Abstract
Organic biomolecules that have retained their basic chemical structures over geological periods (molecular fossils) occur in a wide range of geological samples and provide valuable paleobiological, paleoenvironmental, and geochemical information not attainable from other sources. In rare cases, such compounds are even preserved with their specific functional groups and still occur within the organisms that produced them, providing direct information on the biochemical inventory of extinct organisms and their possible evolutionary relationships. Here we report the discovery of an exceptional group of boron-containing compounds, the borolithochromes, causing the distinct pink coloration of well-preserved specimens of the Jurassic red alga Solenopora jurassica. The borolithochromes are characterized as complicated spiroborates (boric acid esters) with two phenolic moieties as boron ligands, representing a unique class of fossil organic pigments. The chiroptical properties of the pigments unequivocally demonstrate a biogenic origin, at least of their ligands. However, although the borolithochromes originated from a fossil red alga, no analogy with hitherto known present-day red algal pigments was found. The occurrence of the borolithochromes or their possible diagenetic products in the fossil record may provide additional information on the classification and phylogeny of fossil calcareous algae.
Keywords: fossil red algae, molecular preservation, phenolic boric acid esters, optical activity, liquid chromatography–mass spectrometry
The striking pink coloration of specimens of the fossil calcareous red alga Solenopora jurassica has been a matter of debate for decades and is well known from the Jurassic of Great Britain (“Beetroot Stone”) (1–3) and France (4). Solenopora specimens at the reported localities are well preserved and exhibit preserved tissue structures and regular alternating bands (2–4) that have been interpreted as seasonal growth structures (3). The characteristic coloration associated with these bands is generally more intense in the inner portions of the algal nodules (2, 3). Given that no traces of the pigments can be found in the surrounding sediment, consisting of white oolitic limestones at both locations, there can be no doubt that the pigments are of endogenous origin. Previous reports speculated that the pigments from the Beetroot Stone likely are porphyrins (2), generally known from bituminous sediments and petroleum (5), whereas the coloration of specimens from France has been attributed to fossil hypericinoid pigments (fringelites) (6), polycyclic quinones described from purple-colored fossil crinoids (7, 8). Here we provide evidence that the pink coloration of S. jurassica from both occurrences is in fact due to the presence of a unique class of complicated boron-containing organic pigments, which we name borolithochromes.
Results and Discussion
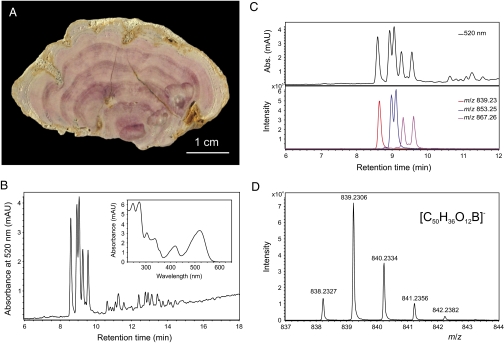
We analyzed distinctly pink-colored S. jurassica specimens (Fig. 1A) from two of the localities reported in the literature, including a part of the neotype from the Beetroot Stone. Following dissolution of the carbonate matrix with HCl, crude extracts were obtained by extraction of the residues with dimethyl sulfoxide (DMSO). The reddish-colored extracts were purified by solid-phase extraction and characterized by HPLC–diode array detection–electrospray ionization–mass spectrometry (HPLC-DAD-ESI-MS). From a large Solenopora sample (102.5 g) from France, 1.1 mg of crude pigment isolate was obtained as an intensely crimson-colored organic residue (Fig. S1). HPLC analysis of the pigments revealed numerous compounds with similar UV-visible spectra, with the prominent group at retention time of 8.0–10.0 min showing a major broad absorption band at 520 nm and a minor one at 420 nm (Fig. 1B), but no Soret band at ~400 nm (which is characteristic of porphyrins). In the negative-ion mass spectra, corresponding ions at mass-to-charge ratios (m/z) of 839, 853, and 867 were detected, indicating that the pigments consist of a homologous series of compounds and accompanying isomers (Fig. 1C). Moreover, all compounds exhibited a characteristic isotope pattern indicative of the presence of a single boron atom (Fig. 1D). Based on accurate mass data obtained by HPLC-MS and additional measurements using Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) for the ions at m/z 839, 853, and 867, the molecular formulae C50H36O12B, C51H38O12B, and C52H40O12B were determined (Fig. 1D and Fig. S2).
