Abstract
An important mechanism by which IFN-γ primes macrophages for enhanced innate immune responses is abrogation of feedback inhibitory pathways. Accordingly, IFN-γ abrogates endotoxin tolerance, a major negative feedback loop that silences expression of inflammatory cytokine genes in macrophages previously exposed to endotoxin/Toll-like receptor (TLR) ligands. Mechanisms by which IFN-γ inhibits endotoxin tolerance have not been elucidated. Here, we show that pretreatment with IFN-γ prevented tolerization of primary human monocytes and restored TLR4-mediated induction of various proinflammatory cytokines, including IL-6 and TNFα. Surprisingly, IFN-γ did not alter proximal TLR4 signaling defects in tolerized monocytes. Instead, IFN-γ blocked tolerance-associated down-regulation of IL6 and TNF transcription, RNA polymerase II recruitment, and NF-κB and CCAAT/enhancer-binding protein β transcription factor binding to the IL6 and TNF promoters in tolerized monocytes. The mechanism by which IFN-γ restored IL6 expression was by facilitating TLR4-induced recruitment of chromatin remodeling machinery to the IL6 promoter and promoting IL6 locus accessibility in tolerized monocytes. Our results suggest that IFN-γ overcomes endotoxin tolerance by facilitating TLR-induced chromatin remodeling to allow expression of proinflammatory genes. These results identify a mechanism by which IFN-γ promotes activation of macrophages and highlight the importance of chromatin remodeling and transcriptional control in the regulation of inflammatory cytokine production in tolerant and activated macrophages.
Keywords: inflammation, innate immunity, macrophage
Endotoxin tolerance is a phenomenon whereby previous exposure of cells or organisms to microbial products, such as Toll-like receptor (TLR) ligands, induces a transient period of hyporesponsiveness on subsequent endotoxin challenge and is characterized by diminished release of proinflammatory cytokines, such as IL-6 and TNFα (1, 2). This negative feedback mechanism is important for protecting the host against tissue damage and lethality caused by excessive inflammation.
Tolerance results from a complex interplay among many factors involved in multiple levels of TLR signal transduction that altogether reinforce the refractory state. Most previous work has focused on regulation of proximal TLR signaling and has shown that tolerant macrophages exhibit decreased TLR-induced MAPK and NF-κB activation because of defective assembly of TLR adaptor proteins and induction of negative regulators of TLR signaling, such as IL-1 receptor-associated kinase M (IRAK-M), SH2-containing inositol phosphatase (SHIP), and MAPK phosphatase 1 (MKP-1) (1–6). However, mechanisms that globally suppress TLR signaling cannot explain why certain genes are nontolerizable and are induced after TLR stimulation of tolerant cells (7). More recent work has highlighted the importance of gene-specific regulation in tolerant macrophages, which is mediated by modification of chromatin to silence a subset of TLR-inducible genes. Silencing is achieved by acquisition of nonpermissive histone modifications and a block in TLR-induced nucleosome remodeling, resulting in decreased accessibility of gene loci to transcription factors (7, 8). Such gene-specific regulation allows differential expression of subsets of TLR-inducible genes (for example, induction of antimicrobial genes important for host defense concomitant with silencing of inflammatory genes that can cause excessive toxicity) (7, 9, 10).
IFN-γ is a potent endogenous activator of macrophages that augments responses to activating stimuli such as TLR ligands by various mechanisms (11). One important function of IFN-γ is reversal of endotoxin tolerance and restoration of inflammatory cytokine production, which has been shown in vitro and in vivo in mice and humans (12–16). Importantly, the in vivo biological significance of IFN-γ–mediated reversal of endotoxin tolerance has been established in human patients. IFN-γ treatment overcomes the down-regulation in serum IL-6 and TNFα levels in cancer patients exposed to repeated LPS injections given as antitumor therapy and restores monocyte host defense capabilities in sepsis patients (17, 18). The latter is significant, because it has become increasingly clear that sepsis patients exhibit tolerance-related defects in monocyte and macrophage functions, leading to immunosuppression and increased susceptibility to secondary infections that represent a leading cause of mortality in this population (19). Despite these numerous observations establishing the importance of IFN-γ–mediated abrogation of macrophage tolerance, underlying molecular mechanisms have not been previously investigated. In addition, little is known about mechanisms of tolerance in primary human macrophages, because most studies have used human cell lines or murine systems.
In this study, we wished to investigate mechanisms by which IFN-γ abrogates endotoxin tolerance. We used primary human monocytes to maximize relevance of results for human subjects and compare tolerance mechanisms with those previously described in mice. Because IFN-γ–mediated augmentation of TLR responses has conventionally been viewed to occur through positive enhancement of TLR signal transduction, we tested the hypothesis that IFN-γ blocks tolerance by overcoming tolerance-associated defects in TLR signaling. Unexpectedly, IFN-γ did not alter upstream TLR pathway signaling defects in tolerized monocytes. Instead, we found that IFN-γ inhibited tolerance-mediated down-regulation of IL6 and TNF by promoting recruitment of transcription factors NF-κB and CCAAT/enhancer-binding protein β (C/EBPβ) and RNA polymerase II (Pol II) to endogenous gene promoters. IFN-γ restored accessibility of the IL6 promoter in tolerized monocytes by facilitating TLR-induced chromatin remodeling. These results provide the first insights into mechanisms that block endotoxin tolerance and highlight the importance of gene-specific regulation in determining the tolerant state of macrophages.
