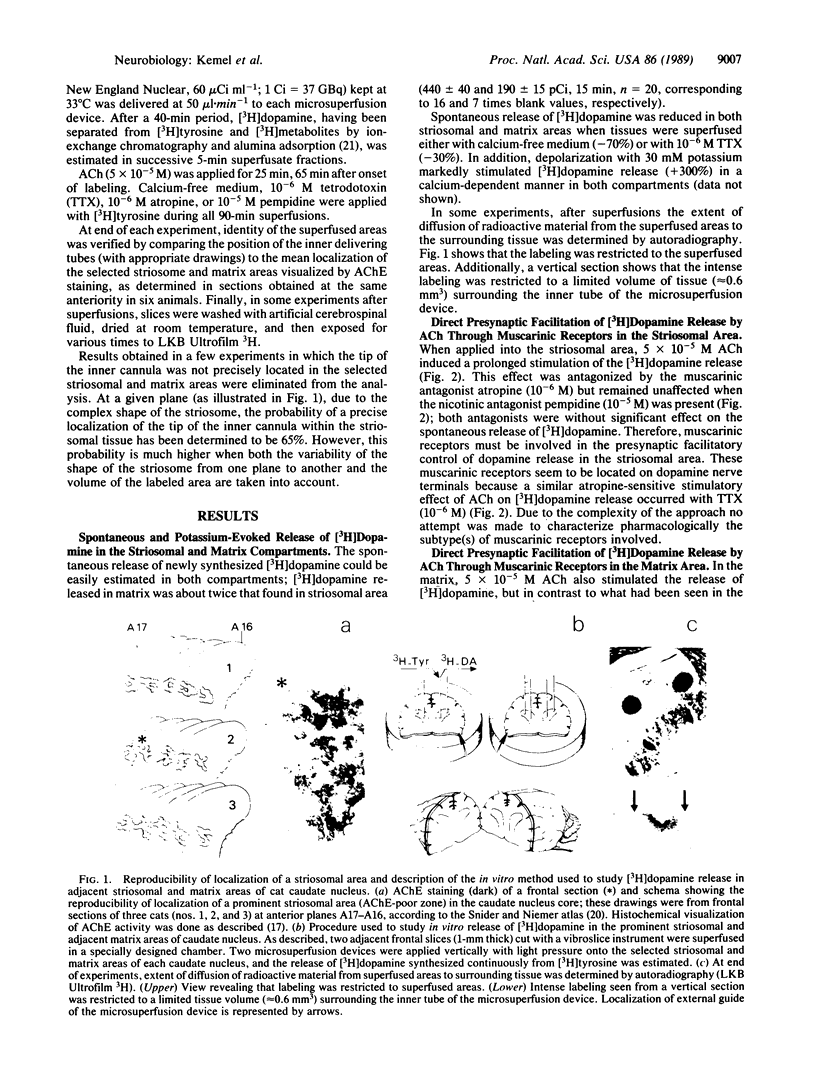
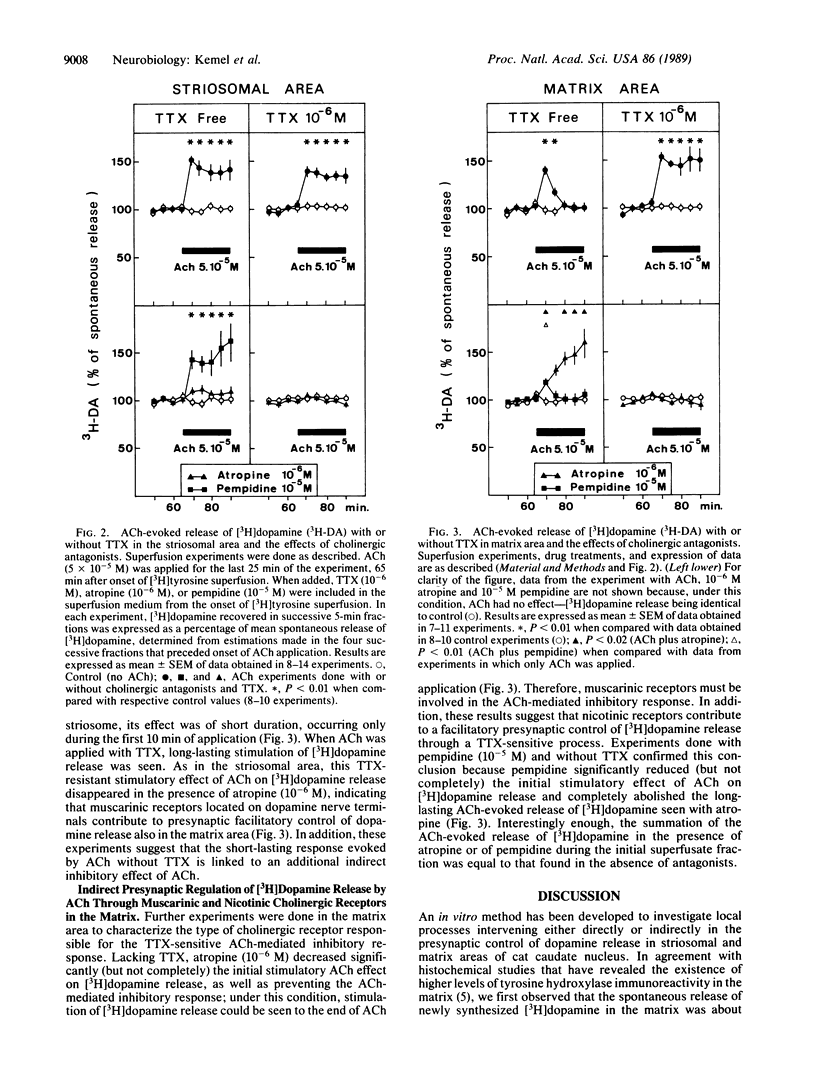
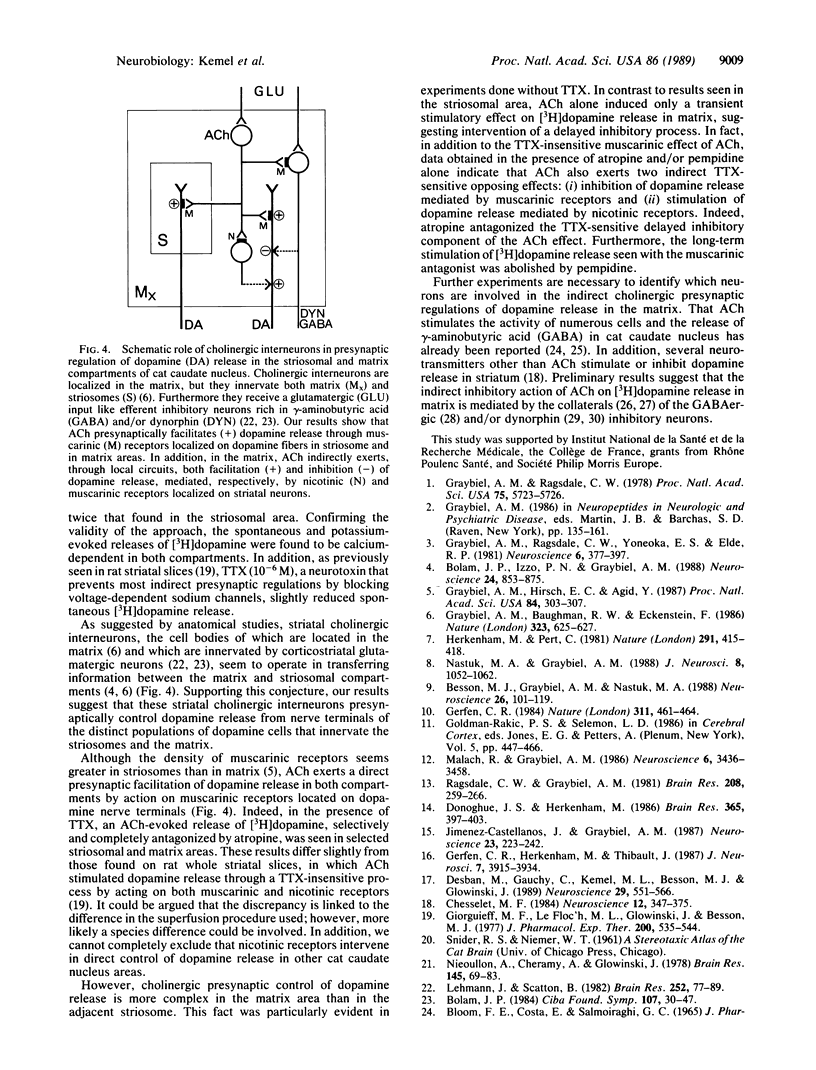
Abstract
By use of a sensitive in vitro microsuperfusion method, the cholinergic prsynaptic control of dopamine release was investigated in a prominent striosome (areas poor in acetylcholinesterase activity) located within the core of cat caudate nucleus and also in adjacent matrix area. The spontaneous release of [3H]dopamine continuously synthesized from [3H]tyrosine in the matrix area was found to be twice that in the striosomal area; the spontaneous and potassium-evoked releases of [3H]dopamine were calcium-dependent in both compartments. With 10(-6) M tetrodotoxin, 5 x 10(-5) M acetylcholine stimulated [3H]dopamine release in both striosomal and matrix areas, effects completely antagonized by atropine (10(-6) M), thus showing the involvement of muscarinic receptors located on dopaminergic nerve terminals. Experiments without tetrodotoxin revealed a more complex regulation of dopamine release in the matrix: (i) In contrast to results seen in the striosome, acetylcholine induced only a transient stimulatory effect on matrix dopamine release. (ii) Although 10(-6) M atropine completely abolished the cholinergic stimulatory effect on [3H]dopamine release in striosomal area, delayed and prolonged stimulation of [3H]dopamine release was seen with atropine in the matrix. The latter effect was completely abolished by the nicotinic antagonist pempidine (10(-5) M). Therefore, in the matrix, in addition to its direct (tetrodotoxin-insensitive) facilitatory action on [3H]dopamine release, acetylcholine exerts two indirect (tetrodotoxin-sensitive) opposing effects: an inhibition and a stimulation of [3H]dopamine release mediated by muscarinic and nicotinic receptors, respectively.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besson M. J., Graybiel A. M., Nastuk M. A. [3H]SCH 23390 binding to D1 dopamine receptors in the basal ganglia of the cat and primate: delineation of striosomal compartments and pallidal and nigral subdivisions. Neuroscience. 1988 Jul;26(1):101–119. doi: 10.1016/0306-4522(88)90130-3. [DOI] [PubMed] [Google Scholar]
