Abstract
Light therapy can be an effective treatment for mood disorders, suggesting that light is able to affect mood state in the long term. As a first step to understand this effect, we hypothesized that light might also acutely influence emotion and tested whether short exposures to light modulate emotional brain responses. During functional magnetic resonance imaging, 17 healthy volunteers listened to emotional and neutral vocal stimuli while being exposed to alternating 40-s periods of blue or green ambient light. Blue (relative to green) light increased responses to emotional stimuli in the voice area of the temporal cortex and in the hippocampus. During emotional processing, the functional connectivity between the voice area, the amygdala, and the hypothalamus was selectively enhanced in the context of blue illumination, which shows that responses to emotional stimulation in the hypothalamus and amygdala are influenced by both the decoding of vocal information in the voice area and the spectral quality of ambient light. These results demonstrate the acute influence of light and its spectral quality on emotional brain processing and identify a unique network merging affective and ambient light information.
Keywords: melanopsin, emotion processing, hypothalamus, functional MRI
Light therapy is the treatment of choice for seasonal affective disorder (SAD) and is a promising treatment for other major affective disorders (1, 2), suggesting that light can modulate mood in the long term. To better understand this effect and because neural networks involved in emotional behavior have been implicated in mood disorders (3), we first assessed whether light can acutely influence normal brain emotional processing. Indeed, ambient light is known to regulate processes other than vision, such as hormone secretion, body temperature, and sleep, but also alertness and cognition (4–8). These nonclassical [also called “non–image-forming” or “nonvisual” response, but see recent findings (9)] responses to light are mediated through a nonclassical photoreception system, which is maximally sensitive to blue light (≈480 nm), as opposed to the classical photopic luminance visual pathways, maximally sensitive to green light (≈550 nm), and recruits the recently discovered intrinsically photosensitive retinal ganglion cells (ipRGC) expressing the photopigment melanopsin, in addition to rods and cones (7, 10–13).
The impact of ambient light is detected in the longer term through the regulation of circadian rhythms (4, 7), and the benefit of light therapy on mood has been proposed to be mediated through a long-term circadian effect (14). However, nonclassical responses to ambient light also result in acute physiological changes. For example, ambient light significantly modulates ongoing cognitive brain function, including attention, working memory, updating, and sensory processing, within a few tens of seconds (6, 15–18). The amygdala, a core component of the emotional brain (3, 19) that receives sparse direct projections from ipRGC (20), is one of the brain areas acutely affected by changes in ambient light (18). This result raises the intriguing possibility that ambient light directly influences emotional brain processing. Although this hypothesis may have major implications in basic and clinical neuroscience, it has not yet been tested experimentally, and the brain mechanisms underpinning the influence of light on emotional processing are unknown.
Here we used functional MRI (fMRI) and a validated (nonvisual) auditory emotional task to characterize such a direct effect of ambient light exposure on brain responses to emotional stimuli.
Results
Seventeen young and healthy participants (Table S1) performed in the morning hours a gender discrimination task on verbal but meaningless emotional and neutral auditory stimuli (21). Common (but not completely identical) pathways are activated regardless of the direction of the emotional valence (22). However, our experience in the study of emotional responses is that negative valence elicits greater responses, which are less influenced by interindividual variability in valence perception (23). Therefore, half of the stimuli were pronounced with an angry negative prosody and the other half with a neutral prosody. It is known that these negative sounds elicit larger responses than neutral ones in the voice-sensitive area (24) of the temporal cortex and to a lesser extent in the amygdala (25, 26). Importantly, this effect is detected even when attention is directed toward the gender classification task and not the emotional content. The task thus allowed us to separate the known effect of ambient light on attention from its potential influence on emotion processing.
While performing the auditory task, participants were exposed to 12 40-s periods of monochromatic blue (473 nm) alternating with 12 40-s periods of green (527 nm) light of equal photon density (Fig. 1). To identify modulation of brain activity by ambient light, brain responses recorded during blue light exposure, to which the nonclassical photoreception system is maximally sensitive, were compared with the activity recorded during green light exposure, to which the classical photopic luminance visual pathway is maximally sensitive.
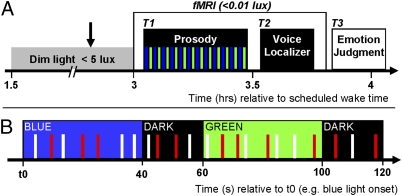
Fig. 1.
Experimental design. (A) General protocol. Arrow indicates pupil dilator administration. Time relative to scheduled wake time (h). T1 (task 1): first fMRI task, consisting of a gender discrimination of auditory vocalizations while exposed to alternating blue and green monochromatic ambient light (see B for details). T2 (task 2): second fMRI task (voice localizer); its main aim was to identify the voice-sensitive area of the temporal cortex. Participants performed a 1-back task with the voice stimuli from task 1 (anger and neutral pseudoword) and nonvoice white-noise auditory stimuli replicating the envelope (EN) or the mean of the fundamental (F0) of the original voice stimuli from task 1. T3 (task 3): emotional judgment task performed outside the MRI scanner, in which the emotions of all of the auditory stimuli presented in T1 were evaluated by the participants on a five-item Likert scale. (B) Detailed experimental procedures of the gender discrimination task (T1). Time (s) relative to t0, a time point arbitrarily chosen as a blue light onset of the session. Monochromatic [blue (473 nm) or green (427 nm)] ambient light exposures lasted 40 s and were separated by 15- to 25-s periods of darkness (mean duration, 20 s). Anger (red bars) and neutral (white bars) prosody vocalizations (meaningless word-like sounds; half neutral, half anger) were pseudorandomly and evenly administered in each light condition throughout the entire session (interstimuli interval, 3–11 s; mean, 4.8 s).