Results
IFN-γ Efficiently Blocks Tolerization of IL-6 and TNFα.
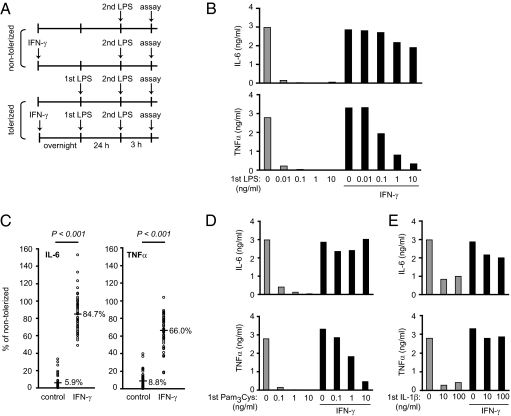
We first used primary human monocytes to extend previous work showing that IFN-γ can block the development of endotoxin tolerance. Control and IFN-γ–activated human monocytes were tolerized with increasing doses of LPS for 24 h before being restimulated with a second dose of LPS, and IL-6 and TNFα produced in response to LPS rechallenge were measured (Fig. 1A). LPS tolerization doses as low as 10 pg/mL were sufficient to severely blunt the ability of control monocytes to produce both IL-6 and TNFα proteins on LPS rechallenge (Fig. 1B and Fig. S1A). In contrast, IFN-γ pretreatment efficiently counteracted tolerance-induced down-regulation of IL-6 and TNFα. Interestingly, tolerization of IL-6 was readily prevented by IFN-γ, even at high tolerizing doses of LPS, whereas tolerization of TNFα was more resistant to inhibition by IFN-γ at these high LPS doses. These results were highly reproducible in experiments with more than 50 independent blood donors; tolerized control monocytes produced 5.9% of the IL-6 and 8.8% of the TNFα produced by their nontolerized counterparts, whereas tolerized monocytes preactivated with IFN-γ produced 84.7% of the IL-6 and 66.0% of the TNFα produced by their nontolerized counterparts (Fig. 1C). IFN-γ also inhibited the establishment of cross-tolerance of TLR4 responses by the TLR2 ligand Pam3Cys or the proinflammatory cytokine IL-1β (Fig. 1 D and E and Fig. S1 B and C). Thus, IFN-γ is highly effective at blocking the development of endotoxin tolerance in primary human monocytes.
Fig. 1.
IFN-γ blocks tolerization of IL-6 and TNFα. (A) Experimental design. (B) Control and IFN-γ–activated monocytes were tolerized with increasing doses of LPS (first LPS: 0.01–10 ng/mL) for 24 h and then challenged with 10 ng/mL LPS for 3 h. IL-6 and TNFα levels in culture supernatants were measured by ELISA. (C) Control and IFN-γ–activated monocytes were tolerized with 0.1 ng/mL LPS for 24 h and then challenged with 10 ng/mL LPS for 3 h. Percent tolerization of IL-6 and TNFα was calculated by dividing cytokine production of tolerized cells by that of nontolerized cells for both control and IFN-γ–treated groups. Data are a summary of 51 independent donors, with each circle representing an independent donor. Numbers show mean percent tolerization of cytokines. P values were calculated by paired Student’s t test. P = 2.4 × 10−28 for IL-6; P = 3.0 × 10−27 for TNFα. (D and E) Control and IFN-γ–activated monocytes were tolerized with increasing doses of (D) Pam3Cys (0.1–10 ng/mL) or (E) IL-1β (10–100 ng/mL) for 24 h and then challenged with 10 ng/mL LPS for 3 h. IL-6 and TNFα levels in culture supernatants were measured by ELISA. Data in B, D, and E show cytokine production in response to the second LPS stimulation and are representative of three independent donors. Cumulative results for different donors are shown in Fig. S1.
IFN-γ Does Not Alter Upstream TLR Signaling Defects in Tolerized Monocytes.