- Besson M. J., Kemel M. L., Gauchy C., Glowinski J. Bilateral asymmetrical changes in the nigral release of [3H]GABA induced by unilateral application of acetylcholine in the cat caudate nucleus. Brain Res. 1982 Jun 10;241(2):241–248. doi: 10.1016/0006-8993(82)91060-5. [DOI] [PubMed] [Google Scholar]
- Bolam J. P., Izzo P. N., Graybiel A. M. Cellular substrate of the histochemically defined striosome/matrix system of the caudate nucleus: a combined Golgi and immunocytochemical study in cat and ferret. Neuroscience. 1988 Mar;24(3):853–875. doi: 10.1016/0306-4522(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Bolam J. P. Synapses of identified neurons in the neostriatum. Ciba Found Symp. 1984;107:30–47. doi: 10.1002/9780470720882.ch3. [DOI] [PubMed] [Google Scholar]
- Chesselet M. F. Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neuroscience. 1984 Jun;12(2):347–375. doi: 10.1016/0306-4522(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Desban M., Gauchy C., Kemel M. L., Besson M. J., Glowinski J. Three-dimensional organization of the striosomal compartment and patchy distribution of striatonigral projections in the matrix of the cat caudate nucleus. Neuroscience. 1989;29(3):551–566. doi: 10.1016/0306-4522(89)90130-9. [DOI] [PubMed] [Google Scholar]
- Donoghue J. P., Herkenham M. Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res. 1986 Feb 19;365(2):397–403. doi: 10.1016/0006-8993(86)91658-6. [DOI] [PubMed] [Google Scholar]
- Fonnum F., Gottesfeld Z., Grofova I. Distribution of glutamate decarboxylase, choline acetyl-transferase and aromatic amino acid decarboxylase in the basal ganglia of normal and operated rats. Evidence for striatopallidal, striatoentopeduncular and striatonigral GABAergic fibres. Brain Res. 1978 Mar 17;143(1):125–138. doi: 10.1016/0006-8993(78)90756-4. