Abstract
Increased endoplasmic reticulum (ER) stress is one of the central mechanisms that lead to dysregulated metabolic homeostasis in obesity. It is thus crucial to understand the underpinnings of the mechanisms that lead to the development of ER stress. A high level of ER Ca2+ is imperative for maintenance of normal ER function and this high Ca2+ concentration of ER is maintained by sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA). Here, we show that SERCA2b protein and mRNA levels are dramatically reduced in the liver of obese mice and restoration of SERCA2b in the liver of obese and diabetic mice alleviates ER stress, increases glucose tolerance, and significantly reduces the blood glucose levels. Furthermore, overexpression of SERCA2b in the liver of obese mice significantly reduces the lipogenic gene expression and the triglyceride content in the liver. Our results document the importance of SERCA2b in dysregulated glucose and lipid homeostasis in the liver of obese mice and suggest development of drugs to increase SERCA2b activity for treatment of type 2 diabetes and nonalcoholic steatohepatitis.
Keywords: obesity, type 2 diabetes, unfolded protein response, insulin resistance, calcium
Obesity is one of the greatest public health concerns (1) and it is a major underlying pathology for development of several serious medical conditions, such as type 2 diabetes, nonalcoholic steatohepatitis (NASH), and cardiovascular disease (2–4). Despite intense research efforts, the molecular links among obesity, type 2 diabetes, and NASH are not well understood.
The endoplasmic reticulum (ER) is a cellular organelle where secretory and membrane proteins are folded into their low-energy, 3D structures. In addition to protein folding, the ER also plays a central role in lipid and cholesterol biosynthesis (5–7). Conditions that interfere with the folding capacity of the ER, or that increase the folding demand beyond the levels that ER can cope with, create a condition known as ER stress, which in turn leads to an initiation of a complex signaling cascade called the unfolded protein response (UPR) (5–7). Three ER membrane proteins, PKR-like ER kinase (PERK), inositol-requiring enzyme-1 (IRE1), and activating transcription factor-6 (ATF6), are the main players in the initiation of the main signaling arms of the UPR (5–8).
In recent years, we and others have shown that increased ER stress is a key contributor to the development of glucose intolerance and insulin resistance in obesity (9–14). ER stress and activated UPR signaling pathways also create severe leptin resistance in the hypothalamus and play a significant role in the development of obesity (15). Considering the contribution of ER stress to obesity-related pathologic processes and the development of obesity itself, it is of crucial importance to understand the pathological mechanisms that create ER stress in obesity conditions. We also demonstrated that ER folding capacity is significantly reduced in obese mice, preventing the ER from responding properly to metabolic overload and resulting in activation of the UPR in the fed state (13). Moreover, the chaperone response is completely blunted in obese mice as a result of the inability of X-Box binding protein 1 (XBP1), a master regulator of ER folding capacity, to move to the nucleus as a result of the loss of interaction between XBP1 and p85α and p85β, the regulatory subunits of PI3K (13).
In addition to being the main organelle in which free calcium is stored, operations in ER lumen depend heavily upon intraluminal calcium concentrations (6, 16, 17): high calcium levels are critical for optimum activity of different enzymes and chaperones that have key roles in protein folding and ER homeostasis (6, 16, 17). Perturbations in ER-luminal Ca2+ inhibit chaperone function and agents that disturb calcium homeostasis in the ER create severe stress in this organelle. For example, thapsigargin, an inhibitor of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), blocks reuptake of Ca2+ from the cytoplasm and creates a chaotic environment in the lumen of the ER, leading to ER stress and subsequent initiation of the UPR (18). The same is true for Ca2+ ionophores, such as A23187, which is also known to disrupt ER Ca2+ homeostasis and create severe ER stress (5–7, 17).
SERCA is a Ca2+-transport ATPase, whose only known function is the reuptake of Ca2+ from the cytosol into the ER lumen. In mammals, three different SERCA genes (ATP2A1–3) lead to generation of three different isoforms (SERCA1–3), each of which has at least two further subisoforms. SERCA2 is by far the most widespread of all SERCA isoforms and has two well known subisoforms: SERCA2a and SERCA2b. The main isoform of SERCA2 in the liver is SERCA2b (16, 17).
In the current report, we demonstrate that SERCA2b protein and mRNA levels are dramatically reduced in the liver of ob/ob mice and increasing the levels of SERCA2b greatly reduces ER stress in the liver, increases glucose tolerance, and establishes euglycemia in severely obese and diabetic mice.
Results
SERCA2b Levels Are Dramatically Reduced in the Liver of Obese Mice.