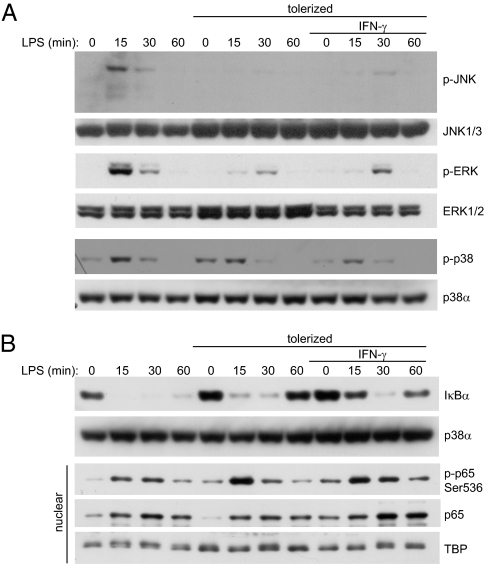
Because endotoxin tolerance is widely reported to be associated with altered TLR signal transduction, we first asked whether IFN-γ restored proinflammatory cytokine production in tolerized monocytes by restoring upstream signaling events. Control monocytes responded to LPS stimulation with robust activation of JNK, ERK, and p38 MAPKs, whereas tolerized monocytes showed diminished activation of MAPKs (Fig. 2A). Unexpectedly, IFN-γ did not alter the defects in MAPK activation in tolerized monocytes (Fig. 2A and Fig. S2 A–C), despite the ability of these monocytes to produce high levels of IL-6 and TNFα. IFN-γ did not alter the MAPK dependence of these cytokines, because specific inhibitors of MAPKs still diminished IL-6 and TNFα production by tolerized monocytes that had been preactivated with IFN-γ (Fig. S2D), suggesting that low-level MAPK signaling is sufficient to activate cytokine production in IFN-γ–treated cells. Control monocytes rapidly degraded IκBα in response to LPS stimulation, leading to NF-κB p65 phosphorylation on Ser536 and translocation into the nucleus (Fig. 2B). Although tolerized monocytes showed slightly slower kinetics in LPS-induced IκBα degradation and more rapid IκBα resynthesis, p65 was still phosphorylated in these monocytes and translocated into the nucleus (Fig. 2B). Higher tolerizing doses of LPS (10 ng/mL) were required to inhibit IκBα degradation. IFN-γ did not have a substantial effect on IκBα degradation or p65 activation in tolerized monocytes (Fig. 2B).
Fig. 2.
IFN-γ does not alter upstream TLR signaling defects in tolerized monocytes. Control and IFN-γ–activated monocytes were tolerized with 0.1 ng/mL LPS and then stimulated with 10 ng/mL LPS for the indicated times. (A) JNK, ERK, and p38 phosphorylation were assessed by Western blot of cytoplasmic extracts. Data are representative of more than nine independent donors. Cumulative results for different donors are shown in Fig. S2 A–C. (B) IκBα degradation, NF-κB p65 Ser536 phosphorylation, and p65 nuclear translocation were assessed by Western blot. Nuclear, nuclear extracts. Data are representative of five to seven independent donors.
A previous study suggested that IFN-γ prevented endotoxin tolerance by inhibiting LPS-induced down-regulation of IRAK-1 expression and promoting IRAK-1/MyD88 complex formation (20). In our system, down-regulation of IRAK-1 was variable and did not occur in half of the donors tested (Fig. S3A). Although we confirmed that IFN-γ restores IRAK-1 expression in tolerized monocytes in a subset of donors (Fig. S3A), the lack of correlation between IRAK-1 expression and either tolerance induction or downstream signaling events suggests that the induction and regulation of tolerization in our system are not predominantly regulated by IRAK-1. The expression of several negative regulators of TLR signaling, including IRAK-M, SHIP, and MKP-1, is up-regulated during endotoxin tolerance in murine macrophages (4–6). In human monocytes, these TLR signaling inhibitors were not induced in tolerized monocytes, although IRAK-M was induced in a subset of donors (Fig. S3B). Consistent with IFN-γ having little effect on upstream signaling pathways in tolerized monocytes, we found that IFN-γ also did not inhibit the expression of these reported mediators of endotoxin tolerance (Fig. S3B). Taken together, these results suggest that IFN-γ is able to abrogate endotoxin tolerance in a manner that is independent of regulation of proximal TLR signaling pathways.
IFN-γ Blocks Tolerization of IL-6 and TNFα at the Level of Transcription.
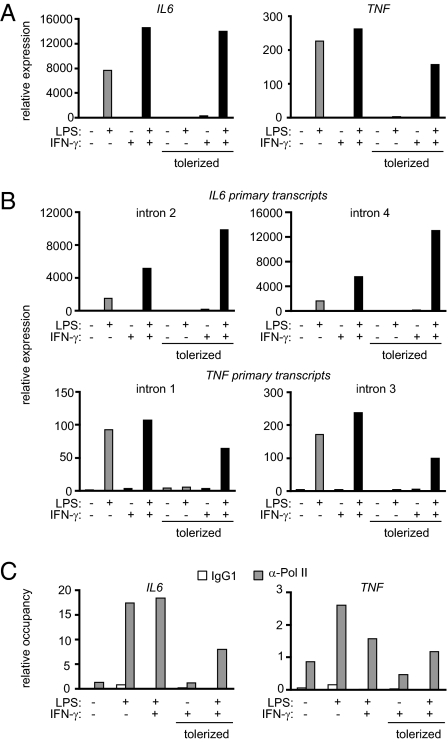
The observation that IFN-γ inhibited endotoxin tolerance without altering proximal TLR signaling defects prompted us to examine downstream nuclear events. LPS strongly induced IL6 and TNF steady-state mRNA in control monocytes, whereas this induction was severely attenuated in tolerized monocytes (Fig. 3A and Fig. S4A). IFN-γ effectively restored LPS-induced IL6 and TNF gene expression in tolerized monocytes to levels comparable with those in control monocytes. Similarly, IFN-γ restored expression of IL1B, IL12A, IL12B, and IL23A but not IL10 in tolerized monocytes (Fig. S5 A and B), suggesting that IFN-γ broadly restores inflammatory gene expression in human monocytes.
