sirius red. In all experiments, four mice per group were used.
Taken together, our results clearly demonstrate the exclusive role of FcγRIV for the activity of IgG2a and IgG2b in models of ADCC and autoimmunity in vivo. In contrast, despite the capacity of FcγRIV to bind to mouse IgE in vitro, the FcγRIV knockout mouse did not show an impaired IgG-dependent Arthus or IgE-dependent passive cutaneous anaphylaxis reaction (Fig. 3). This might be most easily explained by the exclusive role of mast cells in these model systems that lack FcγRIV expression. Thus, the FcγRIV knockout mouse together with all of the other available single, double, and triple knockout mouse strains will be a valuable tool to further delineate the role of the individual FcγRs for the activity of the individual IgG subclasses in mice in vivo. With respect to the transferability of these results to the human system, it should be kept in mind that, despite a certain level of convergence such as the amino acid sequence homology between human CD16A and mouse FcγRIV, the capacity of both receptors to recognize IgG without fucose with a higher affinity and their important role for ADCC reactions, there are distinguishing features as well. Notably, mouse FcγRIV is not expressed on natural killer cells (which express FcγRIII), on which CD16A is expressed at high levels in humans. In addition, mouse FcγRIV but not human CD16A can recognize IgE (18, 19). The same applies for the ligands of FcγRs in mice and humans. Although both have four IgG subclasses, it is hard to define functional equivalents. Complicating the situation even further, several allelic variants of the low-affinity FcγRs, CD32A, and CD16A exist in humans and influence the outcome of antibody therapy. To further elucidate the role of these individual FcγRs for the activity of the different human IgG subclasses, humanized mice carrying a deletion of all mouse FcγRs and transgenes for the human FcγRs will be helpful. The feasibility of this approach is shown by the availability of mouse strains carrying individual human FcγRs (30–32).
Methods
Mice.
C57BL/6 and non obese diabetic (NOD) mice were purchased from the Jackson Laboratory. FcγRI−/− mice were provided by P. Hogarth (Burnet Institute, Melbourne) and backcrossed to the C57BL/6 background for 12 generations. KRN TCR transgenic mice on a C57BL/6 background (K/B) were gifts from D. Mathis and C. Benoist (Harvard Medical School, Boston, MA) and were bred to NOD mice to generate K/BxN mice. FcRγ−/− mice (γ−/−), FcγRIII−/−, and FcγRI/III−/− mice were generated in J. V. Ravetch's laboratory and backcrossed for 12 generations to the C57BL/6 background. The FcγRIV-deficient mouse strain was generated by targeted deletion of the first three exons of FcγRIV in C57BL/6 embryonic stem cells. For all experiments, female mice at 6–12 wk of age were used and maintained at the animal facilities of the University of Erlangen and The Rockefeller University. All experiments were done in compliance with federal laws and institutional guidelines.
Antibodies and Reagents.
TA99-IgG2a was produced by transient transfection of 293T cells followed by purification of the secreted antibody from the culture supernatant via protein G as described (14). Anti-biotin-alkaline phosphatase-coupled IgG, mouse IgE anti-DNP, DNP-HSA, ovalbumin, GPI, and Evans blue were purchased from Sigma. The polyclonal rabbit anti-ovalbumin IgG preparation was from Abcam. The FcγRIV specific antibody 9E9 was generated in J. V. Ravetch's laboratory and used as described before (15). B220-, Ly6G-, and CD11b-specific antibodies were from BD Biosciences, and the CD16-specific antibody was from R&D Systems. For immunohistochemistry, streptavidin-alkaline phosphatase (BD Biosciences) and FITC-specific antibodies coupled to HRP (DAKO) were used as detection reagents. To detect mouse serum IgG subclasses, HRP-labeled secondary antibodies (Bethyl) were used.
B16-F10 Tumor Model.