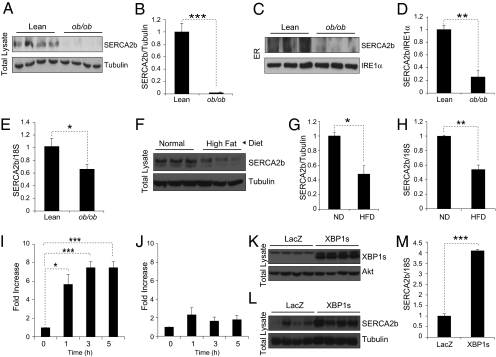
In light of the central role of Ca2+ in ER homeostasis and major contribution of SERCA2b in keeping ER calcium levels at optimally high concentrations, we investigated whether perturbations in the protein and gene expression levels of SERCA2b can be detected in obese states compared with lean conditions. For this purpose, we first determined the SERCA2b protein in whole liver lysates of lean and obese mice; we observed a significant (P < 0.001) reduction in protein levels in the liver of ob/ob mice relative to lean controls (Fig. 1 A and B). Next, we isolated ER fractions from the liver of lean and ob/ob mice and determined SERCA2b protein levels by immunoblotting. In parallel to the results obtained from whole liver lysates, SERCA2b protein levels were significantly (P < 0.01) reduced in the isolated liver ER fractions of the ob/ob mice compared with those of lean controls (Fig. 1 C and D). As a loading control, we analyzed the level of IRE1α that resides in ER-membrane (Fig. 1C). We also determined levels of SERCA2b mRNA levels in the liver of lean and ob/ob mice; SERCA2b mRNA was significantly (P < 0.05) down-regulated in the liver of ob/ob mice (Fig. 1E). Next, we analyzed the SERCA2b protein and mRNA levels in the liver of normal diet-fed lean and high-fat diet-fed obese mice. In parallel to the results obtained from ob/ob mice, protein and mRNA levels of SERCA2b were also markedly reduced in the liver of the diet-induced obesity model (Fig. 1 F–H).
Fig. 1.
SERCA2b protein and mRNA levels are decreased in the liver of obese mice. (A) SERCA2b and tubulin immunoblotting from the whole liver lysates of lean WT and ob/ob mice. (B) Quantification of the Western blot showing the ratio of SERCA2b to tubulin. (C) SERCA2b and IRE1α immunoblotting in the isolated liver ER fractions of WT and ob/ob mice. (D) Quantification of the blot in the ratio of SERCA2b to IRE1α. (E) mRNA levels of SERCA2b in the liver of WT and ob/ob mice. 18S was used as a control. (F) Western blot for SERCA2b and tubulin in the whole liver lysates of mice that were kept on normal or high fat diet for 16 wk. (G) Quantification of the blot showing the ratio of SERCA2b/tubulin. (H) Quantitative PCR for SERCA2b in the liver of normal diet and high-fat diet mice. (I) Fold increase of SERCA2b mRNA expression during 1, 3, and 5 h of refeeding after 24 h of fasting in lean WT mice. (J) Fold increase of SERCA2b mRNA expression during refeedings after 24 h of fasting in ob/ob mice. (K–M) Seven-week-old male ob/ob mice were injected with Ad-LacZ or Ad-XBP1s (4 × 108 pfu/g) through a tail vein. (K) Western blots for the levels of XBP1s and Akt as a control in the liver of adenovirus-injected ob/ob mice on day 7 after injection. (L) SERCA2b and tubulin levels in the liver of Ad-LacZ or Ad-XBP1s injected mice on day 7 after injection. (M) mRNA levels of SERCA2b in the liver of Ad-LacZ and Ad-XBP1s injected mice with 18S level as a control. Error bars are SEMs; P values were determined by Student t test (*P < 0.05, **P < 0.01, ***P < 0.001).
Regulation of SERCA2b Levels During Metabolic Overload Is Blunted in Obese Mice.
We have previously shown that components of ER machinery are up-regulated in the liver of WT mice during refeeding after a fasting period to cope with the increasing demand (13). In the mean time, this response was completely lost in the obese mice (13). To investigate whether SERCA2b, which is one of the most crucial elements for ER homeostasis in the liver, is also regulated during refeeding, we fasted 8-wk-old lean WT mice for 24 h and refed for 1, 3, and 5 h. SERCA2b levels in the liver of WT lean mice were significantly increased during refeeding (Fig. 1I). We next analyzed SERCA2b levels at 24 h fasting and after 1, 3, and 5 h of refeeding in the liver of ob/ob mice. Of interest, up-regulation of SERCA2b levels during refeeding was lost in the liver of the ob/ob mice (Fig. 1J). These results prompted us to test whether SERCA2b is regulated by XBP1s, as we have previously shown that XBP1s nuclear translocation is defective in obesity conditions and restoration of the ability of XBP1s to translocate to the nucleus has reinstated the chaperone response (13). For this purpose, we overexpressed XBP1s in the liver of ob/ob mice by tail vein injection of XBP1s-expressing adenovirus (Ad-XBP1s). LacZ-expressing adenovirus (Ad-LacZ) were used as a control. Injection of Ad-XBP1s into the tail vein of ob/ob mice led to a significant increase in the expression of XBP1s in the liver (Fig. 1K). Analysis of protein and mRNA levels of SERCA2b revealed a dramatic augmentation in the XBP1s-overexpressed livers, indicating that XBP1s is one of the regulators of SERCA2b in the liver (Fig. 1 L and M).
SERCA2b Increases ER Folding Capacity.
