Abstract
N2-fixing cyanobacteria play an essential role in sustaining primary productivity in contemporary oceans and freshwater systems. However, the significance of N2-fixing cyanobacteria in past nitrogen cycling is difficult to establish as their preservation potential is relatively poor and specific biological markers are presently lacking. Heterocystous N2-fixing cyanobacteria synthesize unique long-chain glycolipids in the cell envelope covering the heterocyst cell to protect the oxygen-sensitive nitrogenase enzyme. We found that these heterocyst glycolipids are remarkably well preserved in (ancient) lacustrine and marine sediments, unambiguously indicating the (past) presence of N2-fixing heterocystous cyanobacteria. Analysis of Pleistocene sediments of the eastern Mediterranean Sea showed that heterocystous cyanobacteria, likely as epiphytes in symbiosis with planktonic diatoms, were particularly abundant during deposition of sapropels. Eocene Arctic Ocean sediments deposited at a time of large Azolla blooms contained glycolipids typical for heterocystous cyanobacteria presently living in symbiosis with the freshwater fern Azolla, indicating that this symbiosis already existed in that time. Our study thus suggests that heterocystous cyanobacteria played a major role in adding “new” fixed nitrogen to surface waters in past stratified oceans.
Keywords: nitrogen fixation, intact polar lipids, cyanobacterial biomarkers, symbiosis, saproprel
The global nitrogen cycle largely relies on biological nitrogen fixation to maintain biological productivity with some 1.4 to 2.4 Teramole of combined nitrogen being annually added to the marine nitrogen budget, counteracting the loss of bioavailable nitrogen through processes such as denitrification and anaerobic ammonium oxidation (anammox) (1). The filamentous cyanobacterium Trichodesmium sp. (2) and unicellular cyanobacteria (3) are considered to be the major N2-fixers in the contemporary open ocean. Because the nitrogenase enzyme, utilized to convert N2 into 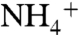 , is sensitive towards molecular oxygen, some groups of N2-fixing cyanobacteria perform photosynthesis separate in time from N2-fixation, while another subgroup performs the fixation of N2 in specialized cells, so-called heterocysts, to protect the oxygen-sensitive nitrogenase enzyme. Heterocystous cyanobacteria are often dominant diazotrophs in many freshwater and brackish environments such as the Baltic Sea (4) whereas in the marine environment they mainly occur in symbiosis with diatoms (5). Although the importance of N2-fixing cyanobacteria in sustaining primary productivity in modern aquatic environments is clearly evident, their importance in ancient nitrogen cycling is less clear. Microfossils of heterocystous cyanobacteria have been found in a number of ancient rocks, documenting their evolutionary history (e.g., 6) but their preservation potential, especially in open ocean settings, is relatively poor. Cyanobacteria have been suggested to be major primary producers during times of strong ocean stratification such as during the formation of Pleistocene Mediterranean sapropels (7) and Cretaceous black shales (8, 9) based on the depletion of 15N of bulk organic matter and chlorins, which is commonly associated with the relatively small isotopic fractionation of diazotrophs (10). For the Cretaceous black shales, elevated concentrations of 2-methyl hopanes, general markers for cyanobacteria (11), have also been reported (8). In combination, these proxies suggest that diazotrophic cyanobacteria may have been important in replenishing the reservoir of combined nitrogen during the formation of these organic-rich marine deposits.
, is sensitive towards molecular oxygen, some groups of N2-fixing cyanobacteria perform photosynthesis separate in time from N2-fixation, while another subgroup performs the fixation of N2 in specialized cells, so-called heterocysts, to protect the oxygen-sensitive nitrogenase enzyme. Heterocystous cyanobacteria are often dominant diazotrophs in many freshwater and brackish environments such as the Baltic Sea (4) whereas in the marine environment they mainly occur in symbiosis with diatoms (5). Although the importance of N2-fixing cyanobacteria in sustaining primary productivity in modern aquatic environments is clearly evident, their importance in ancient nitrogen cycling is less clear. Microfossils of heterocystous cyanobacteria have been found in a number of ancient rocks, documenting their evolutionary history (e.g., 6) but their preservation potential, especially in open ocean settings, is relatively poor. Cyanobacteria have been suggested to be major primary producers during times of strong ocean stratification such as during the formation of Pleistocene Mediterranean sapropels (7) and Cretaceous black shales (8, 9) based on the depletion of 15N of bulk organic matter and chlorins, which is commonly associated with the relatively small isotopic fractionation of diazotrophs (10). For the Cretaceous black shales, elevated concentrations of 2-methyl hopanes, general markers for cyanobacteria (11), have also been reported (8). In combination, these proxies suggest that diazotrophic cyanobacteria may have been important in replenishing the reservoir of combined nitrogen during the formation of these organic-rich marine deposits.
