Abstract
Humans with inherited mutations in BRCA2 are at increased risk for developing breast and ovarian cancer; however, the relationship between BRCA2 mutation and these cancers is not understood. Studies of Brca2 mutation by gene targeting in mice are limited, given that homozygous Brca2 mutation typically leads to early embryonic lethality. We established a zebrafish line with a nonsense mutation in brca2 exon 11 (brca2Q658X), a mutation similar in location and type to BRCA2 mutations found in humans with hereditary breast and ovarian cancer. brca2Q658X homozygous zebrafish are viable and survive to adulthood; however, juvenile homozygotes fail to develop ovaries during sexual differentiation. Instead, brca2Q658X homozygotes develop as infertile males with meiotic arrest in spermatocytes. Germ cell migration to the embryonic gonadal ridge is unimpaired in brca2Q658X homozygotes; thus, failure of ovarian development is not due to defects in early establishment of the embryonic gonad. Homozygous tp53 mutation rescues ovarian development in brca2Q658X homozygous zebrafish, reflecting the importance of germ cell apoptosis in gonad morphogenesis. Adult brca2Q658X homozygous zebrafish are predisposed to testicular neoplasias. In addition, tumorigenesis in multiple tissues is significantly accelerated in combination with homozygous tp53 mutation in both brca2Q658X homozygous and brca2Q658X heterozygous zebrafish. These studies reveal critical roles for brca2 in ovarian development and tumorigenesis in reproductive tissues.
Keywords: gonad development, meiosis, sex determination, fancd1
Inherited mutations in the human breast cancer 2 gene (BRCA2) are a well-established risk factor for developing ovarian cancer (1, 2). The link between mutations in BRCA2 and ovarian cancer susceptibility is not understood. Epidemiologic evidence suggests that mutations in the “ovarian cancer cluster region” in BRCA2 exon 11 are associated with an increased incidence of ovarian cancer relative to breast cancer in affected families (3, 4). Cancer risk occurs in heterozygous carriers of BRCA2 mutations, but loss of heterozygosity is frequently observed in cancer cells (5).
BRCA2 is a component of the DNA repair machinery and mediates homologous recombination in somatic cells and meiotic recombination in germ cells (6, 7). Mutations in BRCA2 perturb double-strand DNA break repair, and various chromosomal aberrations are observed in BRCA2-deficient cells (6–8). In vivo studies of Brca2 mutation in mouse models are limited, given that homozygous loss of Brca2 by gene targeting leads to decreased cellular proliferation, developmental arrest, and early embryonic lethality in most instances (9, 10).
To investigate the role of brca2 in development and tumorigenesis, we established a zebrafish line with a nonsense mutation in brca2 exon 11 (brca2Q658X), a mutation similar in location and type to BRCA2 mutations in humans with hereditary breast and ovarian cancer (11). We report that brca2 is essential for zebrafish ovarian development. Homozygous brca2Q658X mutation leads to an all-male phenotype in zebrafish, which can be abrogated by concomitant loss of tp53. brca2 also functions as a tumor-suppressor gene in zebrafish. Adult brca2Q658X homozygotes develop testicular tumors, and tumorigenesis is accelerated in multiple tissues by homozygous tp53 mutation in both brca2Q658X heterozygotes and homozygotes. Thus, brca2 plays critical roles in both ovarian development and cancer susceptibility.
Results
brca2 Is Expressed During Embryogenesis and in Adult Gonads.
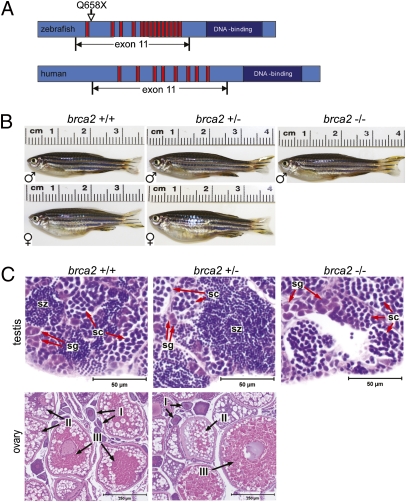
Zebrafish brca2 is homologous to human BRCA2, with conserved structural organization and a large exon 11 in both species (12). The characteristic BRC repeats located in exon 11 of human BRCA2 are also found in exon 11 of the zebrafish brca2 gene (Fig. 1A and Fig. S1).