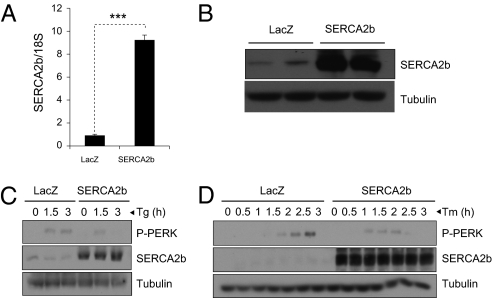
It is very well known that a reduction in SERCA function leads to development of ER stress (6, 16, 17). We thus sought to investigate whether an increase in SERCA function leads to a corresponding up-regulation of ER folding capacity and makes the ER more resistant to various stress-causing stimuli. To test this, we created an adenovirus that encodes mouse SERCA2b (Ad-SERCA2b). Infection of mouse embryonic fibroblasts (MEFs) with Ad-SERCA2b increased levels of mRNA and protein for SERCA2b, relative to infection with LacZ-expressing adenovirus (Ad-LacZ) controls (Fig. 2 A and B). To explore whether overexpression of SERCA2b increases resistance of the MEFs to ER stress, we infected the cells with Ad-LacZ or Ad-SERCA2b; following overnight starvation in 0.5% FBS-containing medium, we stimulated the MEFs with DMSO, thapsigargin (0.5 nM), or tunicamycin (0.0625 μg/mL) for various amounts of time and analyzed phosphorylation of PERK as an indicator of ER stress. SERCA2b overexpression dramatically reduced the thapsigargin- (Fig. 2C) and tunicamycin-induced ER stress (Fig. 2D) as evinced by delayed PERK phosphorylation. We had expected that overexpression of SERCA2b would make the cells more resistant to thapsigargin-induced ER stress because this agent is a direct inhibitor of SERCA. However, the ability of SERCA2b to increase the resistance of the cells even to tunicamycin-induced ER stress indicated that SERCA2b can increase ER folding capacity, as tunicamycin creates ER stress by blocking N-glycosylation and increasing the amount of unfolded proteins in the ER lumen.
Fig. 2.
SERCA2b increases resistance of cells to ER stress. (A) qPCR analysis of the mRNA levels of SERCA2b in the Ad-LacZ and Ad-SERCA2b-infected cells. (B) SERCA2b immunoblotting in the whole lysates of MEFs infected with Ad-LacZ or Ad-SERCA2b. Tubulin was used as a control. (C) MEFs was infected with Ad-LacZ or Ad-SERCA2b for 12 h and treated with thapsigargin (0.5 nM) for 0, 1.5, and 3 h. Immunoblotting was performed for phospho-PERKThr980, SERCA2b, and tubulin levels. (D) Ad-LacZ or Ad-SERCA2b-infected MEFs were stimulated with tunicamycin (0.0625 μg/mL) for 0, 0.5, 1, 1.5, 2, 2.5, and 3 h, and total lysates were blotted against phospho-PERKThr980, SERCA2b, and tubulin.
Overexpression of SERCA2b in the Liver of Obese Mice Reduces ER Stress and Improves Glucose Homeostasis.
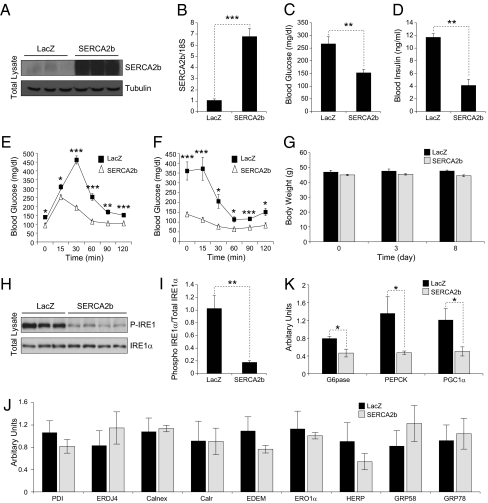
Considering the central role of ER stress in glucose homeostasis and the role of SERCA in keeping ER capacity intact and the observation that SERCA2b levels were dramatically reduced in the liver of ob/ob mice, we asked whether increasing the levels of SERCA2b in the liver of obese mice would have an influence on glucose homeostasis. For this purpose, 8-wk-old male ob/ob mice were injected with 5.8 × 107 pfu/g of Ad-SERCA2b or Ad-LacZ. Analysis of the expression levels for SERCA2b on postinjection day 8 confirmed the successful up-regulation of protein as well as mRNA in the liver of ob/ob mice (Fig. 3 A and 3B). Consistent with our hypothesis, overexpression of SERCA2b in the liver of ob/ob mice significantly lowered blood glucose levels as early as 3 d after injection (Fig. 3C) and analysis at 8 d after injection revealed a significant decrease in circulating insulin levels in the Ad-SERCA2b-injected ob/ob mice relative to the Ad-LacZ controls (Fig. 3D). We next performed a glucose tolerance test (GTT) on postinjection day 4 and showed that the hyperglycemic response to the i.p. glucose challenge was significantly reduced in SERCA2b-overexpressing ob/ob mice compared with the Ad-LacZ–injected group (Fig. 3E). Also, an insulin tolerance test (ITT) done on day 7 demonstrated that insulin-stimulated disposal of glucose was enhanced in the Ad-SERCA2b-injected ob/ob mice compared with control levels (Fig. 3F). The body weight of the Ad-LacZ– and Ad-SERCA2b–injected mice remained unchanged throughout the course of the experiment (Fig. 3G).
Fig. 3.