Results
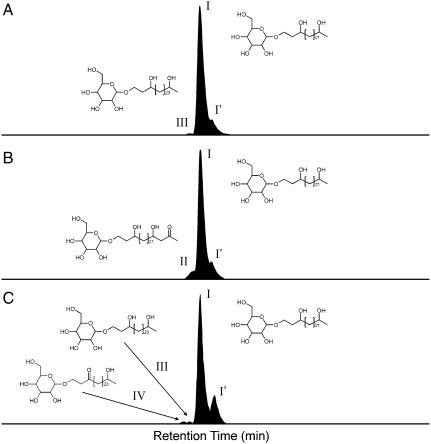
In this study, we employed biological markers specific for heterocystous N2-fixing cyanobacteria to examine the importance of these diazotrophs in recent and ancient nitrogen cycling. In heterocystous cyanobacteria the oxygen-sensitive nitrogenase enzyme is protected by laminated layers of heterocyst glycolipids (HGs) that are part of the heterocyst cell envelope (12). These components consist of long-chain diols, triols, keto-ols, and keto-diols that are glycosidically bound to hexose molecules (see Fig. S1) (13, 14). To the best of our knowledge, HGs have not been reported in any other organism and thus they represent excellent tracers not only for heterocystous cyanobacteria but also for the N2 fixation process itself. In order to establish the applicability of heterocyst glycolipids as biological markers, we developed a sensitive HPLC-MS2 technique based on previously published methods (14) to allow their detection in complex matrices (see Materials and Methods). In this way, C26 (I; Fig. S1) and C28 HG diols (III) with distributions similar to those found in pure cultures of the heterocystous cyanobacteria Anabaena spp. and Nodularia spp. (15) were detected in microbial mats from the North Sea barrier island Schiermonnikoog (Table S1). We then determined the fate of HGs by screening sediments from a number of modern environments, known to host heterocystous cyanobacteria, for the presence of HGs (see SI Text). Analysis of particulate organic matter from surface waters of an East African crater lake (Lake Challa), where heterocystous cyanobacteria are part of the phytoplankton community, revealed the presence of the C26 HG diol (I; Fig. 1). This HG was also present in Lake Challa’s sinking particulate organic matter collected by a sediment trap at 35 m water depth and in the upper 10 m of sediments of this lake (Fig. 1; Table S1), revealing that HGs are preserved in the sedimentary record. We also detected HGs in Baltic Sea sediments buried up to 34 m deep (Table S1). In this brackish coastal sea, heterocystous cyanobacteria form an important component of the phytoplankton community in present-day surface waters (4). In contrast, HGs were not detected in sediments of contemporary open oceans, in agreement with the observation that heterocystous cyanobacteria represent only a small fraction of the phytoplankton assemblage in open marine environments (16). Hence, the presence of HGs in sediments reflects the (past) presence of heterocystous N2-fixing cyanobacteria.
Fig. 1.
HPLC/ESI-MS/MS summed ion chromatograms of SRM transitions of heterocyst glycolipids in (A) particulate organic matter (1 m water depth), (B) sediment trap (35 m water depth), and (C) sediment (10 m depth) taken from Lake Challa. The heterocyst glycolipid distribution is dominated 1-(O-hexose)-3,25-hexacosanediol (I), while 1-(O-hexose)-3-keto-25-hexacosanol (II), 1-(O-hexose)-3,27-octacosanediol (III), 1-(O-hexose)-3-keto-27-octacosanol (IV) and an unknown isomer (possibly with a different sugar moiety) of 1-(O-hexose)-3,25-hexacosanediol (I′) were detected in lower abundances. Note that the heterocyst glycolipid distribution is relatively similar in the particulate organic matter, sediment trap material, and subsurface sediment suggesting no preferential degradation of individual glycolipids.