Fig. 1.
Zebrafish brca2 structure and zebrafish brca2Q658X mutation. (A) Schematic of zebrafish brca2 (Upper) compared with human BRCA2 (Lower). The zebrafish brca2 gene [National Center for Biotechnology Information (NCBI) sequence NC_007126.4] has 16,583 base pairs with 27 exons and encodes a 2874-aa protein (NCBI sequence NP_001103864). Conserved domains include the Rad51-binding BRC repeats (red bars) and the DNA-binding domain (dark-blue box). The zebrafish brca2Q658X mutation (C1971T;Q658X) described herein is shown relative to its position within exon 11. (B) WT and brca2Q658X heterozygotes exhibit distinctly male or female phenotypes, whereas brca2Q658X homozygotes are phenotypically male. (C) Gonads from zebrafish of each genotype reflect observed male or female phenotypes in B. Spermatogenesis is complete in testes from WT and brca2Q658X heterozygotes. Testes from brca2Q658X homozygotes contain only spermatogonia and primary spermatocytes. Ovaries from WT and brca2Q658X heterozygotes are similar and contain developing and mature oocytes. sg, spermatagonia; sc, spermatocytes; sz, spermatozoa; I, stage I oocyte; II, stage II oocyte; III, stage III oocyte. (Scale bars: 50 μm for sections of testis; 250 μm for sections of ovaries.)
Whole-mount in situ hybridization (WISH) for brca2 on WT embryos from the two-cell stage through 24 h postfertilization (hpf) showed abundant expression of brca2 in early cleavage-stage embryos (Fig. S2A), corresponding to maternal mRNA produced during oogenesis (13). Thus, maternally provided brca2 is available for DNA repair before zygotic transcription begins. At 24 hpf, brca2 expression was highest in the developing brain, eye, and ear (Fig. S2A). These findings complement a previous study of brca2 expression during zebrafish embryogenesis (14). In situ hybridization for brca2 in adult WT zebrafish ovaries demonstrated brca2 expression in developing and mature oocytes, with the strongest expression seen in the most mature oocytes (Fig. S2B). In adult WT testes, brca2 was expressed in spermatogonia and developing spermatocytes, but not in mature spermatozoa (Fig. S2B).
brca2 Mutant Zebrafish Are Phenotypically Male and Exhibit Aberrant Spermatogenesis.
To establish a brca2 mutant zebrafish model, we screened an ethylnitrosourea (ENU)-mutagenized library for mutations in brca2 exon 11 by resequencing and identified a zebrafish line carrying a C-to-T point mutation that produces a glutamine (Q) to stop codon (X) change (Fig. 1A). Multiple incrosses of brca2Q658X heterozygotes yielded WT, heterozygous, and homozygous mutant zebrafish in Mendelian ratios, indicating that brca2Q658X homozygotes are fully viable (Table S1).
At sexual maturity, all brca2Q658X homozygotes were phenotypically male, whereas both the WT and brca2Q658X heterozygous cohorts included similar ratios of male and female zebrafish (Fig. 1B). brca2Q658X homozygotes induced egg laying in fertile WT female zebrafish, but eggs collected from these crosses were unfertilized.
On histological examination of the gonads, adult WT and brca2Q658X heterozygotes had either mature ovaries or testes, and gonadal sex was consistent with predicted sex based on physical features and behavior (Fig. 1 B and C). In contrast, all brca2Q658X homozygotes had only testes (Fig. 1C). Spermatogenesis was arrested in testes from brca2Q658X homozygotes, with only spermatogonia and primary spermatocytes present (Fig. 1C). Spermatogenesis was complete in testes from male WT and brca2Q658X heterozygotes (Fig. 1C). The ovaries from female WT and brca2Q658X heterozygotes were morphologically similar, with complete oocyte development and maturation (Fig. 1C).
brca2 Mutant Zebrafish Do Not Develop Juvenile Ovaries.