Fig. 1.
Specimen of the Jurassic red alga S. jurassica with exceptional preservation of fossil boron-containing organic pigments (borolithochromes) and analytical data of extracted pigments (DMSO extract). (A) Polished slab of S. jurassica (MNHN 40091), Upper Jurassic, Tannay, France. (B) HPLC chromatogram (detection at 520 nm) of extracted fossil pigments (MNHN 23874) and UV-visible spectrum of the first peak in the chromatogram (Inset). (C) Section from the HPLC chromatogram shown in B (Upper) and extracted ion chromatograms (negative-ion ESI-MS) (Lower) of fossil pigments. (D) Mass spectrum of the main single isomeric pigment showing the characteristic isotope pattern of boron, observed m/z 839.2306 [M]−, calculated for C50H36O1211B: 839.2305). Note the difference of 0.9979 Da (calculated for 11B − 10B: 0.9964) between m/z 838 and 839 and the difference of 1.0028 Da (calculated for 13C − 12C: 1.0034) between m/z 839 and 840.
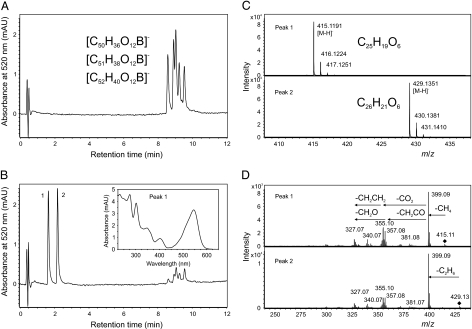
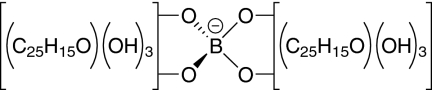
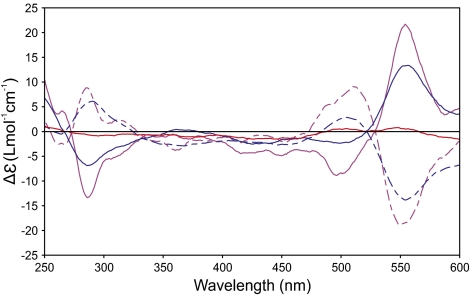
The boron could be readily removed from the borolithochromes by reacting the pigments in methanol containing 0.1% trifluoroacetic acid. Several series of homologous pigments were isolated by HPLC for further analysis. Solvolysis (methanolysis/hydrolysis) of a fraction containing various isomers of the pigments [C50H36O12B]−, [C51H38O12B]−, and [C52H40O12B]− ([M]−) (Fig. 2A) resulted in the formation of only two HPLC peaks (Fig. 2B) with ions at m/z 415 and 429 ([M–H]−), which could be assigned to C25H19O6 and C26H21O6 (Fig. 2C). Accordingly, solvolysis of a fraction containing isomers of the pigments [C48H32O8B]−, [C49H34O8B]−, and [C50H36O8B]− resulted in the formation of two HPLC peaks with ions at m/z 369 and 383 ([M–H]−), which could be assigned to C24H17O4 and C25H19O4 (Fig. S3 A–C). Obviously, two equivalent homologous ligands with different substitution patterns give rise to the combinatorial multitude of homologous and isomeric borolithochromes. All solvolysis products revealed UV-visible spectra similar to the boron-containing precursors (Fig. 2B, Inset) with a distinct bathochromic shift of the long-wavelength absorption. Furthermore, all borolithochromes and all solvolysis products exhibited deuterium-exchangeable protons, indicating the presence of multiple hydroxy groups (Fig. S4). For the homologous borolithochromes [C50H36O12B]− to [C52H40O12B]− ([M]−), six H/D exchanges were observed, compared with two H/D exchanges for homologs [C48H32O8B]− to [C50H36O8B]− ([M]−), suggesting a difference of four hydroxy groups between the two series (Fig. S4 A and B). The distinct hypsochromic shift of 15 nm observed in the UV-visible spectra of the two series indicates that the hydroxy groups are phenolic (Fig. S5). Accordingly, the corresponding products C25H19O6 and C24H17O4 ([M–H]−) (and their homologs) showed four and two H/D exchanges, respectively (Fig. S4 C and D), suggesting a difference of two hydroxy groups between the products. These data strongly imply that the borolithochromes are boric acid esters with two phenolic moieties as boron ligands, representing a unique class of spiroborate pigments (Fig. 3). This was also confirmed by 11B NMR spectroscopy of a crude DMSO-d6 extract, which displayed a single peak at 2.7 ppm (Fig. S6) characteristic of borates (9). Moreover, the tetrahedral coordination of the spiroborates is expressed by their chiroptical properties, as revealed by circular dichroism (CD) spectroscopy of individual pigments (Fig. 4). Whereas the single isomeric [C50H36O12B]− is chiroptically inactive, the CD spectra of the chromatographically resolvable isomers of [C51H38O12B]− and [C52H40O12B]− are opposite in sign, indicating that the latter compounds are diastereomers (due to a chiral center in addition to their inherent C2 symmetry; SI Text) (10). Because MS data suggest that the borolithochromes are negatively charged from the borate but the solvolysis products form [M–H]− ions, based on the number of H/D exchanges, a quinoid structure can be excluded. Characteristic