HGs were also detected in a number of ancient lacustrine deposits, ranging in age from Holocene to Eocene (Table S2 and Fig. S2), again attesting to the excellent preservation potential of these components. Sediments from all these locations accumulated under anoxic conditions in stratified freshwater lakes that were oligotrophic and likely permitted proliferation of N2-fixing cyanobacteria. The presence of HGs in these ancient sediments is remarkable, given that glyceride lipids with glycosidic head groups are thought to be rapidly degraded and not preserved over geological time scales (17). However, microbially mediated degradation experiments and theoretical modelling shows that glycosidic ether lipids are more recalcitrant towards degradation than membrane lipids with a phospho-head group (18, 19). Interestingly, the dominant intact polar lipids found in subsurface sediments are glycosidic derivatives of archaeol and glycerol dibiphytanyl glycerol tetraether lipids (20), which contain glycosidically bound sugar head groups similar to those in heterocyst glycolipids. The presence of fossil HGs in sediments, thus, raises the question whether certain intact polar lipids, especially those with glycosidic head groups, may be more recalcitrant than previously thought and possibly constitute a significant fossil component of the subsurface sedimentary intact polar lipid pool (21).
Discussion
The ability to detect specific markers for the past presence of N2-fixing cyanobacteria raises the exciting possibility to determine their paleoecology and their role in past nitrogen cycling. We illustrate this approach by examining the role of heterocystous cyanobacteria in the Pleistocene Eastern Mediterranean Sea and the Eocene Arctic Ocean.
Heterocystous Cyanobacterial N2-Fixation in the Pleistocene Eastern Mediterranean.
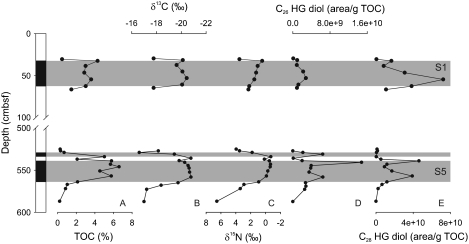
During the Quaternary, the Mediterranean Sea was occasionally strongly impacted by the input of large amounts of freshwater, leading to the development of a low salinity surface layer and hence strong stratification of the upper part of the water column (22, 23). These conditions have led to nutrient depletion and thus possibly favored the proliferation of N2-fixing cyanobacteria (7). Indeed, the C26 (I) and C28 HG diols (III) were detected in sediments deposited at the onset of sapropel S1 and S5 formation, while they were either absent or only present in trace amounts in the adjacent organic-poor sediments (Table S2; Fig. 2). Maxima in the HG abundances within the S5 sapropel layer correlate well with lowest bulk nitrogen isotope values (δ15N = -0.5‰), providing direct proof for past N2 fixation by heterocyst cyanobacteria during the formation of Pleistocene sapropels. HGs were also identified within the boundaries of the S1 sapropel, albeit in lower abundances, in agreement with less depleted bulk δ15N values (+0.4‰). Some differences in the distribution of the individual HGs in the S1 and S5 sapropels are noted (Table S2), which may either reflect adaptations to different environmental conditions, such as temperature and oxygen concentration (15), or contributions of different heterocystous cyanobacteria (15) at times of sapropel formation.
Fig. 2.
Depth profile of the Eastern Mediterranean piston core MS66PC, showing the core and stratigraphic profiles of (A) TOC, (B) stable carbon isotopes or organic matter 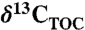 ), (C) bulk stable nitrogen isotopes (δ15N), concentrations of (D) 1-(O-hexose)-3,25-hexacoasanediol (C26 HG diol), and (E) 1-(O-hexose-)-3,27-octacosanediol (C28 HG diol). Gray shaded intervals represent S1 and S5 sapropel layers. The increased concentrations of the heterocyst glycolipids at times of sapropel deposition coincide with a depletion of 15N suggesting enhanced dinitrogen fixation (7).
), (C) bulk stable nitrogen isotopes (δ15N), concentrations of (D) 1-(O-hexose)-3,25-hexacoasanediol (C26 HG diol), and (E) 1-(O-hexose-)-3,27-octacosanediol (C28 HG diol). Gray shaded intervals represent S1 and S5 sapropel layers. The increased concentrations of the heterocyst glycolipids at times of sapropel deposition coincide with a depletion of 15N suggesting enhanced dinitrogen fixation (7).