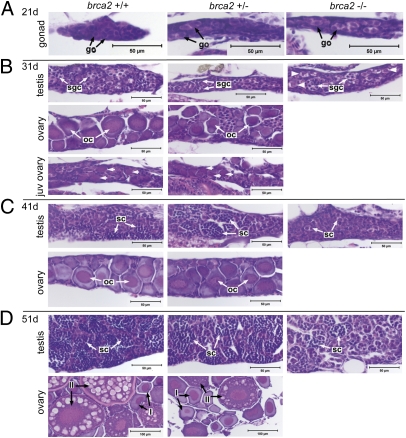
To determine the stage at which brca2 mutation affects sexual differentiation, we examined gonads from juvenile zebrafish of each brca2 genotype by histology at intervals between 21 d and 51 d postfertilization (dpf). During this period, all zebrafish normally develop “juvenile ovaries” that contain gonocytes, early meiotic oocytes, and perinucleolar oocytes (15, 16). With maturation, males undergo oocyte loss and proliferation of spermatogonia and spermatocytes, whereas females continue to experience oocyte proliferation and differentiation (15, 16).
Gonadal development in WT and brca2Q658X heterozygotes was similar at all time points. At 21 dpf, the gonads from zebrafish of both genotypes were undifferentiated and contained gonocytes and scant stroma (Fig. 2A). At 31 dpf, gonads were classified into four morphologic types: undifferentiated (similar to gonads at 21 dpf), juvenile ovary, presumptive ovary, and presumptive testis (Fig. 2B). Juvenile ovaries were distinguished by the presence of perinucleolar oocytes (Fig. 2B, short arrows). Presumptive ovaries exhibited maturing oocytes, whereas presumptive testes exhibited developing spermatogonial cysts with reduction or loss of perinucleolar oocytes (Fig. 2B). By 41 and 51 dpf, definitive ovarian or testicular differentiation was apparent in WT and brca2Q658X heterozygotes (Fig. 2 C and D).
Fig. 2.
Failure of ovarian development occurs in brca2Q658X homozygotes during gonadal development. (A) Gonads from WT, brca2Q658X heterozygotes, and brca2Q658X homozygotes are indistinguishable at 21 dpf. (B) At 31 dpf, WT and brca2Q658X heterozygotes exhibit juvenile ovaries, early testicular differentiation, or early ovarian differentiation, whereas brca2Q658X homozygotes exhibit only early testicular differentiation. Perinucleolar oocytes in juvenile ovaries are indicated by short arrows; putative premeiotic germ cells are indicated by arrowheads. (C and D) At 41 and 51 dpf, WT and brca2Q658X heterozygotes exhibit continued maturation of testes or ovaries, whereas brca2Q658X homozygotes exhibit only testicular development. go, gonocyte; sgc, spermatogonial cyst; oc, oocyte; sc, spermatocyst; I, stage I oocyte; II, stage II oocyte. (Scale bars: 50 μm; 100 μm in 51 dpf ovaries.)
In contrast, oocyte differentiation was not observed in gonads from brca2Q658X homozygotes at any time point. At 21 dpf, gonads from brca2Q658X homozygotes were similar to gonads from WT and brca2Q658X heterozygotes (Fig. 2A). At 31 dpf, gonads from brca2Q658X homozygotes were classified as undifferentiated or presumptive testes (Fig. 2B). The presumptive testes contained germ cells and clusters of round cells encircled by somatic cells, consistent with developing spermatogonial cysts. Gonads from some brca2Q658X homozygotes contained putative premeiotic germ cells (Fig. 2B, arrowheads), which were not observed in gonads from WT or brca2Q658X heterozygotes. Few spermatocysts were observed in developing testes at 41 dpf in brca2Q658X homozygotes compared with WT and brca2Q658X heterozygotes (Fig. 2C). By 51 dpf, brca2Q658X homozygous testes contained only spermatogonia and primary spermatocytes, with fewer spermatocysts compared with WT and brca2Q658X heterozygotes (Fig. 2D). No perinucleolar oocytes were observed in developing gonads from brca2Q658X homozygotes at any time point.