fragmentation patterns were obtained by collision-induced dissociation of the ions at m/z 415 and 429 by means of ESI tandem MS (Fig. 2D). Compounds demonstrated elimination of CH4 and C2H6, indicating the presence of alkyl side chains, followed by elimination of CH2CO and CO2. Fragmentation of the ions at m/z 369 and 383 also led to the elimination of CH4 and C2H6 (Fig. S3D), however, followed by elimination of CHO, C2H2, and CO, consistent with a phenolic structure. Based on the molecular formulae of the solvolysis products, all of which require 16 degrees of unsaturation, as well as the lack of significant fragments below m/z 200, it can be concluded that the basic structure of the borolithochrome ligands is a highly condensed aromatic system. The extremely complex composition of organic matter in the samples from Great Britain and France (SI Text, Fig. S7, and Table S1) with very low concentrations of individual compounds, along with the limited availability of fossil specimens with distinct coloration, excluded a detailed structural elucidation of the fossil pigments by means of 1H-, 13C-, and 2D NMR.
Fig. 2.
Solvolysis of borolithochromes and formation of free phenolic compounds. (A) HPLC chromatogram (detection at 520 nm) of a HPLC fraction containing only isomers of the pigments [C50H36O12B]−, [C51H38O12B]−, and [C52H40O12B]− ([M]− at m/z 839, 853, and 867) analyzed immediately after dissolution in methanol containing 0.1% trifluoroacetic acid. (B) HPLC chromatogram of the same solution after storage for 30 min at 50 °C with reaction products (peaks 1 and 2) and UV-visible spectrum of peak 1 (Inset). (C) Mass spectra of the products shown in B (peak 1: observed m/z 415.1191 [M–H]−, calculated for C25H19O6: 415.1187; peak 2: observed m/z 429.1351 [M–H]−, calculated for C26H21O6: 429.1344). (D) Collision-induced dissociation mass spectra of m/z 415.12 and 429.13 shown in C (peaks 1 and 2).
Fig. 3.
Structure of the main single isomeric borolithochrome (C50H36O12B, [M]− at m/z 839).
Fig. 4.
CD spectra of borolithochrome racemate and diastereomers in DMSO [(M)-C50H36O12B + (P)-C50H36O12B], [M]− at m/z 839 (red solid line; first peak in Fig. 1C), (M,R)-C51H38O12B [with an arbitrarily assigned configuration (R) of one of the ligands], [M]− at m/z 853 (blue dashed line; second peak in Fig. 1C), (P,R)-C51H38O12B, [M]− at m/z 853 (blue solid line; third peak in Fig. 1C), (M,R)-C52H40O12B, [M]− at m/z 867 (magenta dashed line; fourth peak in Fig. 1C), and (P,R)-C52H40O12B, [M]− at m/z 867 (magenta solid line; fifth peak in Fig. 1C).
The borolithochromes represent a previously unknown class of organic pigments, fossil as well as recent, and are exceptional in containing the element boron. Because the borolithochromes are found only within pink-colored specimens of Solenopora, and specific chirality as observed in the pigments is a characteristic signature of life, the borolithochrome subchromophores are of unequivocal biological origin. However, two hypotheses could conceivably account for the presence of boron in the pigments: (i) Although present-day natural compounds containing boron are rare (11, 12), the fossil pigments might represent original boron-containing natural products, and (ii) boron might have been introduced during diagenesis. Because of the thermodynamic situation of phenolic spiroborates (13, 14), the diagenetic formation of these spiroborates from unboronated precursor compounds would be rather unlikely. This is consistent with the low boron content of the surrounding limestone (4.5–15.7 ppm), which is within the lower range of other marine carbonates (15), as well as the lack of intermediate single ligand esters in the fossils. It also should be noted that no diagenetic complexation process with boron has been reported in the literature. However, although the present data point to the preservation of primary boron-containing pigments, the latter hypothesis cannot be fully excluded, because diagenetic complexation processes (involving predominately transition metals) are well known for porphyrins (5).