Fig. 3.
IFN-γ blocks tolerization of IL-6 and TNFα at the level of transcription. Control and IFN-γ–activated monocytes were tolerized with 0.1 ng/mL LPS and then stimulated with 10 ng/mL LPS for 1 or 3 h for analysis of TNF mRNA and IL6 mRNA, respectively. Real-time PCR was used to measure (A) steady-state mRNA levels and (B) primary transcript levels. Data are representative of four to seven independent donors. Cumulative data from multiple donors are shown in Fig. S4. (C) RNA polymerase II recruitment to the IL6 and TNF promoters was assessed by ChIP after stimulation with LPS for 3 h. Data are representative of three independent donors.
To determine whether IFN-γ–mediated restoration of IL6 and TNF mRNA expression in tolerized monocytes occurred at the level of transcription, we measured primary transcripts using primers specific for intronic regions of the IL6 and TNF genes. The regulation of IL6 and TNF primary transcripts essentially followed the same trend as the steady-state mRNA, and this was confirmed by two independent intronic primer sets for each gene (Fig. 3B and Fig. S4B). We also examined the binding of Pol II by ChIP. LPS-induced recruitment of Pol II to the IL6 and TNF promoters was dramatically blunted in tolerized monocytes and restored by IFN-γ (Fig. 3C). These results suggest that IFN-γ abrogates tolerization of IL6 and TNF at the level of gene transcription. The results also show that IFN-γ can restore expression of a primary response gene (TNF) and a secondary response gene (IL6) that are tolerized by different mechanisms (7, 8, 21, 22).
IFN-γ Restores Transcription Factor Binding to the IL6 and TNF Promoters in Tolerized Monocytes.
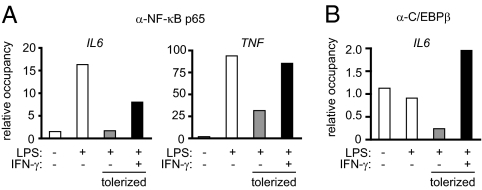
Because IFN-γ restored Pol II recruitment to the IL6 and TNF promoters in tolerized monocytes, we next investigated whether IFN-γ differentially regulated transcription factor binding to these promoters. ChIP assays showed that LPS-induced recruitment of NF-κB p65 to the IL6 and TNF promoters was considerably diminished in tolerized monocytes but efficiently restored by IFN-γ (Fig. 4A). In addition, occupancy of C/EBPβ, another transcription factor important for IL6 gene activation, at the IL6 promoter was defective in tolerized monocytes and was restored by IFN-γ (Fig. 4B). These results suggest that, by facilitating the binding of transcription factors to the IL6 and TNF promoters, IFN-γ can restore Pol II recruitment and subsequent gene transcription in tolerized monocytes.
Fig. 4.
IFN-γ restores transcription factor binding to the IL6 and TNF promoters in tolerized monocytes. Control and IFN-γ–activated monocytes were tolerized with 0.1 ng/mL LPS and then stimulated with 10 ng/mL LPS for 1 or 3 h for analysis of the TNF and IL6 promoters, respectively. (A) NF-κB p65 recruitment to the IL6 and TNF promoters and (B) C/EBPβ binding to the IL6 promoter were assessed by ChIP. Data are representative of three independent experiments per ChIP using cells from different human donors.
IFN-γ Promotes Chromatin Accessibility at the IL6 Promoter in Tolerized Monocytes.
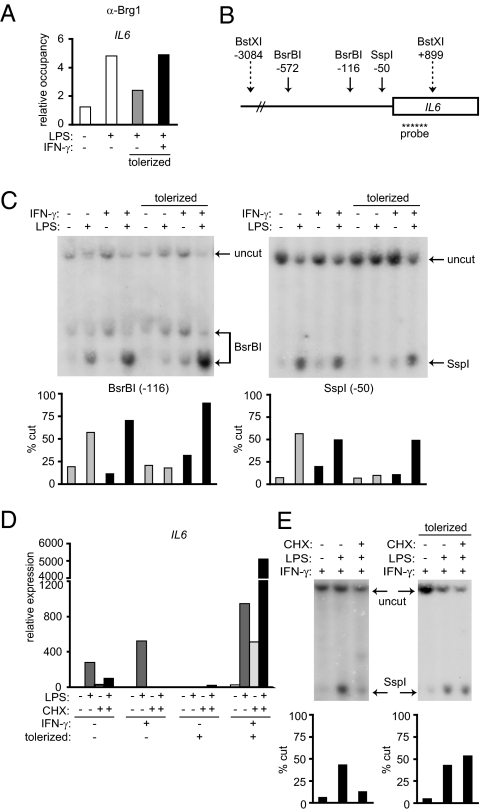
Induction of secondary response genes such as IL6 depends on new protein synthesis and nucleosome remodeling by Brahma-related gene 1 (Brg1)-containing switch/sucrose nonfermenting (SWI/SNF) complexes, which result in increased chromatin accessibility to transcription factors (23, 24). We then investigated whether diminished NF-κB p65 binding to the IL6 promoter in tolerized monocytes (Fig. 4A), despite robust activation and nuclear translocation of p65 (Fig. 2B), could be explained by lack of chromatin accessibility at the IL6 locus and whether chromatin accessibility was regulated by IFN-γ. Consistent with a previous report that used murine macrophages (7), ChIP assays showed that LPS-induced Brg1 recruitment to the IL6 promoter was attenuated in tolerized human monocytes (Fig. 5A). IFN-γ restored Brg1 binding to the IL6 promoter in tolerized monocytes (Fig. 5A), suggesting that restored chromatin accessibility may explain increased transcription factor and Pol II binding to the IL6 promoter in IFN-γ–treated tolerized monocytes.