The presence of HGs in the sapropel layers is direct proof that heterocystous N2-fixing cyanobacteria played a prominent role in the N-cycle in the eastern Mediterranean Sea during sapropel formation. These heterocystous cyanobacteria may have been free-living species as found in freshwater and brackish environments like the Baltic Sea (5). Under open marine conditions, however, free-living heterocystous species have only been reported in a few occasions (24), but they can form massive blooms as endosymbionts of diatoms such as Rhizosolenia sp. and Hemiaulus sp. (5). Reconstruction of the sea surface salinity of the Mediterranean Sea demonstrated that although surface salinity decreased substantially during sapropel deposition, it did not become truly brackish, remaining between 39‰-33‰ (25). Given these still typical marine salinities of the surface waters at the time of sapropel deposition, it is likely that heterocystous cyanobacteria living in association with diatoms, rather than free-living heterocystous species, were the important class of N2-fixers. High concentrations of diatoms have previously been reported from the S5 layer and invoked to explain the organic-rich nature of this deposit (26). Therefore, it is likely that symbiotic heterocystous cyanobacteria played a major role in sustaining primary production during sapropel formation by providing a source of “new” combined nitrogen.
Azolla-Nostocaceae Symbiosis in the Eocene Arctic Ocean.
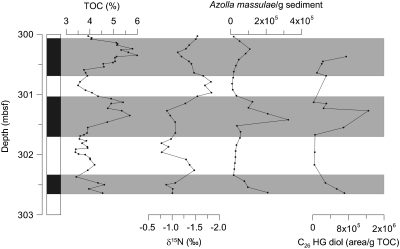
Early/Middle Eocene sediments from the central Arctic Ocean were found to contain abundant remains of Azolla megaspores and microspore massulae as well as Azolla-specific biomarkers (27, 28). The fact that the free floating aquatic fern Azolla grew and reproduced in the Eocene Arctic (27, 29) is strong evidence that the surface waters of the Arctic Basin freshened considerably as Azolla cannot thrive at high salinities (> 3‰; 29). At the same time, bottom waters appear to have remained saline, facilitating deep-water anoxia and salinity stratification (27, 29, 30). The Azolla interval is characterized by total organic carbon (TOC) values between 3 and 6 wt%, covarying with Azolla spore abundance (Fig. 3). Bulk sedimentary nitrogen isotope ratios are persistently low, between -0.7% and -2.4%, throughout the Azolla interval, and average around -1% at peak Azolla occurrences (Fig. 3). These values are consistent with the reported nitrogen isotopic composition of diazotrophic cyanobacteria (10) and cultured Azolla biomass with a similarly low average δ15N of -1.5% (28). The bulk sedimentary nitrogen isotope ratios are also substantially lower than δ15N-values in the Early Eocene Arctic Ocean preceding the Azolla interval which range from +1 to +4% (31). Hence, the encountered δ15N values point towards the presence of N2-fixing organisms during the Early/Middle Eocene Azolla interval in the Arctic Ocean.
Fig. 3.
Depth profile of the ACEX, IODP leg 302 Hole 4A, Core 11× from the Middle Eocene Arctic ocean showing the stratigraphic profiles of (A) TOC, (B) Azolla megaspore counts, (C) bulk stable nitrogen isotopes (δ15N), and concentrations of (D) 1-(O-hexose)-3,25-hexacoasanediol (C26 HG diol). Gray shaded intervals represent layers of enhanced Azolla abundance (see 29 for further details).
Extant Azolla species are known to live in symbiosis with N2-fixing heterocystous cyanobacteria of the genera Nostoc and Anabaena (32). These symbionts are located inside a highly specialized cavity in the dorsal leaf lobe of Azolla and provide the fern with fixed organic nitrogen. Through the symbiosis the aquatic fern Azolla is not limited by fixed nitrogen availability (32), thus facilitating rapid growth (33, 34). Indeed, analyses of extracts of extant Azolla filiculoides show the presence of 1-(O-hexose)-3,25-hexacosanediol (C26 HG diol) (I) and its corresponding keto-ol (II) (Table S1), the predominant glycolipids in the order of Nostocaceae (14).