Most fossil organic compounds, particularly those of pre-Cenozoic age, lost their original functional groups during diagenesis, retaining only their basic chemical structures (5). The exceptional preservation of the highly functionalized borolithochromes might be explained by rapid burial of the algae in a high-energy depositional environment (Materials and Methods) and by the occurrence of the pigments within a calcium carbonate matrix. The pigments can be extracted only with organic solvents after dissolution of the carbonate matrix by either the use of a strong acid (HCl or HF) or calcium complexation (EDTA). Because salt formation with divalent ions, such as Ca2+, is believed to be a key factor in the preservation of fossil hypericinoid pigments (due to the strong acidity of specific hydroxy groups) (8, 16), the extraordinary stability of the borolithochromes might be due to salt formation of the negatively charged borates with Ca2+ from the calcitic Solenopora material.
The occurrence of borolithochromes in Middle and Upper Jurassic specimens of Solenopora from different localities in Europe suggests that these compounds were common in solenoporacean algae, a family with a fossil record from the early Paleozoic to the Miocene generally assigned to the Rhodophyta. Even though Solenoporaceae are currently considered a heterogenous group and appear to include chaetetid sponges and receptaculids in addition to red algae (17, 18), S. jurassica and similar fossils from the Jurassic most likely are red algae, given the presence of typical features of coralline algae (e.g., filaments with well-developed cross partitions) (2, 18). Although no indications of the presence of phycoerythrin, the present-day red algal light-harvesting pigment (19), or its possible degradation products were found in S. jurassica, and no boron-containing phenolic pigments are hitherto known from recent organisms, other phenolic pigments within the red algae are represented by the floridorubin pigments (20, 21). The presence of the borolithochromes in S. jurassica neither supports nor call into question the status of this species and related forms as red algae, but offers the possibility of clarifying their assignment if similar pigments were to be found in recent organisms. In any case, the specific fossil pigments are of potential value as biomarkers for a coherent group of organisms. It would be rather unlikely for such stereochemically complicated and highly unusual pigments to have evolved independently in different groups of organisms, such as red algae and sponges. Furthermore, horizontal gene transfers, which might account for the occurrence of similar secondary metabolites in phylogenetically distant organisms, have been documented between eukaryotes only rarely. Thus, the occurrence of the borolithochromes or their possible diagenetic products in the fossil record may have implications for the phylogenetic status of individual taxa and may provide additional information on the relationship between coralline red algae with their Mesozoic and Paleozoic ancestors.
Materials and Methods
Fossil Material and Geological Setting.
Specimens of S. jurassica with distinct pink coloration from two localities reported in the literature were selected from collection material or were collected at the original localities (2, 4): (i) two pink-colored specimens (MNHN 21556 and MNHN 23874; Muséum National d'Histoire Naturelle Paris) from Les Petites-Armoises (Département Ardennes, France) and one intensely pink-colored specimen (MNHN 40091) from Tannay (~2 km northeast of the locality near Les Petites-Armoises), Upper Jurassic, Oxfordian, and (ii) two pink-colored specimens (BMNH V.60741, representing a part of the neotype, and NHMUK PAL PB V 67825; Natural History Museum London) from Foss Cross Quarry near Chedworth (Gloucestershire, Great Britain), Middle Jurassic, Bathonian, White Limestone Formation, Beetroot Stone.
The investigated specimens of S. jurassica from both localities came from oolitic limestones deposited in shallow marine environments. At the French location, Solenopora specimens are found in proximity to coral reefs (4), whereas the Solenopora-bearing Beetroot Stone in Great Britain has no close reefal association (2). The disturbed orientation of the majority of algal masses suggests that benthic organisms were disrupted and rapidly buried under high-energy conditions (2).
Extraction and Isolation of Pigments.