Overexpression of SERCA2b in the liver of ob/ob mice increases glucose tolerance and establishes euglycemia. (A–J) Eight-week-old male ob/ob mice were injected with Ad-LacZ or Ad-SERCA2b (5.8 × 107 pfu/g) through a tail vein. (A) SERCA2b immunoblotting in the liver of Ad-LacZ or Ad-SERCA2b-injected ob/ob mice. (B) mRNA levels of SERCA2b in the liver of ob/ob mice that were injected with Ad-LacZ or Ad-SERCA2b. (C) Blood glucose level (mg/dL) on day 3 after injection at 6 h of fasting. (D) Serum insulin level (ng/mL) on postinjection day 8. (E) GTT was performed with i.p. injection of 0.5 g/kg of glucose on day 4 after injections. (F) ITT was performed with i.p. injection of insulin (2 IU/kg) on postinjection day 7 after 6 h fasting. (G) Body weight (in g) at postinjection days 3 and 5. (H) Phospho-IRE1αSer724 and total IRE1 protein levels in the liver of Ad-LacZ and Ad-SERCA2b-injected ob/ob mice. (I) Quantification of phospho/total IRE1 ratio. (J) qPCR analysis of the mRNA levels of PDI, ERDJ4, calnexin, calreticulin, EDEM, ERO1α, HERP, GRP58, and GRP78. (K) Gene expression level of G6Pase, PEPCK, and PGC1α was analyzed by qPCR in the liver of Ad-LacZ or Ad-SERCA2b-injected ob/ob mice. Error bars are means SEM; P values were determined by Student t test (*P < 0.05, **P < 0.01, ***P < 0.001).
Our working hypothesis that up-regulation of SERCA2b function increases ER folding capacity by increasing the efficiency of chaperones led us to anticipate that, after overexpression of SERCA2b, ER stress in the liver of ob/ob mice would resolve without up-regulation of the chaperones. This was indeed the case, as shown in Fig. 3 H and I; IRE1αSer724 phosphorylation was significantly reduced, but no accompanying increase was noted in the mRNA levels for chaperones such as protein disulphide isomerase (PDI), ER-localized DnaJ homologue 4 (ERDJ4), calnexin, calreticulin (Calr), ER degradation enhancing α-mannosidase-like protein (EDEM), ER oxidoreductase (ERO1α), homocysteine-inducible ER stress protein (HERP), glucose-regulated protein, 58-kDa (GRP58), and GRP78. In fact, we observed a slight decrease in the mRNA levels of some of these chaperones (Fig. 3J). These results indicate that up-regulation of SERCA2b levels and consequent increased Ca2+ levels in the lumen of ER can establish the homeostasis with already available chaperones and reduce the activation of UPR. Furthermore, we demonstrated that genes that are involved in regulation of glucose homeostasis, such as glucose 6 phosphatase (G6PAse), phosphoenolpyruvate carboxykinase (PEPCK), and peroxisome proliferator-activated receptor γ coactivated-1 α (PGC1α), were significantly reduced in the SERCA2b-overexpressing ob/ob mice (Fig. 3K).
Overexpression of SERCA2b Reduces Steatohepatitis in Obese Mice.
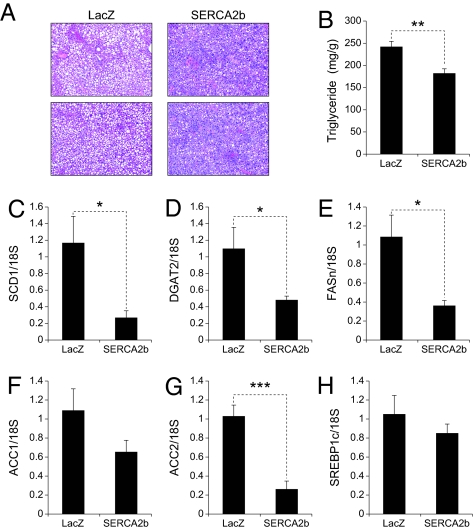
Next, to investigate whether overexpression of SERCA2b would reduce triglyceride (TG) levels in the liver of ob/ob mice, we examined H&E-stained sections taken from the liver of ob/ob mice that were injected with Ad-LacZ or Ad-SERCA2b. We noted a marked decrease in the amount of lipid droplets in the liver of SERCA2b overexpressing ob/ob mice compared with the controls (Fig. 4A). Evaluation of TG content in the liver of Ad-LacZ and Ad-SERCA2b-injected ob/ob mice revealed a significant reduction in TG levels (in mg/g) associated with the Ad-SERCA2b overexpression (Fig. 4B). Finally, the expression profile of lipogenic genes showed that mRNA levels for stearoyl-CoA desaturase-1 (SCD1), diacylglycerol acyltransferase 2 (DGAT2), fatty acid synthase (FASn), and acetyl co-A carboxylase 2 (ACC2) were dramatically reduced, without significant alterations in ACC1 and sterol regulatory element binding protein 1c (SREBP1c) levels (Fig. 4 C–H).
Fig. 4.
SERCA2b reduces lipogenic gene expression and TG levels in the liver of ob/ob mice. Eight-week-old male ob/ob mice were injected with Ad-LacZ or Ad-SERCA2b (5.8 × 107 pfu/g) through a tail vein. (A) Sections of the liver from Ad-LacZ or Ad-SERCA2b-injected ob/ob mice were stained with H&E. (B) TG content (mg/g) in the liver of Ad-LacZ or Ad-SERCA2b-injected ob/ob mice. mRNA levels of (C) SCD1, (D) DGAT2, (E) FASn, (F) ACC1, (G) ACC2, and (H) SREBP1c were quantified by qPCR. Error bars represent SEM; P values were determined by Student t test (*P < 0.05, **P < 0.01, ***P < 0.001).