Experiments were performed essentially as described (14). Mice were injected with 5 × 104 B16-F10 tumor cells subcutaneously followed by treatment with PBS or i.p. application of 200 μg TA99-IgG2a on days 0, 2, 4, 7, 9, and 11. Starting from day 6–7, mice were analyzed for tumor growth. The tumor size was measured with an automated caliper, and mice were killed if the tumor size exceeded 150 mm2.
Passive Cutaneous Anaphylaxis and Arthus Reaction.
Passive cutaneous anaphylaxis was induced by intradermal injection of 20 ng of DNP-specific IgE into the ear, followed by i.v. injection of DNP-HSA in 1% Evans blue solution 12 h later as described before (25). The Arthus reaction was performed by intradermal injection of 30 μg of a polyclonal rabbit anti-ovalbumin (OVA) IgG preparation or PBS as a control followed by i.v. injection of 20 mg/kg ovalbumin in 1% Evans blue solution. Three hours later animals were killed, and the skin was prepared to evaluate edema formation as described (33).
Serum Transfer, Arthritis Scoring, and Histology.
Serum was prepared as described previously (29). Briefly, serum was separated from blood collected from the K/BxN mice (6–12 wk old). Serum collected over several weeks was pooled and frozen in aliquots. Arthritis was induced by one i.v. injection of 250 μl K/BxN serum. Arthritis was scored by clinical examination, and the index of all four paws was added: 0 (unaffected), 1 (swelling of one joint), 2 (swelling of more than one joint), and 3 (severe swelling of the entire paw). For histological examination, ankle joints were fixed in 10% formalin, decalcified for 48 h in Decal solution, and embedded in paraffin. Four- to 6-μm sections were stained with hematoxylin/eosin (Carl Roth) and evaluated by light microscopy using a Zeiss Axiovert 200M microscope and the AxioVision software.
Nephrotoxic Nephritis Model.
Nephrotoxic nephritis was induced as described before (24). Briefly, mice were preimmunized intraperitoneally with 200 μg of sheep IgG (AbD Serotec) in complete Freunds adjuvant, followed by i.v. injection of 2.5 μL sheep anti-glomerular basement membrane antiserum (generously provided by M. Madaio, Medical College of Georgia, Augusta, GA) per gram of mouse weight 14 d later. To evaluate kidney function, blood urea nitrogen levels were quantified 4 days after anti-glomerular basement membrane serum injection with an enzymatic urea nitrogen kit (Stanbio Laboratory). For histological analysis, kidneys were removed, fixed in 10% buffered formalin, and embedded in paraffin. Four-micrometer paraffin sections were stained with Sirius red to detect collagen deposits and evaluated by light microscopy. Circulating sheep IgG-specific mouse IgG2b antibodies were measured as described (24).
Statistical Analysis.
Statistical differences of clinical scores were calculated with Mann–Whitney's U test. All other statistical differences were determined with Student's t test. A P-value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We are grateful to Michael Madaio (Medical College of Augusta, Augusta, GA) and Peter Hogarth (Burnet Institute, Melbourne) for providing reagents and mice. This study was supported by the German Research Foundation Grants SFB 643 and SPP 1468 (to F.N.) and Grant DU548/2-1 (Emmy-Noether Program; to D.D.), the Bavarian Genome Research Network BayGene Grant (to D.D. and F.N.), the Bavarian Academy of Sciences (D.D.), and the National Institutes of Health (J.V.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014515107/-/DCSupplemental.