Fragments of pink-colored Solenopora material (8.2–31.1 g) were cleaned with acetone. After dissolution of the carbonate with 10 M HCl, the residues were separated by centrifugation, washed thoroughly with distilled water, and dried overnight at room temperature under vacuum (~10 Torr). Residues were then sequentially extracted by sonication (10 min at 40 °C) and centrifugation in toluene (3×), tetrahydrofuran (3×), and DMSO (1×). Toluene extracts contained no pigments and were not analyzed in detail. The reddish-colored tetrahydrofuran and DMSO extracts were cleaned up by solid-phase extraction. The sorbent (Bondesil C18, 40 μm) was conditioned by washing with acetonitrile. The extracts then were loaded onto the column, and compounds were eluted with acetonitrile. Analysis of tetrahydrofuran and DMSO extracts showed that both extracts contained the same pigments.
Isolation of pigments was done using a 102.5-g sample from of a large specimen (MNHN 23874) from S. jurassica with distinct coloration from Les Petites-Armoises. The material was treated with 10 M HCl and extracted sequentially as described above, but using only toluene and successive portions of DMSO. The dark, reddish-brown DMSO extract was further purified using a modified solid-phase extraction method. The sorbent (Bondesil C18, 40 μm) was conditioned by washing with acetonitrile, followed by acetonitrile/20 mM aqueous ammonium acetate (50:50). The DMSO extract then was loaded onto the column, and the sorbent was washed with acetonitrile/20 mM aqueous ammonium acetate (50:50) to remove organic matrix compounds (brown-colored fraction) and then with water. The pink-colored compounds were eluted with acetonitrile, and the solvent was removed under vacuum, leaving an intensely crimson-colored residue (1.1 mg). Several borolithochrome fractions were obtained by semipreparative HPLC on a Phenomenex Gemini C18 column (150 × 4.6 mm i.d., 5 μm) at 30 °C. The HPLC program consisted of a linear gradient of acetonitrile/20 mM aqueous ammonium acetate (60:40) to 100% acetonitrile in 15 min, followed by isocratic elution at 100% acetonitrile at a flow rate of 1.0 mL min−1. Finally, individual borolithochromes were isolated using a Phenomenex Gemini C18 column (250 × 4.6 mm i.d., 5 μm) at 30 °C. The HPLC program consisted of a linear gradient of acetonitrile/20 mM aqueous ammonium acetate (65:35) to 85% acetonitrile in 40 min, followed by a linear gradient to 100% acetonitrile in 2 min and isocratic elution at 100% acetonitrile at a flow rate of 1.0 mL min−1.
HPLC-MS Analysis.
HPLC-MS measurements were carried out using an Agilent 1100 Series HPLC system with a diode array detector coupled to an Agilent 6520 Q-TOF LC/MS mass spectrometer equipped with an ESI source. Separation was performed at 30 °C on an Agilent Zorbax Eclipse XDB-C18 column (50 × 4.6 mm i.d., 1.8 μm). The HPLC program consisted of a linear gradient of acetonitrile/20 mM aqueous ammonium acetate (50:50) to 100% acetonitrile in 20 min, followed by isocratic elution at 100% acetonitrile at a flow rate of 1 mL min−1. The DAD wavelength was 520 nm, and UV-visible spectra of each peak were recorded in the 200- to 800-nm wavelength range. Extracts were filtered before injection using 0.2-μm polytetrafluoroethylene filters (ReZist; Schleicher & Schuell). Mass spectra were acquired in the negative-ion mode (nebulizer gas pressure, 60 psi; drying gas flow, 12 L min−1; drying gas temperature, 350 °C; capillary voltage, 4.0 kV) over an m/z range of 100–1,300. Mass calibration was obtained using purine and the HP-0921 acetate adduct (C20H21O8N3P3F24) introduced via a reference sprayer. For tandem MS experiments, precursor ions measured at defined retention times during the HPLC run were mass-selected in the quadrupole and fragmented in the collision cell operated at various collision offset voltages. Borolithochromes were fragmented at 80, 120, and 160 V, and the solvolysis products were fragmented at 55 V (m/z 415.12 and 429.13) and 70 V (m/z 369.11 and 383.13).
FT-ICR-MS.