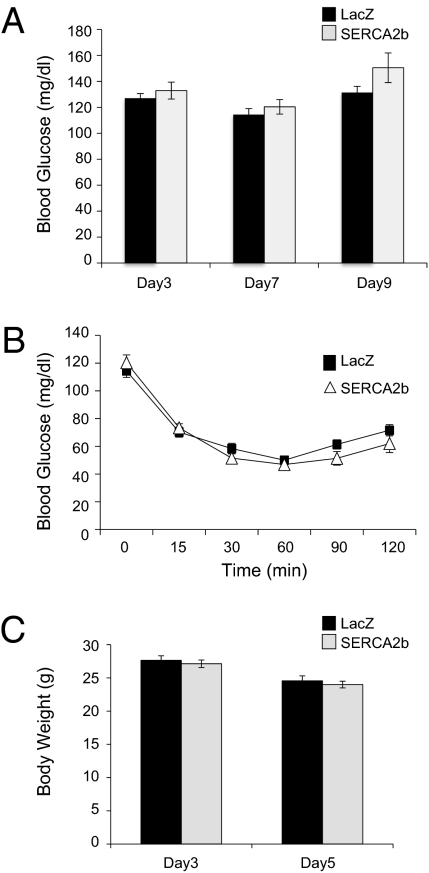
Overexpression of SERCA2b Does Not Alter Glucose Homeostasis in Lean Mice.
One of the remaining questions was whether overexpression of SERCA2b in lean and healthy mice could lead to development of hypoglycemia, which would be an obstacle in the approaches to activate SERCA2b as a therapeutic option for the treatment of type 2 diabetes. For this purpose, 8-wk-old male WT mice were injected with 5.8 × 107 pfu/g of Ad-SERCA2b or Ad-LacZ through a tail vein. Blood glucose levels were measured on postinjection days 3, 7, and 9 (Fig. 5A). No differences were observed in the blood glucose levels of the LacZ and SERCA2b-expressing WT mice. Performance of ITT on postinjection day 6 (Fig. 5B) demonstrated that insulin sensitivity was not altered. Body weight of both groups during the course of experiment displayed no difference (Fig. 5C).
Fig. 5.
Overexpression of SERCA2b in the liver of WT lean mice does not alter the glucose levels and insulin sensitivity. Eight-week-old male mice were injected with Ad-LacZ or Ad-SERCA2b (5.8 × 107 pfu/g) through a tail vein. (A) Blood glucose level at days 3, 7, and 9 after injection. (B) ITT was performed with i.p. injection of insulin (1 IU/kg) on postinjection day 6 after 6 h of fasting. (C) Body weight was measured on days 3 and 5 after injection.
Discussion
In 2005, the World Health Organization reported that 1.6 billion adults worldwide were overweight and 400 million were obese. Obesity is the leading cause of several debilitating diseases such as type 2 diabetes, NASH, cardiovascular disease, and stroke (2–4). In particular, type 2 diabetes and NASH are most prominently associated with obesity (1). The World Health Organization estimated that 171 million people worldwide had type 2 diabetes in 2000, and this number is projected to increase to 366 million by 2030 as a result of an uncontrolled increase in the incidence of obesity. To develop new and effective therapeutic agents to treat these diseases, it is of crucial importance to understand the pathological mechanisms that link obesity to type 2 diabetes and NASH.
During the past decade, we have witnessed the emerging role of ER stress in the pathology of several obesity-related diseases: increased ER stress in obesity is linked to glucose intolerance, insulin resistance, and ultimately to type 2 diabetes, and chemical reduction of ER stress greatly enhances metabolic homeostasis in severely obese and diabetic mice (10–12, 14, 15). These observations provide support for the view that the ER system is an attractive target for the treatment of obesity and related pathologic processes such as insulin resistance and type 2 diabetes. However, despite increasing efforts in this area of research, the pathogenesis of ER stress in obesity conditions is not well understood.
Our current work indicates that level of SERCA2b, which is one of the key players in the maintenance of ER homeostasis, is severely reduced in the liver of obese mice. Furthermore, we document that, when WT lean mice were challenged with refeeding after a fasting period, the liver SERCA2b level was significantly increased. These results indicate that SERCA2b is also dynamically regulated to enhance the efficiency of ER system to cope with demanding conditions. We have previously shown that obese mice cannot up-regulate the ER chaperones during refeeding compared to lean mice (13). In our current work, we document that SERCA2b up-regulation is also blunted in the liver of obese mice during refeeding. In addition to blunted chaperone response, lack of proper SERCA2b regulation during refeeding could be playing a role in the development of ER stress in obese mice. Indeed, we showed that restoration of SERCA2b abundance in the liver of ob/ob mice alleviates ER stress, significantly improves glucose tolerance, and establishes euglycemia.
One of the important questions that need to be answered is how SERCA2b is regulated in obesity conditions. Our results indicate that XBP1s is one of the factors that regulate SERCA2b expression; XBP1s overexpression in the liver of ob/ob mice significantly increased the levels of SERCA2b. Furthermore, reduced SERCA2b levels also correlate with loss of XBP1s activity in obesity conditions. However, further investigation is necessary to completely understand the regulatory factors for SERCA2b.