Fig. 5.
IFN-γ promotes chromatin accessibility at the IL6 promoter in tolerized monocytes. Control and IFN-γ–activated monocytes were tolerized with 0.1 ng/mL LPS and then stimulated with 10 ng/mL LPS for 3 h. (A) Brg1 recruitment to the IL6 promoter was assessed by ChIP. Data are representative of three independent donors. (B) Schematic representation of the proximal IL6 promoter. Solid arrows show restriction enzyme sites used for analysis of chromatin remodeling at the IL6 promoter; dotted arrows show reference enzyme sites. Location of the Southern blot probe is indicated. (C) Nuclei from control and IFN-γ–treated tolerized monocytes were digested with 50 U of BsrBI or SspI for 30 min at 37 °C. Purified genomic DNA was analyzed by Southern blot. Percent of genomic DNA cut by restriction enzyme was quantified by densitometry using ImageJ software. Data shown are representative of four independent donors. (D) Control and IFN-γ–activated monocytes were tolerized with 0.1 ng/mL LPS. Cells were then pretreated with 20 μg/mL cycloheximide (CHX) for 30 min and stimulated with 10 ng/mL LPS for 3 h, and IL6 mRNA levels were measured by real-time PCR. Data are representative of three independent donors. (E) IFN-γ–activated monocytes were tolerized with 0.1 ng/mL LPS or left nontolerized. Cells were then pretreated with CHX and stimulated with 10 ng/mL LPS for 3 h, and accessibility at the SspI site was analyzed by Southern blot as in C. Data shown are representative of four independent donors.
To directly monitor chromatin accessibility at the IL6 promoter, we used the restriction enzyme accessibility assay, a well-established method for analyzing accessibility at endogenous gene loci (24, 25). Accessibility is reflected by increased cleavage by nucleases in the setting of native chromatin structure. We measured accessibility at the BsrBI and SspI restriction endonuclease sites in the IL6 promoter (Fig. 5B) (26–28). Cleavage at the BsrBI and SspI sites upstream of the transcription start site was substantially increased by LPS in control monocytes (Fig. 5C). In contrast, LPS-induced accessibility at these two sites was markedly attenuated in tolerized monocytes. Remarkably, IFN-γ completely restored BsrBI and SspI accessibility in tolerized monocytes in a manner that correlated with IL6 gene expression and binding of transcription factors and chromatin remodeling proteins to the IL6 promoter. Similar results were obtained with NheI, which cleaves at −225. These results suggest that IFN-γ restores the ability of TLR signals to induce IL6 promoter accessibility in tolerized monocytes to allow expression of IL6 during endotoxin tolerance.
Foster et al. (7) previously showed that a subset of nontolerizable genes that were secondary response genes in naïve macrophages was converted into primary response genes in tolerant macrophages. Because IFN-γ essentially converts IL6 from a tolerizable gene to a nontolerizable gene, we wondered whether IFN-γ also altered the transcriptional requirements for IL6 gene expression. We, therefore, measured IL6 gene expression in control and IFN-γ–activated monocytes in the presence of cycloheximide (CHX), a protein synthesis inhibitor. CHX inhibited the induction of IL6 mRNA by LPS in control monocytes, consistent with IL6 being a secondary response gene in macrophages (Fig. 5D) (23, 24). LPS-induced IL6 expression was also dependent on new protein synthesis in IFN-γ–activated nontolerized monocytes. In striking contrast, CHX treatment did not down-regulate but rather, superinduced LPS-stimulated IL6 expression in tolerized monocytes that had been preactivated with IFN-γ (Fig. 5D). In parallel, induction of IL6 promoter accessibility by LPS no longer required new protein synthesis in IFN-γ–activated tolerized monocytes (Fig. 5E). Thus, proteins required for LPS-induced chromatin remodeling and gene expression at the IL6 locus are expressed in IFN-γ–treated tolerized monocytes but not in naïve or IFN-γ–activated nontolerized monocytes. These results indicate that IFN-γ converts IL6 into a primary response gene in tolerized monocytes and suggest that loss of a requirement for new protein synthesis for chromatin remodeling represents one mechanism by which a secondary response gene can become converted into a primary response gene.