Molecular formulae of the pigments were determined by FT-ICR-MS on a Bruker ApexQe instrument equipped with an ESI source and a 9.4-T superconducting magnet. All spectra were obtained in the negative-ion mode. DMSO extracts were diluted depending on their initial concentration from 1:10 to 1:30 in acetonitrile/water 3:1 (vol/vol) plus 0.1 M ammonia and were delivered to the ESI interface via a syringe pump at 3–6 μL min−1. The solutions were sprayed at 4.5 kV with a nebulizer gas flow of 1.0 L min−1 and a desolvation gas flow of 2.0 L min−1 at 200–220 °C. Depending on the sample concentration and the type of experiment, the ions were accumulated in the collision hexapole for 1.0–4.0 s and then transferred into the ICR cell. The mass spectra were acquired in the broadband mode over an m/z range of 250–1,500 with 2 mega data points. Typically, 32 transients were accumulated for one magnitude spectrum. External mass calibration was performed with a solution of arginine [0.2 mg mL−1 in methanol/water 1:1 (vol/vol)] using [arginine–H]− cluster ions. The same solution was added to the analyte solution to establish internal mass calibration. Generally, a mass accuracy of 1 ppm was achieved.
Solvolysis of Borolithochromes.
Isolated pigments were dissolved in methanol (0.02% H2O, according to Karl Fischer titration) containing 0.1% trifluoroacetic acid (vol/vol). The solution was stored at 50 °C for 30 min, and the reaction was monitored by HPLC-DAD-ESI-MS under the same conditions described above.
H/D Exchange Experiments and MS Analysis.
For H/D exchange experiments, DMSO extracts and isolated fractions dissolved in DMSO were diluted either 3:7 in acetonitrile/H2O 6:1 (vol/vol) or 3:7 in acetonitrile/D2O 6:1 (vol/vol) and delivered to the ESI interface (Agilent 6520 Q-TOF LC/MS mass spectrometer) via a syringe pump at 25 μL min−1. Mass spectra were acquired in the negative-ion mode (nebulizer gas pressure, 20 psi; drying gas flow, 5 L min−1; drying gas temperature, 325 °C; capillary voltage, 3.5 kV) over an m/z range of 100–1,300. Mass calibration was obtained using trifluoroacetic acid and the HP-0921 trifluoroacetic acid adduct (C20H18O8N3P3F27) introduced via a reference sprayer.
11B NMR.
The 11B NMR measurements were done using a part (13.6 g) from the large specimen (MNHN 23874) from Les Petites-Armoises. After dissolution of the carbonate with 10 M HCl, the organic residue was obtained as described above. Then the residue was directly extracted with a minimum volume (0.5 mL) of DMSO-d6. The 11B NMR spectrum (192.54 MHz) was recorded at 298 K on a Bruker Avance DRX 600 NMR spectrometer using a 5-mm inverse triple probe (1H, 13C, broadband) with triple-axis gradient coils. 11B NMR chemical shifts were referenced externally to boron trifluoride etherate. The broad background signal from any boron-containing glasses used in the probe or in the sample tube was removed during the processing using Topspin version 2.1 (Bruker). Conversion of the experimental digital-filtered raw data to analog-filtered data was followed by backward linear prediction of the first 64 data points using 128 coefficients.
CD Spectroscopy.
CD spectra of individual pigments were recorded in DMSO at 20 °C on a Jasco J-810 spectropolarimeter using 1-mm quartz cuvettetes. Spectra were obtained by accumulation of 16 scans over the 250- to 600-nm wavelength range, and then smoothed using a 25-point Savitzky–Golay filter. Concentrations of the sample solutions were in the range of 6.3–11.6 μmol L−1, as determined on a Varian CARY 100 Bio UV-visible spectrophotometer based on the long-wavelength absorption maximum of the crude pigment isolate in DMSO.
Boron Elemental Analysis of Carbonate.
The boron concentration of the oolitic limestone matrix of a S. jurassica specimen (NHMUK PAL PB V 67825) from Foss Cross was determined by laser ablation inductively coupled plasma MS. Data were acquired using a GeoLasC laser ablation system (MicroLas; wavelength 193 nm) and analyzed with a ELAN 6100 DRC quadrupole mass spectrometer (PerkinElmer). Boron concentrations from single hole measurements of matrix components and cement were in the range of 4.5–15.7 ppm.
Supplementary Material
Acknowledgments
We thank H. Kählig (University of Vienna) for 11B NMR measurements, D. Günther (ETH Zurich) for LA-ICP-MS, R. Riding (University of Tennessee) for helpful information on the status of S. jurassica, and P. Davis (Natural History Museum London), J. Dejax (Muséum National d'Histoire Naturelle Paris), and A. E. Richter for fossil samples. Discussions with W. Buchberger (University of Linz) and J. R. Maxwell (University of Bristol) improved the manuscript. This study was supported by Deutsche Forschungsgemeinschaft Grant WO 1491/1-1 (to K.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. V.J.O. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007973107/-/DCSupplemental.