To investigate whether Azolla lived already in symbiosis with N2-fixing cyanobacteria in the Eocene, we analyzed Early/Middle Eocene sediments from the central Arctic Ocean (Integrated Ocean Drilling Program (IODP) site 302, Lomonosov Ridge), containing abundant remains of Azolla, for their HG content. We detected the C26 diol HG in the sediments and found that HG concentrations covaried with Azolla abundance throughout the interval (Fig. 3). The HGs were absent after the last occurrence of Azolla. The good correlation between the Azolla megaspore counts and HG abundance, and the similar distribution of HGs in the Eocene sediments and extant Azolla suggests that the symbiotic relationship between Azolla and diazotrophic cyanobacteria of the order of Nostocaceae was already established in the Early/Middle Eocene. Furthermore, in view of the inferred marine setting of the site during the Eocene, atmospheric nitrogen fixation by these N2-fixing heterocystous symbiotic cyanobacteria probably played an important role in supplying fixed nitrogen and sustaining Azolla growth in the strongly stratified Eocene Arctic Ocean. However, negative 15N-values are also observed during times of low Azolla counts and HG concentrations, as observed previously (29), suggesting that N2-fixation was a persistent feature at these times (29, 31).
Conclusions
Our results show that HGs can be preserved in ancient sediments of up to 49 million years old where they serve as unique biomarker lipids of N2-fixing heterocystous cyanobacteria, enabling to trace the past ecology of these microbes. Screening of ancient sediments showed that these diazotrophs have played an important role in the nitrogen cycling of past stratified marine and freshwater environments, including those in past greenhouse worlds such as the Eocene. Examples from the Pleistocene Eastern Mediterranean and the Eocene Arctic Ocean illustrate that heterocystous cyanobacteria, likely in symbiosis with diatoms and the freshwater fern Azolla, respectively, have played a key role in supplying newly fixed nitrogen to these past stratified marine systems.
Materials and Methods
Sample Collection.
The heterocystous cyanobacterium Anabaena CCY9613 was grown as an axenic batch culture on the freshwater medium BG11 (35). In order to induce the formation of heterocysts, combined nitrogen sources were omitted from the media. The culture was inoculated in 250 mL Erlenmeyer flasks containing 100 mL of sterile medium and maintained at an alternating 12∶12 h light-dark regime with a light intensity ranging between 5 and 30 μmol m-2 s-1. The culture was grown at a temperature of 14 °C. Azolla filiculoides was initially collected from a ditch near arable land in the surroundings of Elst, The Netherlands (N51°55′48′′; E5°50′6′′) and further cultivated under semicontrolled conditions. Also for Azolla the nutrient solution used did not contain a fixed source of nitrogen.
Pleistocene sapropels (S1 and S5) were collected from the piston core MS66PC, recovered from the deep-sea Nile fan, Eastern Mediterranean (location: 33N1.9′ 31E47.9′) in 2004 during the MIMES MEDIFLUX program. The core was subsampled onboard and the sediments were placed in sterile 50 mL Greiner tubes and frozen at -40 °C immediately. Sediment samples from the Eocene Azolla interval were obtained from lithological Unit 2, Core M0004A-11X taken during the IODP 302 Arctic Coring Expedition (ACEX) expedition at the Lomonosov Ridge, 87.87 °N, 136.18 °E (36). In this study we used sediments from 300 to 302.63 mbsf (meters below sea floor), covering part of the Azolla interval (27). The ACEX sediments and sediments of other locations were generally stored at -20 °C prior to analysis.
Analysis of Bulk Geochemical Parameters and Isotopes.
TOC stable carbon isotopes of organic matter (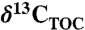 ) and bulk stable nitrogen isotopes (δ15N) were analyzed in duplicate on a ThermoScience Delta Plus isotope ratio mass spectrometer connected on-line to a Carlo Erba Instruments Flash 1112 elemental analyzer. Samples for TOC and
) and bulk stable nitrogen isotopes (δ15N) were analyzed in duplicate on a ThermoScience Delta Plus isotope ratio mass spectrometer connected on-line to a Carlo Erba Instruments Flash 1112 elemental analyzer. Samples for TOC and 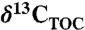 measurements were treated with 2 M hydrochloric acid, neutralized, and freeze-dried prior to analysis. The δ13C is given relative to the Vienna PeeDee Belemnite standard and the δ15N of each sample is expressed relative to atmospheric dinitrogen. Precision is better than ± 0.1‰ for carbon and ± 0.2‰ for nitrogen.
measurements were treated with 2 M hydrochloric acid, neutralized, and freeze-dried prior to analysis. The δ13C is given relative to the Vienna PeeDee Belemnite standard and the δ15N of each sample is expressed relative to atmospheric dinitrogen. Precision is better than ± 0.1‰ for carbon and ± 0.2‰ for nitrogen.