Discussion
Multiple mechanisms of macrophage tolerance have been described, but recent evidence has highlighted the importance of establishing a nucleosome barrier that blocks TLR-induced gene expression at the level of chromatin (7). In this study, we found that IFN-γ abrogates the induction of this chromatin barrier at inflammatory cytokine gene loci such as TNF and IL6 in primary human monocytes and thus, restores the recruitment of transcription factors and Pol II. For the secondary response gene IL6, the mechanism of IFN-γ action was to facilitate recruitment of Brg1 and nucleosome remodeling that is required for IL6 expression. Thus, IFN-γ abrogates a potent TLR-induced feedback inhibitory mechanism that silences inflammatory gene expression. These results identify a new mechanism of IFN-γ action and highlight the importance of reciprocal regulation of chromatin structure by activating and inhibitory signals in the regulation of macrophage gene expression and functional phenotype.
Consistent with previous reports, TLR-induced MAPK signaling was diminished in tolerized monocytes relative to naïve monocytes, although NF-κB activation was mostly intact at the low tolerizing doses of LPS that were used. Proximal TLR-induced signaling was not affected by IFN-γ under conditions where IFN-γ fully restored inflammatory cytokine expression in tolerized monocytes. Thus, in IFN-γ–treated tolerized monocytes, a weak MAPK signal was sufficient to fully induce gene expression. This suggests that TLR-induced changes in chromatin structure are sufficient to convert a weak signal into productive gene output in IFN-γ–activated monocytes and that regulation at the level of chromatin is the rate-limiting step in controlling the magnitude of inflammatory gene activation in tolerized macrophages.
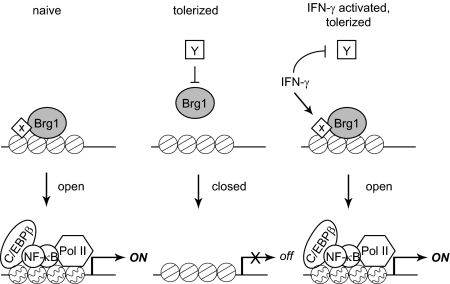
LPS-induced secondary response genes such as IL6 require de novo protein synthesis and subsequent recruitment of Brg1-containing SWI/SNF complexes and nucleosome remodeling for their expression (10). Nucleosome remodeling allows recruitment of transcription factors that are activated by LPS, such as NF-κB and C/EBPβ, and Pol II and subsequent onset of transcription (Fig. 6, Left). Newly synthesized proteins encoded by primary response genes are required for Brg1 recruitment and remodeling of LPS-induced secondary response genes in naïve monocytes (depicted as X in Fig. 6), but the identity of these proteins is not known (10, 24). Similarly, as yet unknown LPS-induced repressors (Fig. 6, Center, Y) have been proposed to silence gene expression in tolerized monocytes, in part by blocking Brg1 recruitment and nucleosome remodeling (7). In the context of this model, which is based on previous reports, IFN-γ can potentially restore gene activation by two mechanisms: (i) induction of factors that recruit Brg1 to the IL6 promoter, and (ii) down-regulation of LPS-induced repressors that establish tolerance and prevent Brg1 recruitment (Fig. 6, Right). Our ability to discriminate between these possibilities is limited because of the lack of knowledge about which factors regulate Brg1 recruitment in LPS-stimulated monocytes (10, 24). We have, however, excluded the possibility that IFN-γ works by down-regulating expression of Bcl3 or RelB, transcription factors that have gene-repressive effects in other tolerance systems (8, 29–31). In addition, silencing of proinflammatory genes such as IL6 in tolerant macrophages has been shown to be associated with loss of TLR-induced histone modifications at specific gene promoters (7). In contrast to murine macrophages, histone H3 and H4 acetylation and H3K4 trimethylation at the IL6 promoter were only weakly and inconsistently affected by LPS stimulation and tolerization in human monocytes, and this precluded obtaining clear results on the effects of IFN-γ. It is not yet clear if the minimal regulation of the histone modifications studied to date reflects a technical issue or species or cell maturation state-related differences, and this will be addressed in future work.
Fig. 6.
Model for IFN-γ–mediated inhibition of endotoxin tolerance. LPS stimulation of naïve monocytes leads to the recruitment of Brg1-containing chromatin remodeling complexes to the IL6 promoter by as yet unknown transcriptional activators (X). Promoter remodeling allows transcription factors and Pol II to access important regulatory elements for gene activation. In contrast, Brg1 is not recruited to the IL6 promoter after stimulation of tolerized monocytes with LPS. Two possibilities are likely: (i) absence of activator X that recruits Brg1, or (ii) presence of transcriptional repressors that block Brg1 recruitment. As a result, the IL6 locus remains closed and inaccessible to transcriptional machinery. IFN-γ abrogates tolerance by restoring the recruitment of Brg1 to the IL6 promoter, possibly through restoration of activator X or antagonism of repressor Y. The IL6 promoter is then remodeled into an open conformation that can bind transcriptional machinery and support gene activation.