References
- 1.Hogarth PM. Fc receptors are major mediators of antibody based inflammation in autoimmunity. Curr Opin Immunol. 2002;14:798–802. doi: 10.1016/s0952-7915(02)00409-0. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. 2010;236:265–275. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 3.Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 4.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 5.Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- 6.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 7.Cartron G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 8.Weng WK, Czerwinski D, Timmerman J, Hsu FJ, Levy R. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22:4717–4724. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Fossati-Jimack L, et al. Markedly different pathogenicity of four immunoglobulin G isotype-switch variants of an antierythrocyte autoantibody is based on their capacity to interact in vivo with the low-affinity Fcgamma receptor III. J Exp Med. 2000;191:1293–1302. doi: 10.1084/jem.191.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med. 2006;203:743–753. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazenbos WL, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 13.Meyer D, et al. FcgammaRIII (CD16)-deficient mice show IgG isotype-dependent protection to experimental autoimmune hemolytic anemia. Blood. 1998;92:3997–4002. [PubMed] [Google Scholar]
- 14.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 15.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: A novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Barnes N, et al. FcgammaRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity. 2002;16:379–389. doi: 10.1016/s1074-7613(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 17.Ioan-Facsinay A, et al. FcgammaRI (CD64) contributes substantially to severity of arthritis, hypersensitivity responses, and protection from bacterial infection. Immunity. 2002;16:391–402. doi: 10.1016/s1074-7613(02)00294-7. [DOI] [PubMed] [Google Scholar]
- 18.Hirano M, et al. IgEb immune complexes activate macrophages through FcgammaRIV binding. Nat Immunol. 2007;8:762–771. doi: 10.1038/ni1477. [DOI] [PubMed] [Google Scholar]
- 19.Mancardi DA, et al. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J Clin Invest. 2008;118:3738–3750. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baudino L, et al. Differential contribution of three activating IgG Fc receptors (FcgammaRI, FcgammaRIII, and FcgammaRIV) to IgG2a- and IgG2b-induced autoimmune hemolytic anemia in mice. J Immunol. 2008;180:1948–1953. doi: 10.4049/jimmunol.180.3.1948. [DOI] [PubMed] [Google Scholar]
- 21.Otten MA, et al. Experimental antibody therapy of liver metastases reveals functional redundancy between Fc gammaRI and Fc gammaRIV. J Immunol. 2008;181:6829–6836. doi: 10.4049/jimmunol.181.10.6829. [DOI] [PubMed] [Google Scholar]
- 22.Syed SN, et al. Both FcgammaRIV and FcgammaRIII are essential receptors mediating type II and type III autoimmune responses via FcRgamma-LAT-dependent generation of C5a. Eur J Immunol. 2009;39:3343–3356. doi: 10.1002/eji.200939884. [DOI] [PubMed] [Google Scholar]
- 23.Giorgini A, et al. Fc gamma RIII and Fc gamma RIV are indispensable for acute glomerular inflammation induced by switch variant monoclonal antibodies. J Immunol. 2008;181:8745–8752. doi: 10.4049/jimmunol.181.12.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med. 2006;203:789–797. doi: 10.1084/jem.20051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 26.Dombrowicz D, et al. Absence of Fc epsilonRI alpha chain results in upregulation of Fc gammaRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between Fc epsilonRI and Fc gammaRIII for limiting amounts of FcR beta and gamma chains. J Clin Invest. 1997;99:915–925. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sylvestre D, et al. Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice. J Exp Med. 1996;184:2385–2392. doi: 10.1084/jem.184.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sylvestre DL, Ravetch JV. Fc receptors initiate the Arthus reaction: Redefining the inflammatory cascade. Science. 1994;265:1095–1098. doi: 10.1126/science.8066448. [DOI] [PubMed] [Google Scholar]
- 29.Ji H, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Wirthmueller U, Ravetch JV. Reconstitution of human Fc gamma RIII cell type specificity in transgenic mice. J Exp Med. 1996;183:1259–1263. doi: 10.1084/jem.183.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenzie SE, et al. The role of the human Fc receptor Fc gamma RIIA in the immune clearance of platelets: A transgenic mouse model. J Immunol. 1999;162:4311–4318. [PubMed] [Google Scholar]
- 32.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 33.Sylvestre DL, Ravetch JV. A dominant role for mast cell Fc receptors in the Arthus reaction. Immunity. 1996;5:387–390. doi: 10.1016/s1074-7613(00)80264-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.