Extraction of Heterocyst Glycolipids.
Heterocyst glycolipids were extracted as previously described by Bauersachs and coworkers (14). Briefly, freeze-dried cell material (30–50 mg) was extracted using a modified Bligh and Dyer extraction procedure (37, 38). Freeze-dried sediments (1–5 g) were extracted using the accelerated solvent extraction technique with a solvent mixture of dichloromethane (DCM):methanol (MeOH; 3∶1 v/v) at high temperature (100 °C) and pressure (20 kPa). The extraction efficiencies of the different extraction methods used in this study were found to be similar for all heterocyst glycolipids (see SI Text and Figs. S4, S5). The bulk of the solvent was first removed by rotary evaporation under vacuum and the remaining extract was subsequently dried under a stream of nitrogen. The residue was dissolved by sonication (10 min) in DCM/MeOH (9∶1, v/v) through a 0.45 μm regenerated cellulose filter (Alltech) prior to HPLC/MS-MS analysis. Procedure blanks were produced with each extraction session and analyzed along with the samples as described below: none of the blanks showed peaks above three times the background level.
Development of HPLC/MS-MS Method.
Normal-phase HPLC analysis of extracts was accomplished using an Agilent 1100 series LC (Agilent) coupled to a Thermo TSQ Quantum ultra Extended Mass triple quadrupole mass spectrometer with an Ion Max Source with electrospray ionization (ESI) probe (Thermo Electron Corporation) operated in positive ion mode following details published earlier (14, 39) with some modifications. Briefly, separation was achieved on a LiChrospher Diol column (250 mm × 2.1 mm internal diameter, 5 μm: Alltech) maintained at 30 °C. Injection volumes ranged from 1 μL for cultures to 10 μL for sediments. Heterocyst glycolipids were eluted using the following linear gradient with a flow rate of 0.2 mL min-1: 90% eluent A to 70% eluent A—30% eluent B in 10 min and held for 20 min, followed by 70% eluent A to 35% eluent A—65% eluent B in 15 min and held for 15 min, subsequently back to 90% eluent A in 1 min and held for 20 min to reequilibrate the column. Eluent A was composed of hexane/isopropanol/formic acid/14.8 M aqueous NH3 (79∶20∶0.12∶0.04, v/v/v/v) and eluent B was isopropanol/water/formic acid/14.8 M aqueous NH3 (88∶10∶0.12∶0.04, v/v/v/v). HPLC/MS-MS analysis was performed in selective reaction monitoring (SRM) mode. SRM transitions were optimized by direct infusion experiments of culture extracts containing the various HGs of interest. Table S3 lists the protonated molecular and selected product ions and respective collision energies for maximal abundance for each of the monitored HG. The selectivity of the newly developed SRM method was demonstrated on cyanobacterial cultures previously shown to contain HGs as dominant compounds, i.e., Anabaena CCY9613, Nostoc CCY0012, and Calothrix CCY9923 (14). Fig. S3 depicts the heterocyst glycolipid distribution of Anabaena CCY 9613 as analyzed in data dependant full scan mode and under SRM conditions, respectively. Retention times of the various HGs were verified by repeated analysis of two cultures containing all analyzed HGs at the start of each analytical sequence. Solvent and procedure blanks were monitored at regular intervals to detect potential cross contamination and prevent false positive identifications of HGs.
Supplementary Material
Acknowledgments.
We thank three anonymous reviewers and the Editor for comments which improved this manuscript. H. Vogel, B. Wagner, M. Melles, P. De Deckker, C. Slomp, H. Mort, J. Werne, Y. van Breugel, J. Weijers, D. Verschuren, G. de Lange, and L. Schwark are acknowledged for supplying a number of the studied sediments; L. Stal and J. Campaore for providing cyanobacterial cultures, and M. van Kempen for providing cultured Azolla. Sediments were recovered from Lake Challa as part of the CHALLACEA project. Sediments from the Eocene Arctic were provided by the Integrated Ocean Drilling Program (IODP). Financial support for this research was provided by the Darwin Center for Biogeosciences, the Royal NIOZ, and the University of Utrecht awarded (to J.S.S.D. and G.J.R.). G.J.R. acknowledges the Statoil Company for additional financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information on-line at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007526107/-/DCSupplemental.