Our current results suggest an interesting possibility that ER calcium levels in the liver cannot be maintained at the desired high concentrations under obesity conditions because the “gatekeeper” for high ER calcium concentration, SERCA2b, is dramatically reduced in the liver and cannot be up-regulated during metabolic overload such as refeeding. Considering the “addictive” nature of ER chaperones for the high calcium level for their optimal activity, we suggest that loss of SERCA2b function/regulation and a consequent decrease in ER calcium levels in the liver reduce chaperone function and ER folding capacity. Indeed, when SERCA2b levels are increased in the liver of ob/ob mice, ER stress is reduced without a corresponding increase in the levels of chaperones: in fact, some chaperones are expressed at slightly lower levels.
In addition, overexpression of SERCA2b increases the resistance of MEFs to stimuli that increase the level of unfolded proteins in the ER lumen. For example, stimulation of SERCA2b-overexpressing MEFs with tunicamycin blocks initiation of the UPR. These data provide further evidence that ER function is enhanced by SERCA2b overexpression.
Our results also indicate that the ER calcium homeostasis is closely related with accumulation of TGs in the liver, as evidenced by the marked reduction in the liver TG content of the ob/ob mice that were injected with Ad-SERCA2b. It is interesting to note that there is also a marked decrease in most lipogenic genes after SERCA2b expression. It is possible that increased efficiency of chaperones after SERCA2b expression leads to retention of SREBP1c in the ER membrane and reduces nuclear abundance of this lipogenic transcription factor; it was previously shown that GRP78 interacts with SREBP1c in the ER membrane and decreases lipogenic gene expression by blocking its cleavage and nuclear translocation (19).
The findings we present here highlight that the level of SERCA2b protein in the liver are critically important in the maintenance of metabolic homeostasis in obese mice. We have previously reported that obese mice are unable to up-regulate the expression levels of chaperone during metabolic overloading (13). Taken together with our previous observations regarding the loss of chaperone response in obesity conditions, the current results led us to propose that the synergy between diminished chaperone response and reduction in ER Ca2+ levels may be the factors most critical in the development of ER stress in obesity conditions. Possible reduced Ca2+ levels in the ER caused by down-regulated SERCA2b levels and the resulting decrease in chaperone function provide further insight into how and why ER stress might develop in obesity.
Furthermore, overexpression of SERCA2b in the liver of euglycemic lean mice did not lead to hypoglycemia, indicating that activation of SERCA2b will be a safe approach that does not alter the glucose homeostasis. Hence, a significant outcome from the current findings could be development of therapeutic agents targeted at effectively enhancing SERCA2b activity, which would in turn decrease ER stress, enhance glucose tolerance, and reduce hepatosteatosis.
Materials and Methods
Biochemical Reagents.
SERCA2b (ATPA2/SERCA2), phospho-PERKThr980, Akt, and lamin A/C-specific antibodies were purchased from Cell Signaling Technology. XBP-1 and tubulin-specific antibodies were from Santa Cruz Biotechnology. Antibodies for phospho-IRE1αSer724 and total IRE1α were purchased from Novus Biologicals. DMEM, FBS, penicillin, and streptomycin were purchased from Gibco. Leupeptin, aproptonin, and PMSF were from Sigma-Aldrich. Nuclear extraction kit was purchased from Thermo Scientific. cDNA synthesis kit was from BioRad and SYBR quantitative PCR (qPCR) Supermix was from Fermentas. TRIzol reagent was from Invitrogen. Free glycerol reagent, triglycerol reagent, glycerol, and ER isolation kit were purchased from Sigma-Aldrich. Chemiluminescence substrates were from Roche. ELISA kit was from Crystal Chem. Tunicamycin and thapsigargin were from Calbiochem. High-fat diet (45 kcal%) was purchased from Research Diets.
Production of Adenovirus.
Mouse SERCA2b cDNA (GenBank ID no. AJ131821) was synthesized and cloned into pENTR3C vector to create pENTR3C-SERCA2b. pAd-SERCA2b was created from pENTR3C-SERCA2b and pAd/CMV/V5-DEST (Invitrogen) by homologous recombination using the manufacturer's gateway system protocol. pAd-SERCA2b was linearized by restriction endonuclease digestion with PacI and transfected to 293A cells. The media was replaced with new media every 3 d until the cytopathic effect was observed. When the cytopathic effect reached 80%, cells were collected and the virus was harvested by repeating freezing and thawing cycles at –80 °C and 37 °C four times. Viral supernatant was obtained by centrifuging at 4,000 × g for 20 min. Ad-XBP1s and Ad-LacZ were created in the same manner as previously reported (15).
Isolation of ER Fractions from Liver Tissue.
Fresh liver tissue (100 mg) was washed twice with PBS solution and then cut into small pieces. Isotonic extraction buffer from ER isolation kit (800 μL, 1×) was added. The sample was homogenized in a glass tube homogenizer and ER fractions were isolated according to the manufacturer's protocol. After ultracentrifuging to obtain ER fraction, the pellet was resuspended in 300 μL of 1× isotonic extraction buffer and homogenized in a glass tube homogenizer.
TG Measurements.