Germ Cell Designation, Development, and Survival in brca2 Mutant Zebrafish.
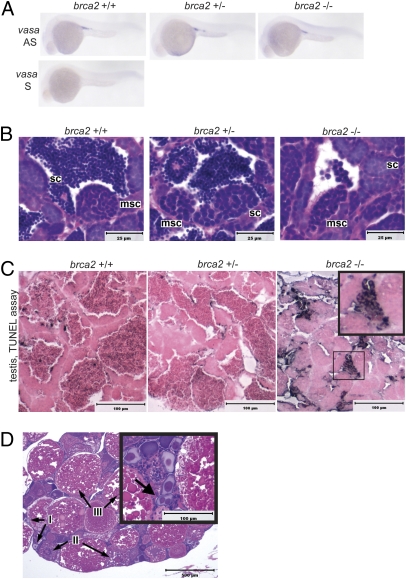
Development of all-male populations has been described in zebrafish with embryonic loss of primordial germ cells (PGCs) or failure of PGCs to migrate to the embryonic gonadal ridge (17, 18). Thus, we evaluated embryos from incrosses of brca2Q658X heterozygous zebrafish for the presence and appropriate localization of PGCs at 24 hpf by WISH for the germ cell marker vasa (Fig. 3A). Expression of vasa in 24-hpf embryos indicated no apparent difference in PGC numbers or localization in WT, brca2Q658X heterozygotes, or brca2Q658X homozygotes (Fig. 3A). Similar results were observed at 4 dpf (Fig. S3).
Fig. 3.
Germ cell specification, development, and survival in embryonic and adult brca2Q658X homozygotes. (A) WISH with antisense (AS) and sense (S) RNA probes for vasa indicates appropriate migration and colonization of the gonadal ridge in WT, brca2Q658X heterozygous, and brca2Q658X homozygous embryos. (B) Testes from WT, brca2Q658X heterozygotes, and brca2Q658X homozygotes show spermatocysts containing meiotic spermatocytes. (C) TUNEL staining of testes from WT, brca2Q658X heterozygotes, and brca2Q658X homozygotes. Multiple clusters of apoptotic spermatocytes are present in testes from brca2Q658X homozygotes (Inset). (D) Homozygous tp53M214K mutation rescues ovarian development in brca2Q658X homozygotes, but female brca2Q658X homozygous;tp53M214K homozygous zebrafish develop binucleate oocytes (Inset). sc, spermatocyst; msc, meiotic spermatocyst; I, type I oocyte; II, type II oocyte; III, type III oocyte. (Scale bars: 25 μm in B, 100 μm in C, and 500 μm in D.)
Although brca2Q658X mutation did not appear to affect embryonic PGC designation, meiotic progression in germ cells was arrested in brca2Q658X homozygotes. Testes from adult brca2Q658X homozygotes showed increased numbers of spermatocysts containing spermatocytes with meiotic nuclear morphology (Fig. 3B). The numbers of spermatocysts containing spermatocytes in meiosis were statistically significantly higher in testes from adult brca2Q658X homozygotes compared with testes from WT (P = 0.0026) and brca2Q658X heterozygotes (P = 0.0009) (Table S2).
Meiotic arrest in testes from adult brca2Q658X homozygotes was accompanied by extensive spermatocyte apoptosis; in contrast, apoptotic cells were rare in testes from adult WT and brca2Q658X heterozygotes. TUNEL assay (Fig. 3C) and immunohistochemistry for cleaved caspase-3 (Fig. S4) demonstrated multiple apoptotic spermatocytes in adult brca2Q658X homozygotes testes, with apoptotic spermatocytes often forming distinct cell clusters (Fig. 3C, Inset).