Behavior.
Accuracy for the gender discrimination task was high and was not affected by emotional or light conditions [performance >87%; F(1,16) < 2.11; P > 0.17; Fig. 2A; SI Results]. In accordance with the literature (25), reaction times were slower for negative relative to neutral stimuli, indicating a significant effect of emotional valence [F(1,16) = 24.69; P = 0.001; Fig. 2B; SI Results]. Individual ratings obtained after the fMRI sessions confirmed that perceived valence differed between emotional and neutral stimuli [F(1,16) = 93.69; P value < 10−6; Fig. 2C; SI Results]. Importantly, reaction times were not influenced by light conditions [F(1,16) < 1.1; P > 0.31], as expected given the short duration of light exposures and the low intensity used in the present experiment (6). Critically, the effects of ambient light on ongoing brain activity can be detected with minimal exposures, so that behavioral differences do not confound neural responses (6).
Fig. 2.
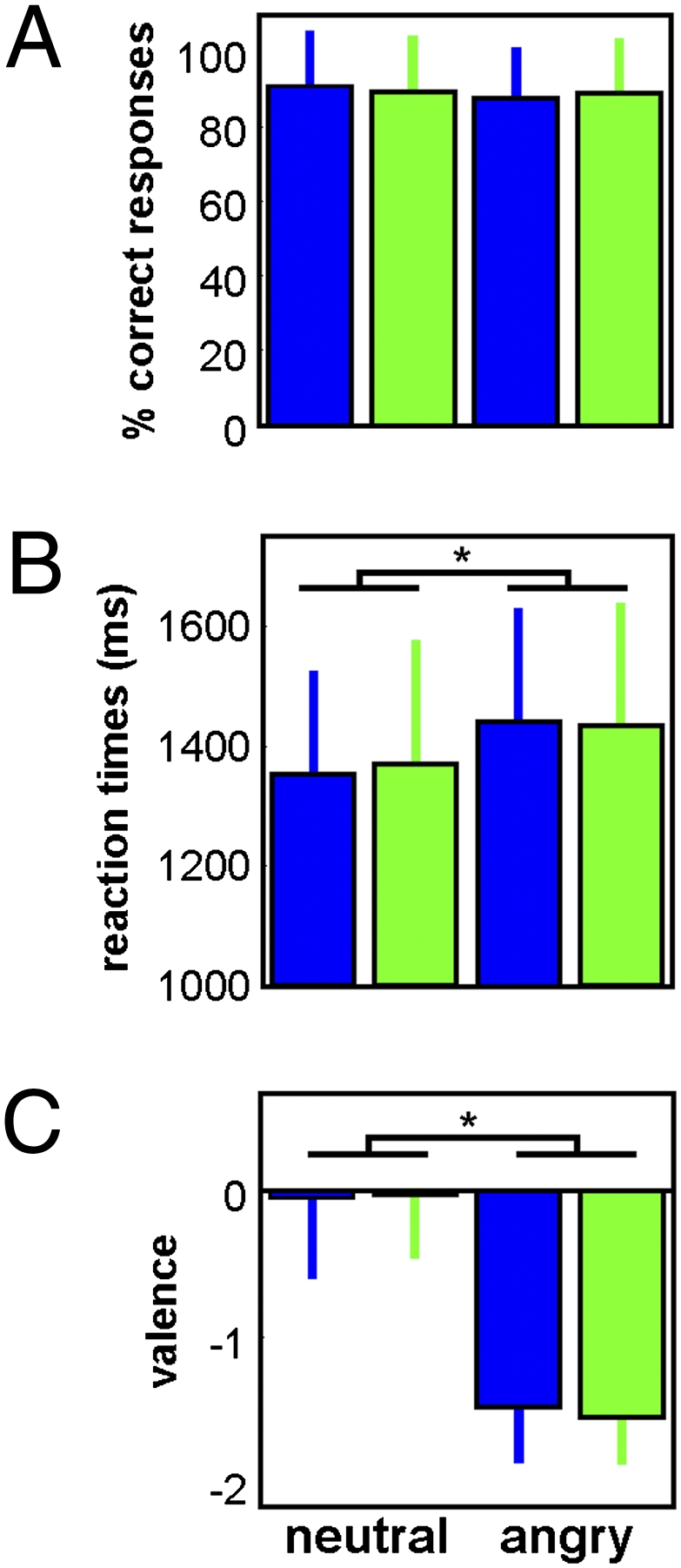
Behavioral results. (A) Accuracy for the gender discrimination task (task 1) (mean ± SD). (B) Reaction times during the gender discrimination task (task 1) (mean ± SD). (C) Emotional judgment of the neutral and anger voice stimuli made by the subjects during task 3 (i.e., after the fMRI procedure and outside the MRI scanner) (mean ± SD). *P ≤ 0.001.