References
- 1.Deutsch C, Sarmiento JL, Sigman DM, Gruber N, Dunne JP. Spatial coupling of nitrogen inputs and losses in the ocean. Nature. 2007;445:163–167. doi: 10.1038/nature05392. [DOI] [PubMed] [Google Scholar]
- 2.Capone DG, et al. Nitrogen fixation by Trichodesmium spp: an important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochem Cy. 2005;19:GB2024. doi: 10.1029/2004GB002331. [Google Scholar]
- 3.Church MJ, Bjorkman KM, Karl DM, Saito MA, Zehr JP. Regional distributions of nitrogen-fixing bacteria in the Pacific Ocean. Limnol Oceanogr. 2008;53:63–77. [Google Scholar]
- 4.Ploug H. Cyanobacterial surface blooms formed by Aphanizomenon sp and Nodularia spumigena in the Baltic Sea: small-scale fluxes, pH, and oxygen microenvironments. Limnol Oceanogr. 2008;53:914–921. [Google Scholar]
- 5.Villareal TA. In: Marine pelagic cyanobacteria: Trichodesmium and other diazotrophs. Carpenter EJ, Capone DG, Rueter JG, editors. Dordrecht, The Netherlands: Kluwer Academic; 1992. pp. 163–175. [Google Scholar]
- 6.Tomitani A, Knoll AH, Cavanaugh CM, Ohno T. The evolutionary diversification of cyanobacteria: molecular phylogenetic and paleontological perspectives. Proc Nat Acad Sci USA. 2006;103:5442–5447. doi: 10.1073/pnas.0600999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs JP, Repeta DJ. Oligotrophy and nitrogen fixation during eastern Mediterranean sapropel events. Science. 1999;286:2485–2488. doi: 10.1126/science.286.5449.2485. [DOI] [PubMed] [Google Scholar]
- 8.Kuypers MMM, van Breugel Y, Schouten S, Erba E, Sinninghe Damsté JS. N2-fixing cyanobacteria supplied nutrient N for Cretaceous oceanic anoxic events. Geology. 2004;32:853–856. [Google Scholar]
- 9.Rau GH, Arthur MA, Dean WA. 15N/14N variations in Cretaceous Atlantic sedimentary sequences: implications for past changes in marine nitrogen biogeochemistry. Earth Planet Sc Lett. 1987;82:269–279. [Google Scholar]
- 10.Wada E, Hattori A. Natural abundance of 15N in particulate organic matter in the North Pacific Ocean. Geochim Cosmochim Acta. 1976;40:249–251. [Google Scholar]
- 11.Summons RE, Jahnke LL, Hope JM, Logan GA. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature. 1999;400:554–557. doi: 10.1038/23005. [DOI] [PubMed] [Google Scholar]
- 12.Walsby AE. Cyanobacterial heterocysts: terminal pores proposed as sites of gas exchange. Trends Microbiol. 2007;15:340–349. doi: 10.1016/j.tim.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Gambacorta A, Pagnotta E, Romano I, Trincone A. Heterocyst glycolipids from nitrogen-fixing cyanobacteria other than Nostocaceae. Phytochemistry. 1998;48:801–805. [Google Scholar]
- 14.Bauersachs T, et al. Rapid analysis of long-chain glycolipids in heterocystous cyanobacteria using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:1387–1394. doi: 10.1002/rcm.4009. [DOI] [PubMed] [Google Scholar]
- 15.Bauersachs T, Schouten S, Compaoré J, Stal LJ, Sinninghe Damsté JS. Distribution of heterocyst glycolipids in cyanobacteria. Phytochemistry. 2009;70:1370–1376. doi: 10.1016/j.phytochem.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann L. Marine cyanobacteria in tropical regions: diversity and ecology. Eur J Phycol. 1999;34:371–379. [Google Scholar]
- 17.Arnosti C, Jørgensen BB. Organic carbon degradation in arctic marine sediments, Svalbard: A comparison of initial and terminal steps. Geomicrobiol J. 2006;23:551–563. [Google Scholar]
- 18.Harvey HR, Fallon RD, Patton JS. The effect of organic matter and oxygen on the degradation of bacterial membrane lipids in marine sediments. Geochim Cosmochim Acta. 1986;50:795–804. [Google Scholar]
- 19.Schouten S, Middelburg J, Hopmans EC, Sinninghe Damsté JS. Fossilization and degradation of intact polar lipids in deep subsurface sediments: a theoretical approach. Geochim Cosmochim Acta. 2010;74:3806–3814. [Google Scholar]