Homozygous Loss of tp53 Rescues Ovarian Development in brca2 Mutant Zebrafish.
To examine how germ cell apoptosis might impact gonad development in brca2 mutant zebrafish, we crossed brca2Q658X heterozygotes with tp53M214K homozygotes (19). We subsequently incrossed brca2Q658X heterozygous;tp53M214K heterozygous zebrafish, and raised the progeny to adulthood.
Histological examination of the gonads from seven adult brca2Q658X homozygous;tp53M214K homozygous zebrafish revealed that two zebrafish had developed ovaries (Fig. 3D). Oogenesis appeared to be complete in the female brca2Q658X homozygous;tp53M214K homozygous zebrafish; however, the ovaries contained multiple binucleate oocytes (Fig. 3D, Inset), which were not seen in female brca2Q658X heterozygotes or WT zebrafish.
brca2 Mutant Zebrafish Are Predisposed to Testicular Neoplasia.
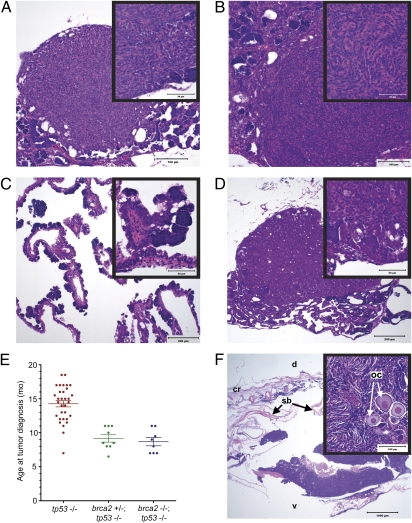
Because BRCA2 mutations in humans are associated predominantly with cancer development in reproductive tissues, we histologically evaluated the testes from aged adult brca2Q658X homozygotes for tumor development. At 10–16 mo of age, 4 out of 13 (31%) brca2Q658X homozygotes developed testicular neoplasia (Fig. 4). Testicular tumors were of both somatic cell and germ cell origin and included undifferentiated stromal cell tumors (Fig. 4 A and B), a papillary cystadenoma (Fig. 4C), and a seminoma (Fig. 4D). Other changes in testes from aged adult brca2Q658X homozygotes included germ cell hyperplasia and dysplasia, as well as segmental testicular degeneration (Fig. S5).
Fig. 4.
brca2Q658X homozygotes are predisposed to testicular neoplasias, and brca2Q658X and tp53M214K mutations have collaborative effects on tumorigenesis. (A) Undifferentiated stromal cell tumor from a brca2Q658X homozygote. (B) Undifferentiated stromal cell tumor from a brca2Q658X homozygote with irregular bundles around poorly defined small tubular structures (Inset). (C) Papillary cystadenoma from a brca2Q658X homozygote. (D) Seminoma from a brca2Q658X homozygote. (E) Age at tumor onset is significantly lower in both brca2Q658X homozygous;tp53M214K homozygous zebrafish and brca2Q658X heterozygous;tp53M214K homozygous zebrafish compared with the tp53M214K homozygous zebrafish parent line. (F) The ovary is a primary site for tumor development in brca2Q658X heterozygous;tp53M214K homozygous zebrafish, with oocyte entrapment (Inset). oc, oocyte; cr, cranial; d, dorsal; v, ventral; sb, swim bladder. (Scale bars: 100 μm in A and B; 200 μm in C and D; 1,000 μm in F; 50 μm in the Insets in A, B, C, and D; 100 μm in the Inset in F.)
In contrast, no testicular tumors were observed in age-matched cohorts of WT (n = 12) and brca2Q658X heterozygotes (n = 12). The hyperplastic, dysplastic, and degenerative changes observed in testes from brca2Q658X homozygotes were not seen in testes from WT and brca2Q658X heterozygotes.