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Herkenham M., Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987 Dec;7(12):3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984 Oct 4;311(5985):461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Giorguieff M. F., Le Floc'h M. L., Glowinski J., Besson M. J. Involvement of cholinergic presynaptic receptors of nicotinic and muscarinic types in the control of the spontaneous release of dopamine from striatal dopaminergic terminals in the rat. J Pharmacol Exp Ther. 1977 Mar;200(3):535–544. [PubMed] [Google Scholar]
- Graybiel A. M., Baughman R. W., Eckenstein F. Cholinergic neuropil of the striatum observes striosomal boundaries. Nature. 1986 Oct 16;323(6089):625–627. doi: 10.1038/323625a0. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Hirsch E. C., Agid Y. A. Differences in tyrosine hydroxylase-like immunoreactivity characterize the mesostriatal innervation of striosomes and extrastriosomal matrix at maturity. Proc Natl Acad Sci U S A. 1987 Jan;84(1):303–307. doi: 10.1073/pnas.84.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr, Yoneoka E. S., Elde R. P. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience. 1981;6(3):377–397. doi: 10.1016/0306-4522(81)90131-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Pert C. B. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature. 1981 Jun 4;291(5814):415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M., Christensson-Nylander I., Sharp T., Staines W., Reid M., Hökfelt T., Terenius L., Ungerstedt U. Striato-nigral dynorphin and substance P pathways in the rat. II. Functional analysis. Exp Brain Res. 1986;64(1):193–207. doi: 10.1007/BF00238214. [DOI] [PubMed] [Google Scholar]
- Jimenez-Castellanos J., Graybiel A. M. Subdivisions of the dopamine-containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience. 1987 Oct;23(1):223–242. doi: 10.1016/0306-4522(87)90285-5. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Scatton B. Characterization of the excitatory amino acid receptor-mediated release of [3H]acetylcholine from rat striatal slices. Brain Res. 1982 Dec 2;252(1):77–89. doi: 10.1016/0006-8993(82)90980-5. [DOI] [PubMed] [Google Scholar]