Functional MRI.
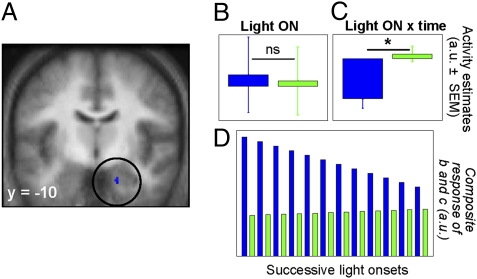
We first considered brain responses associated with the specific time point (“event”) corresponding to light onsets. Confirming our previous findings (18), responses in the right amygdala significantly differed between blue and green light onsets (Fig. 3A and Table 1). This differential response was primarily related to response adaptation across the session (i.e., across the 12 light exposures) (Fig. 3C). The average response to light onset averaged across the 12 exposures did not differ between light conditions (Fig. 3B). A composite representation of the average response and its evolution shows that the amygdala responses to blue light onsets were high at the beginning of the scanning session and monotonically declined throughout the session, indicative of a habituation process, whereas responses remained mostly unchanged for green light onsets (Fig. 3D).
Fig. 3.
Differences in responses to blue and green light onsets in the right amygdala. (A) Statistical results for the blue > green onset contrast modulated by time, overlaid on the population mean structural image (Puncorrected < 0.001). (B) Mean activity estimates [arbitrary units (a.u.) ± SEM] of the constant component of the brain responses associated with blue and green light onsets across the entire session; difference between conditions is nonsignificant (ns). (C) Estimates of the linear change component (a.u. ± SEM) of the brain responses associated with blue and green light onsets across the entire session, showing a significant (*) negative component for blue light onsets, suggesting an adaptation of amygdala responses with time. (D) Composite of both components showing the evolution of the responses to the 12 blue and green light onsets of the session.
Table 1.
Significant fMRI results
| Brain areas | Side | x, y, z | Z score | P value |
| Blue light onset > green light onset, modulated by time | ||||
| Amygdala*† | R | 16, −8, −26 | 3.25 | 0.048 |
| Anger > neutral | ||||
| Superior temporal gyrus/sulcus‡ | R | 58, −2, −8 | 4.54 | 0.001 |
| R | 68, −20, 2 | 4.32 | 0.001 | |
| R | 56, 4, −14 | 3.82 | 0.007 | |
| L | −64, −22, 4 | 3.43 | 0.022 | |
| L | −54, −28, 6 | 3.39 | 0.025 | |
| L | −52, −20, 0 | 3.21 | 0.039 | |
| Inferior frontal gyrus/sulcus§ | R | 44, 32, 2 | 3.67 | 0.011 |
| Anger × (blue > green)¶ | ||||
| Superior temporal sulcus‡ | L | −56, −12, −18 | 4.40 | 0.001 |
| R | 70, −30, 0 | 3.81 | 0.017 | |
| L | −56, −24, −4 | 3.57 | 0.015 | |
| R | 68, −34, 0 | 3.48 | 0.018 | |
| R | 68, −28, 4 | 3.47 | 0.019 | |
| R | 68, −14, −6 | 3.36 | 0.025 | |
| Hippocampus§ | R | 28, −24, −14 | 3.50 | 0.017 |
| L | −26, −24, −14 | 3.33 | 0.027 | |
| PPI with left STG/S: anger × (blue > green)║ | ||||
| Amygdala | L | −20, −16, −28 | 3.99 | 0.028 |
| Hypothalamus | R | 2, −6, −20 | 3.31 | 0.033 |
| PPI with right STG/S: anger × blue**†† | ||||
| Hypothalamus | L | −2, −6, −16 | 3.21 | 0.045 |
R, right; L, left; STG/S, superior temporal gyrus/sulcus.
*Cluster not affected by an inclusive mask (P = 0.001) of the (light onset × blue × time) contrast, indicating that the light condition effect was driven by blue light onset × time.
†Clusters not affected by an exclusive mask (P = 0.05) of the (light onset × green × time) contrast, indicating that the light condition effect was not driven by green light onset × time.
‡Peak voxel surviving an inclusive mask (P = 0.001) of the (voice stimuli > EN) or (voice stimuli > F0) contrasts of the voice localizer session (task 2), thus showing voice-sensitive response.
§Clusters not surviving an inclusive mask (P = 0.001) of the (voice stimuli > EN) or (voice stimuli > F0) contrasts of the voice localizer session (task 2), thus outside voice-sensitive regions.
¶Clusters not affected by an exclusive mask (P = 0.05) of the [neutral × (blue > green)] contrast, indicating that the light condition effect was specific to the emotional (angry prosody) stimuli.
║Clusters not affected by an exclusive mask (P = 0.05) of the [PPI with the left STS × neutral × (blue > green)] contrast, indicating that the light condition effect on functional connectivity was specific to the emotional (angry prosody) stimuli.