- 20.Lipp JS, Morono Y, Inagaki F, Hinrichs K-U. Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature. 2008;454:991–994. doi: 10.1038/nature07174. [DOI] [PubMed] [Google Scholar]
- 21.Pearson A. Biogeochemistry—Who lives in the sea floor? Nature. 2008;454:952–953. doi: 10.1038/454952a. [DOI] [PubMed] [Google Scholar]
- 22.Rossignol-Strick M, Nesteroff W, Olive P, Vergnaudgrazzini C. After the deluge—Mediterranean stagnation and sapropel formation. Nature. 1982;295:105–110. [Google Scholar]
- 23.Thunell RC, Williams DF, Belyea PR. Anoxic events in the Mediterranean Sea in relation to the evolution of Late Neogene climates. Mar Geol. 1984;59:105–134. [Google Scholar]
- 24.Carpenter EJ, Janson S. Anabaena gerdii sp nov., a new planktonic filamentous cyanobacterium from the South Pacific Ocean and Arabian Sea. Phycologia. 2001;40:105–110. [Google Scholar]
- 25.van der Meer MTJ, et al. Hydrogen isotopic compositions of long-chain alkenones record freshwater flooding of the Eastern Mediterranean at the onset of sapropel deposition. Earth Planet Sc Lett. 2007;262:594–600. [Google Scholar]
- 26.Kemp AES, Pearce RB, Koizumi I, Pike J, Rance SJ. The role of mat-forming diatoms in the formation of Mediterranean sapropels. Nature. 1999;398:57–61. [Google Scholar]
- 27.Brinkhuis H, et al. Episodic fresh surface waters in the Eocene Arctic Ocean. Nature. 2006;441:606–609. doi: 10.1038/nature04692. [DOI] [PubMed] [Google Scholar]
- 28.Speelman EN, Reichart GJ, de Leeuw JW, Rijpstra WIC, Sinninghe Damsté JS. Biomarker lipids of the freshwater fern Azolla and its fossil counterpart from the Eocene Arctic Ocean. Org Geochem. 2009;40:628–637. [Google Scholar]
- 29.Speelman EN, et al. The Eocene Arctic Azolla bloom: environmental conditions, productivity and carbon drawdown. Geobiology. 2009;7:155–170. doi: 10.1111/j.1472-4669.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- 30.Stickley CE, Koc N, Brumsack HJ, Jordan RW, Suto I. A siliceous microfossil view of middle Eocene Arctic paleoenvironments: a window of biosilica production and preservation. Paleoceanography. 2008;23 PA1S14, doi: 10.1029/2007PA001485. [Google Scholar]
- 31.Knies J, Mann U, Popp BN, Stein R, Brumsack H-J. Surface water productivity and paleoceanographic implications in the Cenozoic Arctic Ocean. Paleoceanography. 2008;23 PA1S16, doi: 10.1029/2007PA001455. [Google Scholar]
- 32.Braun-Howland EB, Nierzwicki-Bauer SA. Azolla-Anabaena azaollae symbiosis: biochemistry, ultrastructure, and molecular biology. In: Rai AN, editor. Handbook of symbiotic cyanobacteria. Boca Raton, FL: CRC Press; 1990. pp. 65–118. [Google Scholar]
- 33.Peters GA, Meeks JC. Azolla-Anabaena symbiosis: basic biology. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:193–210. [Google Scholar]
- 34.Wagner GM. Azolla: a review of its biology and utilization. Bot Rev. 1997;63:1–26. [Google Scholar]
- 35.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories, and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 36.Backman J, Moran K, McInroy DB, Mayer LA Expedition-Scientists. Arctic coring expedition. Proc Integr Ocean Drill Progr. 2006;302 (doi: 10.2204/iodp.proc.302.2006, Integrated Ocean Drilling Program Management International Inc., Edinburgh) [Google Scholar]
- 37.Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 38.Rütters H, Sass H, Cypionka H, Rullkötter J. Phospholipid analysis as a tool to study complex microbial communities in marine sediments. J Microbiol Meth. 2002;48:149–160. doi: 10.1016/s0167-7012(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 39.Sturt HF, Summons RE, Smith K, Elvert M, Hinrichs KU. Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry—new biomarkers for biogeochemistry and microbial ecology. Rapid Commun Mass Spectrom. 2004;18:617–628. doi: 10.1002/rcm.1378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.