- Malach R., Graybiel A. M. Mosaic architecture of the somatic sensory-recipient sector of the cat's striatum. J Neurosci. 1986 Dec;6(12):3436–3458. doi: 10.1523/JNEUROSCI.06-12-03436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastuk M. A., Graybiel A. M. Autoradiographic localization and biochemical characteristics of M1 and M2 muscarinic binding sites in the striatum of the cat, monkey, and human. J Neurosci. 1988 Mar;8(3):1052–1062. doi: 10.1523/JNEUROSCI.08-03-01052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A., Cheramy A., Glowinski J. Release of dopamine evoked by electrical stimulation of the motor and visual areas of the cerebral cortex in both caudate nuclei and in the substantia nigra in the cat. Brain Res. 1978 Apr 21;145(1):69–83. doi: 10.1016/0006-8993(78)90797-7. [DOI] [PubMed] [Google Scholar]
- Preston R. J., Bishop G. A., Kitai S. T. Medium spiny neuron projection from the rat striatum: an intracellular horseradish peroxidase study. Brain Res. 1980 Feb 10;183(2):253–263. doi: 10.1016/0006-8993(80)90462-x. [DOI] [PubMed] [Google Scholar]
- Ragsdale C. W., Jr, Graybiel A. M. The fronto-striatal projection in the cat and monkey and its relationship to inhomogeneities established by acetylcholinesterase histochemistry. Brain Res. 1981 Mar 16;208(2):259–266. doi: 10.1016/0006-8993(81)90556-4. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Bolam J. P., Smith A. D. Monosynaptic cortical input and local axon collaterals of identified striatonigral neurons. A light and electron microscopic study using the Golgi-peroxidase transport-degeneration procedure. J Comp Neurol. 1981 Feb 1;195(4):567–584. doi: 10.1002/cne.901950403. [DOI] [PubMed] [Google Scholar]
- Vincent S., Hökfelt T., Christensson I., Terenius L. Immunohistochemical evidence for a dynorphin immunoreactive striato-nigral pathway. Eur J Pharmacol. 1982 Nov 19;85(2):251–252. doi: 10.1016/0014-2999(82)90477-0. [DOI] [PubMed] [Google Scholar]