**Cluster not affected by an exclusive mask (P = 0.05) of the (PPI with the right STG × anger x green) contrast, suggesting that the effect is specific to the blue light condition.
††Cluster not affected by an exclusive mask (P = 0.05) of the (PPI with the right STS × neutral x blue) contrast, indicating that this effect was specific to the emotional (angry prosody) stimuli.
We then considered the impact of the emotional valence of the stimuli on brain activity. This also confirmed our findings (25) that negative voices triggered stronger responses (compared with neutral ones) in the right inferior frontal cortex and in bilateral superior temporal gyri (STG) within the voice-sensitive area independently identified during a separate voice-localizer fMRI session (Fig. 1A, Table 1, and Fig. S1) (25, 26). No significant impact of the emotional condition was detected in the amygdala, but this is in line with the observation that response to emotional response in the amygdala is weaker in the auditory than in the visual modality (26).
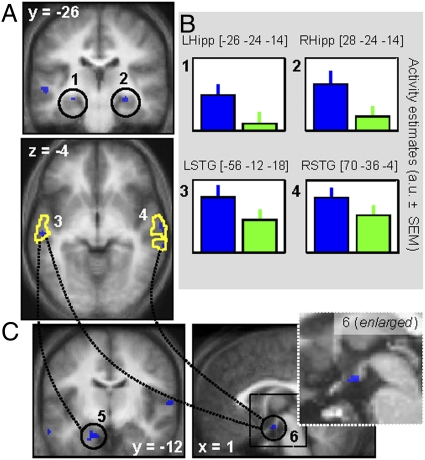
Critically, when considering the impact of ambient light condition on emotional stimuli processing, brain responses elicited by angry voices were enhanced under blue compared with green light exposure bilaterally in the voice-sensitive area of the temporal cortex and in the hippocampus (Fig. 4 A and B and Table 1). In contrast, no brain responses to emotional voices were significantly increased under green (vs. blue) ambient light. Likewise, no significant difference between blue and green ambient light exposures was observed for neutral stimuli, and the sex of the participants did not significantly influence the results (SI Results).
Fig. 4.
Impact of the the wavelength of the ambient illumination context on the brain processing of emotional auditory stimuli. (A) Significant differences between blue and green monochromatic ambient light exposures in the modulation of the brain responses associated with anger prosody stimuli. Yellow lines indicate voice-sensitive regions activated during the voice localizer (task 2). Dotted lines refer to the functional connectivity analysis (see C). Statistical results are overlaid to the population mean structural image (Puncorrected < 0.001). 1, left hippocampus; 2, right hippocampus; 3, left superior temporal gyrus; 4, right superior temporal gyrus. (B) Mean activity estimates [arbitrary units (a.u.) ± SEM] of the brain responses associated with anger prosody during blue and green ambient illumination contexts. (C) Increased functional connectivity with voice-sensitive regions for anger prosody under blue vs. green monochromatic ambient light exposure. Dashed lines/circles indicate higher functional connectivity between left superior temporal gyrus and (5) the left amygdala and (6) the hypothalamus (anterior to the mammilary bodies, posterior to the infundibulum) under blue relative to green ambient light exposure, and increased functional connectivity between the right superior temporal gyrus and (6) the hypothalamus (anterior to the mammillary bodies, posterior to the infundibulum) under blue but not under green ambient light.
We then conducted psychophysiological interaction (PPI) analyses to assess changes in functional connectivity dependent on ambient light condition. In short, PPIs test for condition-dependent modulations of functional connectivity by assessing the impact of experimental conditions (i.e., blue vs. green light exposure) on the regression between the activity time course in a seed region and that of any other brain area (27). We detected an increased functional connectivity between the voice-sensitive area of the left STG and both the left amygdala and a hypothalamic area, selectively for the processing of angry voices in the context of ambient blue light exposure, relative to ambient green light exposure (Fig. 4C and Table 1). Similarly, the functional connectivity between the right STG and the same hypothalamic area was significant during the presentation of angry voices under ambient blue but not under ambient green illumination.
Finally, the protocol included two irradiance levels that were applied to both blue and green light exposures (Methods). Irradiance was not the primary focus of the present research, and only two low irradiance levels were investigated. Statistical analyses did not reveal any compelling effect of irradiance (Table S2), and it seems unwise to derive from the present data a reliable characterization of the impact of irradiance on regional brain responses.
Discussion
These original results demonstrate that ambient light and its spectral quality influences the brain processing of emotional stimuli. Blue (relative to green) light increased responses to emotional stimuli in the voice area of the temporal cortex and in the hippocampus. Furthermore, in the context of blue illumination, emotional processing was associated with an enhanced functional coupling between the voice area, amygdala, and hypothalamus. These effects were detected in the absence of behavioral bias and were not significantly influenced by the sex of the participants.
As in our previous research (18), we reported an impact of the ambient light condition for the onsets of the light exposure, which occurred only once per minute and, critically, were independent of the ongoing task. We interpret this result as an initial high responsiveness to blue light onsets, which decrease with time owing to the habituation process in the emotional system (22).