Collaborative Effects of tp53 and brca2 Mutations on Tumorigenesis.
Analysis of tumorigenesis in zebrafish from an incross of brca2Q658X heterozygous;tp53M214K heterozygous zebrafish revealed that combined mutations in brca2 and tp53 accelerated the onset of tumor development (Fig. 4E and Table S3). The mean age at tumor onset was statistically significantly lower in both brca2Q658X homozygous;tp53M214K homozygous zebrafish and brca2Q658X heterozygous;tp53M214K homozygous zebrafish compared with the tp53M214K homozygous parent line (Table S3). Of all tumor-bearing zebrafish collected from the brca2Q658X heterozygous;tp53M214K heterozygous zebrafish incross, only two were brca2Q658X WT;tp53M214K homozygous zebrafish, and their ages at tumor onset were similar to those observed in the tp53M214K homozygous parent line (Table S4).
The majority of tumors observed in both brca2Q658X heterozygous;tp53M214K homozygous zebrafish and brca2Q658X homozygous;tp53M214K homozygous zebrafish were malignant peripheral nerve sheath tumors (Table S4). Interestingly, in three out of seven female brca2Q658X heterozygous;tp53M214K homozygous zebrafish, the ovaries appeared to be the primary site of tumor development (Fig. 4F). Tumor development in one of two female brca2Q658X homozygous;tp53M214K homozygous zebrafish also appeared to mainly involve the ovary (Table S4). Primary tumorigenesis in the gonads was not typical of a subset of tp53M214K homozygous zebrafish evaluated histologically (Table S4) and was not reported in characterization of the tp53M214K zebrafish line (19). In multiple instances, zebrafish developed tumors in more than one anatomic location and/or developed more than one type of tumor (Table S4).
Discussion
Previous studies of Brca2 mutation in murine models have been limited due to the preponderance of early embryonic lethality associated with homozygous loss of Brca2 (10). In zebrafish, we find that homozygous loss of brca2 does not affect embryonic survival. This difference may reflect the relatively late onset of zygotic gene transcription in zebrafish embryos, which begins in cleavage cycle 10 (20). Given that maternal brca2 is abundant in early-stage zebrafish embryos, it is likely that maternal RNA sustains brca2Q658X homozygotes during early development.
BRCA2 is required in germ cells for the repair of double-strand DNA breaks generated during prophase of meiosis I, with loss of BRCA2 resulting in meiotic arrest (21, 22). Our analyses of brca2Q658X homozygous zebrafish demonstrate a conserved role for brca2 in zebrafish germ cell development and spermatogenesis. Histological analyses of more than 90 zebrafish of each genotype derived from 17 different clutches confirmed that the testicular phenotype described herein was correlated with homozygous brca2Q658X mutation in all cases, and was maintained after three outcrosses to WT zebrafish. The patterns of TUNEL staining and cleaved caspase-3 immunoreactivity in brca2Q658X homozygous testes appear to reflect apoptosis of spermatocyte clones, because zebrafish spermatocytes are clonally derived from a single germ cell and organized into discrete groups known as spermatocysts (23).
These studies also reveal an important role for brca2 in ovarian development, raising questions about the participation of brca2 in PGC–stromal cell signaling in the immature gonad. BRCA2, also known as FANC-D1, is a member of the Fanconi anemia (FA) family of proteins that regulate DNA damage response and repair (24). Interestingly, gonadal dysmorphogenesis and hypogonadism are observed in some patients with mutations in various FA genes (25). Hypogonadism and failure of germ cell maturation also have been reported in mouse models with mutations in several FA genes, including a Brca2-deficient mouse model that was rescued with human BRCA2 (22, 26).
brca2 mutation in zebrafish did not affect PGC specification or gonadal colonization in embryos. However, the finding that homozygous tp53 mutation rescued ovarian development in a small number of brca2Q658X homozygotes indicates that germ cell apoptosis is an important contributor to the failure of ovarian development observed in brca2Q658X homozygotes. PGCs can significantly affect zebrafish gonad development and sex determination. Germ cell apoptosis is an important feature of male gonad development (27), and germ cell depletion by gene mutation or knockdown generates predominantly male zebrafish populations with structurally and functionally male gonads (17, 18).