Liver tissue (100 mg) was homogenized in buffer containing 0.8 mL of H2O and 1.5 mL of chloroform/methanol (2:1 vol/vol) for 1 min. One milliliter of H2O and 0.5 mL of chloroform were added to homogenized sample and the tubes were vortexed, followed by centrifugation at 500 × g for 20 min at 4 °C. The lower chloroform phase was collected in a new Eppendorf tube and 500 μL of collected liquid was dried in a hood for overnight. The dried pellet was dissolved in 200 μL of isopropanol/Triton X-100 (90:10 vol/vol) and a small amount was further diluted 20 times in the same solution. Two microliters of diluted sample was added to 200 μL of free glycerol reagent in a 96-well plate. After 15 min of incubation, the absorbance at a wavelength of 490 nm was recorded for free glycerol content. TG reagent (40 μL) was added and incubated for 15 min. The total glycerol level was measured at a wavelength of 490 nm. Triglycerol level was calculated by subtracting free glycerol level from total glycerol. A standard curve was created with series of dilution of a known concentration of glycerol.
Insulin ELISA.
Blood was collected into a heparinized tube from the retroorbital space and centrifuged at 12,000 × g for 20 min at 4 °C. The serum was collected and diluted three times with sample diluent provided with an ultrasensitive mouse insulin ELISA kit. The insulin level in the serum was measured with a protocol provided by the manufacturer.
RNA Isolation and Real-Time qPCR.
RNA was extracted from the liver of mice by using TRIzol reagent according to the manufacturer's instructions. cDNA was synthesized from 1 μg of RNA using a cDNA synthesis kit with the following conditions: 25 °C for 10 min, 42 °C for 30 min, 85 °C for 5 min. qPCR was performed with SYBR Green qPCR Supermix from Invitrogen according to the manufacturer's instructions. The following primer sets were used for qPCR: 18S (forward), 5′-AGTCCCTGCCCTTTGTACACA-3′; 18S (reverse), 5′-CGATCCGAGGGCCTCACTA-3′; mSERCA2b (forward), 5′-ATGAGCAAGATGTTTGTGAAGG-3′; mSERCA2b (reverse), PDI (forward), 5′-CAAGATCAAGCCCCACCTGAT-3′; PDI (reverse), 5′-AGTTCGCCCCAACCAGTACTT-3′; ERDJ4 (forward), 5′-CCCCAGTGTCAAACTGTACCAG-3′; ERDJ4 (reverse), 5′AGCGTTTCCAATTTTCCATAAATT-3′; Calnexin (forward), 5′-ATGGAAGGGAAGTGGTTACTGT-3′; Calnexin (reverse), 5′-GCTTTGTAGGTGACCTTTGGAG-3′; Calreticulin (forward), 5′-CCTGCCATCTATTTCAAAGAGCA-3′; Calreticulin (reverse), 5′-GCATCTTGGCTTGTCTGCAA-3′; EDEM (forward), 5′-AAGCCCTCTGGAACTTGCG -3′; EDEM (reverse), 5′-AACCCAATGGCCTGTCTGG-3′; ERO1α (forward), 5′-TCAGTGGACCAAGCATGATGA-3′; ERO1α (reverse), 5′-TCCACATACTCAGCATCGGG3′; HERP (forward), 5′-CATGTACCTGCACCACGTCG-3′; HERP (reverse), 5′-GAGGACCACCATCATCCGG-3′; GRP58 (forward), 5′-GAGGCTTGCCCCTGAGTATG-3′; GRP58 (reverse), 5′-GTTGGCAGTGCAATCCACC-3′; GRP78 (forward), 5′-TCATCGGACGCACTT GGAA-3′; GRP78 (reverse), 5′-CAACCACCTTGAATGGCAAGA-3′; G6Pase (reverse), 5′-CAATGCCTGACAAGACTCCA-3′; PEPCK (forward), 5′-ATCATCTTTGGTGGCCGTAG-3′; PEPCK (reverse), 5′-ATCTTGCCCTTGTGTTCTGC-3′; PGC1α (forward), 5′-TGATGTGAATGACTTGGATACAGACA-3′; PGC1α (reverse), 5′-CAATGCCTGACAAGACTCCA-3′; SCD-1 (forward), 3′-AGATCTCCAGTTCTTACACGACCAC-3′; SCD-1 (reverse), 3′-GACGGATGTCTTCTTCCAGGTG-3′; DGAT2 (forward), 5′-TTCCTGGCATAAGGCCCTATT-3′; DGAT2 (reverse), 5′-AGTCTATGGTGTCTCGGTTGAC-3′; FASn (forward), 5′-GGAGGTGGTGATAGCCGGTAT-3′; FASn (reverse), 5′-TGGGTAATCCATAGAGCCCAG-3′; ACC1 (forward), 5′-ATTGGGCACCCCAGAGCT A-3′; ACC1 (reverse), 5′-CCCGCTCCTTCAACTTGCT-3′; ACC2 (forward), 5′-GGGCTCCCTGGATGACAA C-3′; ACC2 (reverse), 5′-TTCCGGGAGGAGTTCTGGA-3′; SREBP1c (forward), 5′-GCGGTTGGCACAGAGCTT-3′; and SREBP1c (reverse), 5′-GGACTTGCTCCTGCCATCAG-3′.
Total Protein Extraction from Tissue.