References
- 1.Garwood EJ. Presidential address. Rep Brit Assoc Adv Sci. 1913;1913:453–472. [Google Scholar]
- 2.Harland TL, Torrens HS. A redescription of the Bathonian red alga Solenopora jurassica from Gloucestershire, with remarks on its preservation. Palaeontology. 1982;25:905–912. [Google Scholar]
- 3.Wright VP. Seasonal banding in the alga Solenopora jurassica from the Middle Jurassic of Gloucestershire, England. J Paleontol. 1985;59:721–732. [Google Scholar]
- 4.Lemoine P. Solenopora from the Jurassic of France. Bull Soc Géol Fr. 1927;27:405–417. (in French) [Google Scholar]
- 5.Peters KE, Walters CC, Moldowan JM. The Biomarker Guide. 2nd Ed. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 6.Falk H, Mayr E, Richter AE. Simple diffuse reflectance UV-Vis spectroscopic determination of organic pigments (fringelites) in fossils. Mikrochim Acta. 1994;117:1–5. [Google Scholar]
- 7.Blumer M. Pigments of a fossil echinoderm. Nature. 1960;188:1100–1101. [Google Scholar]
- 8.Wolkenstein K, Gross JH, Falk H, Schöler HF. Preservation of hypericin and related polycyclic quinone pigments in fossil crinoids. Proc R Soc B. 2006;273:451–456. doi: 10.1098/rspb.2005.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidd RG. In: NMR of Newly Accessible Nuclei, Vol. 2, Chemically and Biologically Important Elements. Laszlo P, editor. New York: Academic; 1983. pp. 49–77. [Google Scholar]
- 10.Lightner DA, Gurst JE. Organic Conformational Analysis and Stereochemistry from Circular Dichroism Spectroscopy. New York: Wiley-VCH; 2000. [Google Scholar]
- 11.Dembitsky VM, et al. Natural occurrence of boron-containing compounds in plants, algae and microorganisms. Plant Sci. 2002;163:931–942. [Google Scholar]
- 12.Carrano CJ, Schellenberg S, Amin SA, Green DH, Küpper FC. Boron and marine life: A new look at an enigmatic bioelement. Mar Biotechnol (NY) 2009;11:431–440. doi: 10.1007/s10126-009-9191-4. [DOI] [PubMed] [Google Scholar]
- 13.Schäfer H. Boric acid and oxy compounds, III: Concerning the equilibria between borate ions, catechol, and catechol borate ions in aqueous solution, and concerning the preparation of mono catechol borates. Z Anorg Allg Chem. 1942;250:127–144. (in German) [Google Scholar]
- 14.Okamoto Y, Kinoshita T, Takei Y, Matsumoto Y. Studies on spiroborate complexes, I: A new synthesis of bis-catechol spiroborate and its analogs using 2-amino-4-methylpyridine borane. Polyhedron. 1986;5:2051–2057. [Google Scholar]
- 15.Hemming NG, Hanson GN. Boron isotopic composition and concentration in modern marine carbonates. Geochim Cosmochim Acta. 1992;56:537–543. [Google Scholar]
- 16.Falk H, Mayr E. Concerning bay salt and peri chelate formation of hydroxyphenanthroperylene quinones (fringelites) Monatsh Chem. 1997;128:353–360. [Google Scholar]
- 17.Taylor TN, Taylor EL, Krings M. Paleobotany: The Biology and Evolution of Fossil Plants. 2nd Ed. Burlington, MA: Academic Press; 2009. [Google Scholar]
- 18.Riding R. Solenopora is a chaetetid sponge, not an alga. Palaeontology. 2004;47:117–122. [Google Scholar]
- 19.Rowan KS. Photosynthetic Pigments of Algae. Cambridge, UK: Cambridge Univ Press; 1989. [Google Scholar]
- 20.Saenger P, Rowan KS, Ducker SC. The water-soluble pigments of the red alga Lenormandia prolifera. Phycologia. 1969;7:59–64. [Google Scholar]
- 21.Chevolot-Magueur A-M, et al. Bromo compounds from Rytiphlea tinctoria (Rhodophyceae) Phytochem. 1976;15:767–771. (in French) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.