Although the auditory task did not explicitly engage declarative memory, the hippocampus, which is also involved in fear conditioning (19), was recruited by the auditory emotional task and significantly more so in the context of ambient blue light exposure relative to green light exposure. Significant activity modulations induced by ambient light were already reported in the hippocampus at the onset of blue light or after bright white light while participants were engaged in attentional processes (16, 18). We surmise that ambient light indirectly influences the hippocampus through projections from the amygdala (28) and brainstem nuclei (29).
As compared with green light, ambient blue light also amplified responses triggered by vocal emotional stimuli in the voice-sensitive area of the temporal cortex. To assess the potential mechanisms by which light can modulate responses in the voice-sensitive area, we tested whether its functional connectivity with the rest of the brain during emotional processing changed as a function of the light condition. Results revealed strengthened functional interactions under ambient blue light exposure between the voice-sensitive area, amygdala, and hypothalamus, which constitute critical areas in emotional and light irradiance information processing. In other words, the responses of the amygdala and hypothalamus to an anger prosody stimulus were linearly dependent on the response in the voice-sensitive area in the context of blue relative to green ambient light exposure. These measures of functional connectivity suggest therefore that through their extensive recurrent connections (28), the temporal cortex and the amygdala interact more strongly to process emotional stimuli in the context of blue rather than green ambient light exposure, thereby instantiating the interaction of light irradiance signal and affective signals.
Functional connectivity changes were mainly apparent in the left hemisphere, in line with the known difference in response dynamics in the left and right amygdala (22). Adaptation is known to occur much faster in the right than in the left amygdala (22), potentially explaining why we did not detect sustained responses to vocal stimuli in the right amygdala in any ambient light condition.
Anatomical connectivity could easily support rapid responses to light in the amygdala, with preferential reaction to blue light. First, the amygdala receives sparse direct inputs from ipRGCs (20). Second, the amygdala also receives indirect retinal inputs through the superior colliculus and pulvinar (30), as well as via other brainstem nuclei (28), and we previously reported a greater sensitivity to blue light of the responses of the thalamus (including in the pulvinar) and brainstem related to auditory cognitive tasks (17, 18). Only brain areas significantly involved in the processing of the vocal stimulation and affected by the light and emotional conditions could be detected in our event-related analyses. Therefore the brainstem or the pulvinar could convey light signal without being detected in the present analyses.
A number of hypothalamic nuclei are located in the surroundings of the detected hypothalamic area, including the ventromedial (VMH) and part of the dorsomedial (DMH) hypothalamus. The amygdala is in a position to control the expression of fear responses through its direct inputs to the paraventricular nucleus and the lateral hypothalamus (19), both receiving direct and indirect inputs, respectively, from the DMH and VMH (31). The hypothalamus is known to receive few inputs from auditory temporal cortex, although auditory inputs can reach it by polysynaptic routes involving the amygdala (19, 32). The present study therefore strongly suggests that, through its structural and functional connectivity, the hypothalamus is a potential site of convergence for emotional (19, 33) and ambient light information (4, 6).
The wavelengths used in the present protocol were chosen according to the spectral sensitivity of the classical photopic luminance visual pathways and of the nonclassical (melanopsin-based) photoreception system, in an attempt to separate their respective influence. However, several mechanisms can be contemplated to explain the spectral influence of ambient light on emotional brain processing. First, the fact that light of shorter wavelength triggered significant modulations of brain activity is an argument in favor of the involvement of the nonclassical photoreception system (4, 6, 10, 11). In addition, we are reporting light modulation of brain responses elicited by auditory stimuli modality, which are in essence nonvisual. To our knowledge there is no evidence that visual responses to light modulate brain responses associated with a gender classification of auditory vocal events in the context of constant diffuse light exposures. Finally, we report sustained responses that span several tens of seconds, whereas visual response are typically transient and time-locked to changes in visual signal at the levels of photoreceptors (10, 34) or neural ensembles of the occipital cortex (35). For these reasons, we favor the implication of the nonclassical photoreception system in our effect. However, this interpretation would entail a significant influence of irradiance. The present data do not allowed us to reliably characterize the impact of irradiance on regional brain responses, and our hypothesis shall await new experimental data to be firmly confirmed.
Second, visual mechanisms could be responsible for the reported effects. For instance, the prevailing preference to blue hues in the general population could have contributed to the differential responses between blue and green light exposures (36). However, performance on detail-oriented visual tasks (i.e., tasks that, as in our experiment, require focused and careful attention) is enhanced by red, relative to blue hues (37). In contrast, blue, compared with red hues, seems to increase creativity and innovation (37). On the basis of these results (which did not include green), one would therefore expect a larger impact for colors associated with longer wavelengths (i.e., green) in our experiment, whereas the contrary was observed in our experiment.
Third, color opponency recruits retinal and cerebral mechanisms (38) that seem to participate in the nighttime suppression of melatonin secretion by light (39) or in pupillary constriction (40) when exposed to polychromatic light. Similar effects might have taken place in the present experiment, although the alternation of both monochromatic light exposures and the 20 s of darkness between the exposures potentially reduce the influence of color opponency in our results.