Liver tissues (100 mg) were homogenized with a bench-top homogenizer from Polytron (PT2100) in 3 mL of ice-cold tissue lysis buffer (25 mM Tris-HCl, pH 7.4, 10 mM Na3VO4, 100 mM NaF, 50 mM Na4P2O7, 10 mM EGTA, 10 mM EDTA, 1% Nonidet P-40, 10 mg/mL leupeptin, 10 g/mL aproptonin, 2 mM PMSF, and 20 nM okadaic acid) in 50-mL round-bottom tubes. The homogenized samples were centrifuged at 8,000 × g for 20 min at 4 °C. The lipid layer was removed and the supernatant was transferred into Eppendorf tubes. After centrifuging at 16,000 × g for 60 min at 4 °C, the supernatants were normalized to the same concentration and boiled at 100 °C in 1× Laemmli buffer for 5 min. The lysate was cooled to room temperature before loading for Western blot analysis.
Cell Culture.
MEFs were cultured in DMEM with 10% FBS, 10 U/mL penicillin, and 1 mg/mL streptomycin. Cells were maintained at 37 °C with 5% CO2.
Total Protein Extraction from MEFs.
MEFs were lysed in lysis buffer (25 mM Tris, pH 7.4, 2 mM NaVo4, 10 mM NaF, 10 mM Na4P2O7, 1 mM EGTA, 1 mM EDTA, 1% Nonidet P-40, 10 μg/mL leupeptin, 10 μg/mL aproptonin, 2 mM PMSF, and 20 nM okadaic acid). The concentrations of protein were normalized with the lysis buffer to have equivalent amounts of protein (200–400 μg) and volume (300 μL). After adding Laemmli buffer, protein was boiled at 100 °C for 5 min and kept at 25 °C for 15 min.
Western Blotting.
Protein lysate was resolved on SDS polyacrylamide gel and transferred onto PVDF membrane at a voltage of 100 V for 2 h at 4 °C. The membrane was blocked in 10% blocking reagent and incubated overnight with primary antibody in Tris-buffered saline solution/Tween (TBST)/10% blocking reagent at 4 °C. After the incubation, the membrane was washed three times in TBST for 20 min and incubated at room temperature with secondary antibody in TBST/10% blocking reagent for 1 h. The membrane was washed three times for 20 min and developed using a chemiluminescence assay system. To strip a membrane for another primary antibody, the membrane was agitated at 50 °C for 20 min in a box with stripping buffer (2% SDS and 100 mM 2-mercaptoethanol in TBS, pH 7.5). The membrane was washed three times for 20 min before blocking and an incubation with primary antibody.
Tail Vein Infection.
Adenovirus was thawed at 25 °C and diluted in saline solution to a final volume of 100 μL. Mice were restrained in a restrainer and adenovirus was injected into a tail vein with a 30-gauge needle. Mild pressure was applied to the spot of injection to prevent the backflow of virus.
Blood Glucose Level Measurement.
Blood was taken from mice by tail clipping and blood glucose level was measured with a portable glucose meter (Contour; Bayer).
GTT.
For GTT, mice were fasted overnight and 0.5 g/kg of d-glucose at a final volume of 100 μL was administrated intraperitoneally. The blood glucose level was measured 0, 15, 30, 60, 90, and 120 min after glucose administration through a tail vein with use of a portable glucose meter (Contour; Bayer).
ITT.
Mice were fasted for 6 h and 2.0 IU/kg of insulin for ob/ob mice and 1.0 IU/kg of insulin for WT lean mice at a final volume of 100 μL were administrated intraperitoneally. The blood glucose level was measured 0, 15, 30, 60, 90, and 120 min after insulin administration through a tail vein with a portable glucose meter (Contour; Bayer).
Adenovirus Transduction.
MEFs were plated at a density of 3 × 106 cells per 10-cm plate. The following day, the media were replaced with 3 mL of 1% FBS containing media and adenovirus was added. The dish was rotated every 15 min for 1 h and 7 mL of media with 1% FBS was added. Cells were incubated with adenovirus-containing media for 16 h.
High-Fat Diet Experiments.
C57BL/6 mice were started on high-fat diet feeding at the age of 3.5 wk, right after weaning. Following 16 wk of high-fat diet feeding, mice were starved for 4 h and the liver was extracted following establishment of anesthesia.
Statistical Analyses.
We used two-tailed Student t tests to determine P values for statistical significance. Error bars in the figures represent SEM.
Animal Experiments.
All animal experiments were approved by the institutional animal care and use committee at Children's Hospital Boston.
Acknowledgments
We thank the members of the U.O. laboratory for their support during the execution of the experiments and useful discussions. This study was supported by Junior Faculty Start Up Funds, National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health Grant R01DK081009 (to U.O.), and Timothy Murphy funds provided to the Division of Endocrinology, Children's Hospital Boston.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89:2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 2.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 3.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 4.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 6.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 7.Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 9.Bailly-Maitre B, et al. Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem. 2010;285:6198–6207. doi: 10.1074/jbc.M109.056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 11.Ozcan U, et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SW, et al. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozawa K, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–663. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 15.Ozcan L, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Ashby MC, Tepikin AV. ER calcium and the functions of intracellular organelles. Semin Cell Dev Biol. 2001;12:11–17. doi: 10.1006/scdb.2000.0212. [DOI] [PubMed] [Google Scholar]
- 17.Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: Cell biological implications. Cell Calcium. 2005;38:291–302. doi: 10.1016/j.ceca.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 18.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- 19.Kammoun HL, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]