We have also found that IFN-γ converted IL6 from a secondary response gene in nontolerized monocytes to a primary response gene in tolerized monocytes that no longer requires new protein synthesis for LPS-induced promoter accessibility. Based on current models (10), this suggests that IFN-γ induced expression of a factor that mediates TLR-induced recruitment of Brg1 to the IL6 locus in tolerized monocytes (24). Notably, LPS stimulation was still required for IL6 chromatin accessibility in IFN-γ–treated tolerized monocytes, indicating that cooperation between IFN-γ and TLR signals is required to open the locus and allow transcription.
IFN-γ blocked the tolerization of inflammatory secondary response genes IL6, IL12A, and IL12B and primary response genes TNF, IL1B, and IL23A but not the antiinflammatory cytokine gene IL10. Thus, IFN-γ restores the expression of proinflammatory factors in tolerized monocytes to promote an inflammatory phenotype. In tolerized monocytes, IFN-γ allowed recruitment of transcription factors to both the primary response gene TNF and the secondary response gene IL6. Tolerization of primary response genes such as TNF that do not require chromatin remodeling for expression proceeds by different mechanisms than tolerization of secondary response genes, and in monocytic THP-1 cells, it involves repressive histone modifications and enrichment of silencing proteins that promote formation of facultative heterochromatin (8, 21, 22). Thus, IFN-γ–mediated prevention of tolerization of primary response genes like TNF likely involves different mechanisms from those described herein for IL6. We have not detected changes in expression or recruitment of transcriptional repressors by IFN-γ. Mechanisms by which IFN-γ restores expression of primary response genes in tolerized monocytes will be investigated in future work.
In summary, we have identified regulation of chromatin remodeling that overcomes barriers to transcription as a mechanism by which IFN-γ antagonizes the establishment of endotoxin tolerance in primary human monocytes. Although tolerance may be a protective mechanism to curb deleterious inflammation in acute settings, it also contributes to the delayed immunosuppression that is an important cause of mortality in patients with sepsis (19). Our findings provide mechanistic insights that can be used to modulate macrophage activation and cytokine production in settings of infection and inflammation.
Materials and Methods
Cell Culture.
Peripheral blood mononuclear cells (PBMCs) were obtained from the blood of healthy donors by density gradient centrifugation using Ficoll (Invitrogen) and a protocol approved by the Hospital for Special Surgery Institutional Review Board. CD14+ human monocytes were purified from PBMCs by positive selection using anti-CD14 magnetic beads as recommended by the manufacturer (Miltenyi Biotec). Monocytes were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen) supplemented with 10% heat-inactivated defined FBS (HyClone), penicillin/streptomycin (Invitrogen), l-glutamine (Invitrogen), and 10 ng/mL human macrophage colony-stimulating factor (M-CSF; Peprotech) in the presence or absence of 100 U/mL human IFN-γ (Roche). For induction of tolerance, monocytes were incubated with 0.1 ng/mL LPS for 24 h, washed and cultured with fresh media for 3 h, and challenged with 10 ng/mL LPS for the indicated times.
ChIP.
ChIP was performed using the ChIP Assay Kit (Millipore) according to the manufacturer's instructions. Immunoprecipitated DNA was analyzed by quantitative real-time PCR and normalized relative to 28S rRNA-encoding gene segments that reflect amounts of nonspecific background DNA precipitation in each reaction. Anti-RNA polymerase II and anti-Brg1 were from Millipore, anti–NF-κB p65 was from Abcam, anti-C/EBPβ was from Santa Cruz Biotechnology, and mouse IgG1 was from BD Biosciences.
Restriction Enzyme Accessibility Assay.
Restriction enzyme accessibility assays were performed as described (25), with slight modifications. Briefly, nuclei were isolated and resuspended in the recommended New England Biolabs buffer containing 50 U of the specified restriction enzymes for 30 min at 37 °C. Digested genomic DNA was purified using the DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer's instructions. Equal amounts of purified DNA were digested to completion with 50 U BstXI overnight at 37 °C, precipitated, and analyzed by Southern blot using a radiolabeled probe specific for the IL6 gene (+51 to +614). BsrBI, SspI, and BstXI restriction enzymes were purchased from New England Biosciences. Densitometry of Southern blots was performed as described in SI Materials and Methods.
Additional methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Stephen T. Smale for providing advice and the protocol for the restriction enzyme accessibility assay. This work was supported by predoctoral fellowships from the Cancer Research Institute and the National Institutes of Health (to J.C.) and National Institutes of Health grants (to L.B.I.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007816107/-/DCSupplemental.