It is even more challenging to assign the reported effects to the recruitment of specific photoreceptors. All retinal photoreceptors are probably involved in the effect we describe (11, 13, 41, 42), and we have no means to isolate the contribution of any of them. Melanopsin-expressing ipRGCs play a key role in nonclassical responses to ambient light exposure. Their maximal sensitivity (460–480 nm) is close to the peak wavelength of blue light (473 nm) used in the present experiment, and the light levels we used are compatible with the threshold identified in rodents for their recruitment (43). Our results are therefore compatible with the contribution of melanopsin-expressing ipRGC. In rodents, rods also mediate nonclassical effects of light, at irradiance levels higher than previously expected (43, 44). Their contribution cannot be ruled out, although their maximal sensitivity (505 nm) is intermediate between the blue and green (527 nm) lights used in the present experiment, reducing their potential influence. We cannot exclude a potential contribution of short-wavelength cones (S-cones), maximally sensitive to ≈420-nm wavelength, which have been integrated in previous models of the nonclassical impact of ambient light (45) and shown to send input to some ipRGC (34). Finally M- and L- cones (maximally sensitive to ≈530 nm and ≈560 nm, respectively), as well as S-cones, could have contributed through the putative color opponency mechanism already mentioned. Novel experimental designs are required to evaluate the respective impact of the visual or nonclassical photoreception systems and of the different retinal photoreceptors in the modulation of emotional brain responses.
As a whole, our results support the view that ambient blue light promotes affective arousal and associated mnemonic processing, which may favor a rapid turnover of limbic reactivity to emotional challenges, and thus could participate in a rapid behavioral adaptation to the environment (6). Emotional responses are acute transient phenomena triggered by external stimulations, whereas mood is a sustained emotional state. Changes or alterations in mood, such as in mood disorders, modify emotional brain responses, whereas responses to emotional stimuli can have a great impact on (subsequent) mood (3). Importantly, mood disorders, such as major depressive disorders and bipolar disorders, are characterized by altered structural or functional changes in areas involved in emotional processing, such as the amygdala, hypothalamus, and hippocampus (3), and we observed an impact of ambient light exposure on responses related to auditory emotional stimulation of these areas. By strengthening emotional brain reactivity in these areas, ambient blue light in the responses of the thalamus (including in the pulvinar) and in the brainstem related to auditory cognitive tasks might promote accurate and contrasting responses to emotional signals, which could ultimately enhance efficient mood regulation processes. In fact, recent reports showed that prolonged darkness or lack of light in rodents induces a depression-like state associated with structural brain changes (46), whereas complete blindness increases depression risk (47). Likewise, long-term changes in ambient light daily profile, such as light therapy, seem to restore normal mood regulation (1, 2), and the spectral composition of light changes across the seasons (48). Interestingly, blue-enriched light seems to be equally effective as (visually) brighter white light in the treatment of SAD (49), and a polymorphism in the melanopsin gene has recently been associated with SAD (50).
Collectively, the data show that the spectral composition of ambient light influences the processing of emotional stimuli, with a superiority of blue light in recruiting a network merging affective and ambient light information. For auditory (voice) stimuli, this circuit involves the amygdala, the voice-sensitive area, and the hypothalamus. Although acute effects of ambient light on emotional processing might differ from its longer-lasting effects on mood, the present findings in healthy subjects may have important implications for our understanding of the mechanisms by which changes in lighting environment improve mood not only in mood disorders using light therapy (1, 2) but also in the general population using blue-enriched light in the work environment (51). Although traditionally these effects of light were thought to be related to changes in circadian rhythm parameters, such as the timing of the melatonin rhythm, our data suggest that they could also depend on brain mechanisms that can swiftly modify brain emotional processing, potentially through (melanopsin-based) nonclassical photoreception.
Methods
More details are presented in SI Methods.
Subjects.
Participants were right-handed, young, and healthy (n = 17; 9 female; age 20–26 y; Table S1). They gave their written informed consent, and the study was approved by the local ethics committee. A semistructured interview (including questionnaires) established the absence of addiction and medical, psychiatric, and sleep disorders, as well as auditory impairments and color blindness. Volunteers followed a regular sleep schedule during the 7-d period preceding the laboratory experiment (verified using actigraphy and sleep diaries).
Experimental Protocol.
Participants arrived in the laboratory 1.5 h after waking up and were maintained in dim light (<5 lx) for 1.5 h (Fig. 1A). They were scanned during two consecutive sessions. During the first session (24 min), subjects were exposed to 40-s periods of monochromatic illumination, alternating between blue (473 nm) and green (527 nm) light, separated by 15- to 25-s periods of darkness (<0.01 lx; T1; Fig. 1 A and B). Each wavelength was presented 12 times. The second session (12 min) was conducted in near complete darkness (<0.01 lx; T2; Fig. 1A).
Auditory Stimuli.
The 262 auditory stimuli used were produced by eight professional actors (four female) and taken from a validated database (21). To avoid semantic processing, we used three different tokens of nonsense syllable sequences (pseudowords: “goster,” “niuvenci,” and “figotleich”) extracted from meaningless sentence-like utterances. These voice stimuli expressed anger or neutral prosody, as validated by extensive behavioral assessments (21) and in previous experiments (25, 26). Male and female speakers were equally counterbalanced across emotional conditions (anger, neutral) and across token types. Each token was equally represented in each emotional condition (anger, neutral). Stimuli were matched in terms of duration (750 ms) and mean acoustic energy to avoid loudness effects. For the second task we created two additional sets of white-noise stimuli matched for the amplitude envelope (EN) and the fundamental frequency (F0) of each of the vocal stimuli in task 1.
Task 1: Main Task.
Participants were required to use a keypad to indicate the gender of the person pronouncing each token. They were not told that the stimuli were pronounced with negative or neutral prosodies.
Task 2: Voice Localizer.
This task was conducted to confirm that differential emotional effects on brain activity in task 1 were driven by vocal prosody rather than being related to low-level acoustic features. Voice stimuli of task 1, F0, and EN were presented in separate blocks, during which participants indicated on an MR-compatible keypad whether the current auditory stimulus was identical to the preceding one (1-back task).
Task 3: Emotional Judgment.
After the fMRI sessions and while they were outside the scanner, participants were asked to evaluate the emotional valence of each stimulus heard in session 1 on a five-item Likert scale, including three negative rates (−3, −2, −1), a neutral rate (0), and a positive rate (+1).
Light Exposure.
In accordance with our previous studies (17, 18) and work of others (4, 5, 7), the photon densities of the two monochromatic light exposures were identical to allow the assessment of the relative contribution of the photoreception system maximally sensitive to each wavelength. However, in an attempt to extend the validity of our previous investigations, we used two photon densities in all subjects so that half of the blue and green exposures were set at 7 × 1012 and the other half at 3 × 1013 photons per cm2 per s [instead of using 1013 (18) or 3 × 1013 (17) photons per cm2 per s for all exposures]. At these levels, nonclassical responses at night and during the day depend on the wavelength of the light exposure (4, 5, 7, 17, 18).
In each 40-s period of monochromatic light exposure, three to four angry prosody stimuli and three to four neutral prosody stimuli were presented in a pseudorandom order. A total of 90 stimuli (50% anger; 50% neutral) were distributed across the two wavelengths (i.e., a total of 180 distinct voice stimuli). For each wavelength, stimuli were equally distributed across the two photon densities (50% angry; 50% neutral). Each of the 24 darkness periods separating each light exposure contained three to four stimuli for a total of 82 stimuli (50% of angry prosody).
fMRI Data Acquisition.
Functional MRI time series were acquired using a 3T MR scanner (Allegra; Siemens). Multislice T2*-weighted fMRI images were obtained with a gradient echo-planar sequence using axial slice orientation (32 slices; voxel size, 3.4 × 3.4 × 3 mm3 with 30% of gap; matrix size, 64 × 64 × 32; repetition time, 2,130 ms; echo time, 40 ms; flip angle, 90°). Structural T1-weighted brain images were also acquired.
fMRI Data Analysis.
Functional volumes were analyzed using Statistical Parametric Mapping (SPM5; http://www.fil.ion.ucl.ac.uk/spm). They were corrected for head motion, spatially normalized, and smoothed. The analysis of fMRI data was conducted in two steps, accounting respectively for fixed and random effects.
For task 1, linear contrasts tested (i) wavelength effects (blue vs. green) on brain responses to light onset; (ii) wavelength effects (blue vs. green) on the brain responses to light onset modulated by time; (iii) main effect of emotion (anger vs. neutral stimuli, irrespective of light condition); (iv) wavelength effect (blue vs. green) on brain responses to anger prosody; and (v) wavelength effect (blue vs. green) on brain responses to neutral stimuli. The same contrasts (except iii) were computed with irradiance as factor (SI Results). For task 2, two contrasts of interest identified difference in activations between the voice stimuli and (i) EN and (ii) F0. The resulting set of voxel values for each contrast constituted maps of the t statistics thresholded at Puncorrected = 0.001. Statistical inferences were performed after correction for multiple comparisons at a threshold of P = 0.05. A description of the PPI analyses can be found in the SI Methods.
Supplementary Material
Acknowledgments
We thank Drs. J. Carrier, P. Rainville, C. Schmidt, and V. Sterpenich for their help. This study was supported by the Belgian Fonds National de la Recherche Scientifique (FNRS), Fondation Médicale Reine Elisabeth, University of Liège, Inter-university Attraction Poles (PAI/IAP) P6/29, and Wellcome Trust Grant GR069714MA (to D.J.D.), and by the Swiss National Science Foundation (D.G. and S.S.). P.M. and C.P. are supported by the FNRS. G.V. was supported by the FNRS during acquisition and by the Fonds québécois de la recherche sur la nature et les technologies (FQRNT) and Fonds de la Recherche en Santé du Québec (FRSQ) during analyses.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010180107/-/DCSupplemental.