References
- 1.Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: Molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- 2.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Piao W, et al. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. J Leukoc Biol. 2009;86:863–875. doi: 10.1189/jlb.0309189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi K, et al. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 5.Nimah M, et al. Contribution of MKP-1 regulation of p38 to endotoxin tolerance. Shock. 2005;23:80–87. doi: 10.1097/01.shk.0000145206.28812.60. [DOI] [PubMed] [Google Scholar]
- 6.Sly LM, Rauh MJ, Kalesnikoff J, Song CH, Krystal G. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity. 2004;21:227–239. doi: 10.1016/j.immuni.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 8.El Gazzar M, Yoza BK, Hu JY, Cousart SL, McCall CE. Epigenetic silencing of tumor necrosis factor alpha during endotoxin tolerance. J Biol Chem. 2007;282:26857–26864. doi: 10.1074/jbc.M704584200. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 10.Smale ST. Selective transcription in response to an inflammatory stimulus. Cell. 2010;140:833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mengozzi M, Sironi M, Gadina M, Ghezzi P. Reversal of defective IL-6 production in lipopolysaccharide-tolerant mice by phorbol myristate acetate. J Immunol. 1991;147:899–902. [PubMed] [Google Scholar]
- 13.Bundschuh DS, et al. Granulocyte-macrophage colony-stimulating factor and IFN-gamma restore the systemic TNF-alpha response to endotoxin in lipopolysaccharide-desensitized mice. J Immunol. 1997;158:2862–2871. [PubMed] [Google Scholar]
- 14.Randow F, et al. In vitro prevention and reversal of lipopolysaccharide desensitization by IFN-gamma, IL-12, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1997;158:2911–2918. [PubMed] [Google Scholar]
- 15.Haas JG, Meyer N, Riethmüller G, Ziegler-Heitbrock HW. Inhibition of lipopolysaccharide-induced in vitro desensitization by interferon-gamma. Eur J Immunol. 1990;20:1181–1184. doi: 10.1002/eji.1830200535. [DOI] [PubMed] [Google Scholar]
- 16.Matic M, Simon SR. Effects of gamma interferon on release of tumor necrosis factor alpha from lipopolysaccharide-tolerant human monocyte-derived macrophages. Infect Immun. 1992;60:3756–3762. doi: 10.1128/iai.60.9.3756-3762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackensen A, Galanos C, Engelhardt R. Modulating activity of interferon-gamma on endotoxin-induced cytokine production in cancer patients. Blood. 1991;78:3254–3258. [PubMed] [Google Scholar]
- 18.Döcke WD, et al. Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 19.Schefold JC, Hasper D, Volk HD, Reinke P. Sepsis: Time has come to focus on the later stages. Med Hypotheses. 2008;71:203–208. doi: 10.1016/j.mehy.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Adib-Conquy M, Cavaillon JM. Gamma interferon and granulocyte/monocyte colony-stimulating factor prevent endotoxin tolerance in human monocytes by promoting interleukin-1 receptor-associated kinase expression and its association to MyD88 and not by modulating TLR4 expression. J Biol Chem. 2002;277:27927–27934. doi: 10.1074/jbc.M200705200. [DOI] [PubMed] [Google Scholar]
- 21.El Gazzar M, et al. G9a and HP1 couple histone and DNA methylation to TNFalpha transcription silencing during endotoxin tolerance. J Biol Chem. 2008;283:32198–32208. doi: 10.1074/jbc.M803446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Gazzar M, et al. Chromatin-specific remodeling by HMGB1 and linker histone H1 silences proinflammatory genes during endotoxin tolerance. Mol Cell Biol. 2009;29:1959–1971. doi: 10.1128/MCB.01862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez-Carrozzi VR, et al. A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell. 2009;138:114–128. doi: 10.1016/j.cell.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez-Carrozzi VR, et al. Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinmann AS, Plevy SE, Smale ST. Rapid and selective remodeling of a positioned nucleosome during the induction of IL-12 p40 transcription. Immunity. 1999;11:665–675. doi: 10.1016/s1074-7613(00)80141-7. [DOI] [PubMed] [Google Scholar]
- 26.Dijsselbloem N, et al. A critical role for p53 in the control of NF-kappaB-dependent gene expression in TLR4-stimulated dendritic cells exposed to Genistein. J Immunol. 2007;178:5048–5057. doi: 10.4049/jimmunol.178.8.5048. [DOI] [PubMed] [Google Scholar]
- 27.Gerlo S, Haegeman G, Vanden Berghe W. Transcriptional regulation of autocrine IL-6 expression in multiple myeloma cells. Cell Signal. 2008;20:1489–1496. doi: 10.1016/j.cellsig.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Ndlovu N, et al. Hyperactivated NF-kappaB and AP-1 transcription factors promote highly accessible chromatin and constitutive transcription across the interleukin-6 gene promoter in metastatic breast cancer cells. Mol Cell Biol. 2009;29:5488–5504. doi: 10.1128/MCB.01657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoza BK, Hu JY, Cousart SL, Forrest LM, McCall CE. Induction of RelB participates in endotoxin tolerance. J Immunol. 2006;177:4080–4085. doi: 10.4049/jimmunol.177.6.4080. [DOI] [PubMed] [Google Scholar]
- 30.Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signaling by NF-kappaB p50 ubiquitination blockade. Science. 2007;317:675–678. doi: 10.1126/science.1142953. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, El Gazzar M, Yoza BK, McCall CE. The NF-kappaB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance. J Biol Chem. 2009;284:27857–27865. doi: 10.1074/jbc.M109.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.