References
- 1.Terman M. Evolving applications of light therapy. Sleep Med Rev. 2007;11:497–507. doi: 10.1016/j.smrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Wirz-Justice A, et al. Brightening depression. Science. 2004;303:467–469. doi: 10.1126/science.303.5657.467c. [DOI] [PubMed] [Google Scholar]
- 3.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brainard GC, Hanifin JP. Photons, clocks, and consciousness. J Biol Rhythms. 2005;20:314–325. doi: 10.1177/0748730405278951. [DOI] [PubMed] [Google Scholar]
- 5.Lockley SW, Gooley JJ. Circadian photoreception: Spotlight on the brain. Curr Biol. 2006;16:R795–R797. doi: 10.1016/j.cub.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Dijk DJ, Archer SN. Light, sleep, and circadian rhythms: Together again. PLoS Biol. 2009;7:e1000145. doi: 10.1371/journal.pbio.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11:453–464. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Ecker JL, et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: Cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berson DM. Strange vision: Ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 11.Hankins MW, Peirson SN, Foster RG. Melanopsin: An exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Provencio I, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Güler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235:352–354. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- 15.Perrin F, et al. Nonvisual responses to light exposure in the human brain during the circadian night. Curr Biol. 2004;14:1842–1846. doi: 10.1016/j.cub.2004.09.082. [DOI] [PubMed] [Google Scholar]
- 16.Vandewalle G, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16:1616–1621. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Vandewalle G, et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex. 2007;17:2788–2795. doi: 10.1093/cercor/bhm007. [DOI] [PubMed] [Google Scholar]
- 18.Vandewalle G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: Prominent role of blue light and the brainstem. PLoS ONE. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Hattar S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banse R, Scherer KR. Acoustic profiles in vocal emotion expression. J Pers Soc Psychol. 1996;70:614–636. doi: 10.1037//0022-3514.70.3.614. [DOI] [PubMed] [Google Scholar]
- 22.Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Sterpenich V, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5:e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- 25.Grandjean D, et al. The voices of wrath: Brain responses to angry prosody in meaningless speech. Nat Neurosci. 2005;8:145–146. doi: 10.1038/nn1392. [DOI] [PubMed] [Google Scholar]
- 26.Sander D, et al. Emotion and attention interactions in social cognition: Brain regions involved in processing anger prosody. Neuroimage. 2005;28:848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 28.Aggleton JP. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley; 1992. [Google Scholar]
- 29.Castle M, Comoli E, Loewy AD. Autonomic brainstem nuclei are linked to the hippocampus. Neuroscience. 2005;134:657–669. doi: 10.1016/j.neuroscience.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 32.Saper CB. Hypothalamus. In: Paxinos G, editor. The Human Nervous System. New York: Academic; 1990. [Google Scholar]
- 33.Schwartz S, et al. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain. 2008;131:514–522. doi: 10.1093/brain/awm292. [DOI] [PubMed] [Google Scholar]
- 34.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 35.Haynes JD, Lotto RB, Rees G. Responses of human visual cortex to uniform surfaces. Proc Natl Acad Sci USA. 2004;101:4286–4291. doi: 10.1073/pnas.0307948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer SE, Schloss KB. An ecological valence theory of human color preference. Proc Natl Acad Sci USA. 2010;107:8877–8882. doi: 10.1073/pnas.0906172107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta R, Zhu RJ. Blue or red? Exploring the effect of color on cognitive task performances. Science. 2009;323:1226–1229. doi: 10.1126/science.1169144. [DOI] [PubMed] [Google Scholar]
- 38.Conway BR. Color vision, cones, and color-coding in the cortex. Neuroscientist. 2009;15:274–290. doi: 10.1177/1073858408331369. [DOI] [PubMed] [Google Scholar]
- 39.Figueiro MG, Bierman A, Rea MS. Retinal mechanisms determine the subadditive response to polychromatic light by the human circadian system. Neurosci Lett. 2008;438:242–245. doi: 10.1016/j.neulet.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 40.Kimura E, Young RS. Sustained pupillary constrictions mediated by an L- and M-cone opponent process. Vision Res. 2010;50:489–496. doi: 10.1016/j.visres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Gooley JJ, et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lall GS, et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altimus CM, et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Brain Res Rev. 2005;50:213–228. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez MM, Aston-Jones G. Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci USA. 2008;105:4898–4903. doi: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bramley T, Peeples P, Walt JG, Juhasz M, Hansen JE. Impact of vision loss on costs and outcomes in medicare beneficiaries with glaucoma. Arch Ophthalmol. 2008;126:849–856. doi: 10.1001/archopht.126.6.849. [DOI] [PubMed] [Google Scholar]
- 48.Thorne HC, Jones KH, Peters SP, Archer SN, Dijk DJ. Daily and seasonal variation in the spectral composition of light exposure in humans. Chronobiol Int. 2009;26:854–866. doi: 10.1080/07420520903044315. [DOI] [PubMed] [Google Scholar]
- 49.Anderson JL, Glod CA, Dai J, Cao Y, Lockley SW. Lux vs. wavelength in light treatment of Seasonal Affective Disorder. Acta Psychiatr Scand. 2009;120:203–212. doi: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 50.Roecklein KA, et al. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114:279–285. doi: 10.1016/j.jad.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viola AU, James LM, Schlangen LJ, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34:297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.