Gonad development in brca2Q658X homozygotes also may be influenced by disrupted meiosis in germ cells, because meiotic progression is required in developing female gonads. In WT animals, including starfish, sea limpets, and Xenopus species, female germ cells initiate meiosis during embryogenesis, whereas male germ cells remain quiescent until sexual maturity (28, 29); thus, the temporal requirements for BRCA2 may differ in female versus male gonads. Given that germ cells from brca2Q658X homozygous embryos are likely to be meiotically incompetent, failure of meiotic progression in brca2-deficient germ cells during embryogenesis might influence stromal differentiation toward testicular development. Thus, the combined effects of germ cell apoptosis and meiotic failure might be the underlying mechanisms that prevent ovarian development in brca2Q658X homozygotes.
Interestingly, multiple binucleate oocytes were present in the rescued brca2Q658X homozygous;tp53M214K homozygous ovaries. A role for BRCA2 in cytokinesis has been suggested, with BRCA2 knockdown or loss resulting in abnormal cell division (30). Although this issue remains controversial (31), our findings support an association between BRCA2 mutation and aberrant cytokinesis.
The impact of BRCA2 mutation on cancer susceptibility, particularly in reproductive tissues, is also conserved in zebrafish. Adult brca2Q658X homozygotes were predisposed to testicular tumors, which were predominantly of somatic cell origin and were not observed in age-matched male siblings. These types of testicular tumors have not been reported previously in zebrafish. Concomitant homozygous tp53M214K mutation accelerates tumorigenesis in multiple tissues in both brca2Q658X heterozygotes and brca2Q658X homozygotes. The majority of zebrafish developed malignant peripheral nerve sheath tumors, similar to those described previously in tp53M214K homozygotes (19); however, a tendency for primary tumor development in the ovary was noted in female brca2Q658X heterozygous;tp53M214K homozygous and brca2Q658X homozygous;tp53M214K homozygous zebrafish. These observations, although limited in number, suggest a specific role for brca2 mutation in gonad tumorigenesis and imply collaborative effects of tp53 and brca2 mutations on tumorigenesis.
Zebrafish with a homozygous mutation of fancl, another member of the FA family, develop as fertile males with some phenotypic similarities to brca2Q658X homozygotes (32). Male zebrafish with a homozygous mutation of the mismatch repair gene mlh1 share some of these characteristics as well (33). Both of these genes are involved in DNA repair; FANCL participates in the FA DNA damage response pathway (34), whereas MLH1 mediates crossover during prophase I (35). Interestingly, an mlh1 mutation in zebrafish does not prevent ovarian development (33), possibly because some germ cells are able to complete meiosis in male and female homozygotes (33, 36). These previous studies, along with the data presented herein, imply that germ cell survival and completion of meiosis are critical factors in zebrafish gonad morphogenesis. Importantly, mutations in fancl and mlh1 are not associated with reproductive tumorigenesis or aberrant ovarian morphology in zebrafish (32, 33). Thus, brca2 mutation uniquely impacts gonadal pathophysiology in zebrafish.
Our findings indicate that brca2 is required for ovarian development in zebrafish and plays an essential role in zebrafish spermatogenesis. They also show that the propensity for tumorigenesis in reproductive tissues in association with BRCA2 mutation appears to be conserved in zebrafish. In humans, the role for BRCA2 in ovarian development and the effects of BRCA2 mutation on intercellular signaling in the gonad remain unknown. Mammalian germ cells appear to play a significant role in both ovarian development and maintenance of the ovarian phenotype (37). Our studies suggest that BRCA2 might be of importance in intercellular interactions in both developing and mature gonads. The implications for BRCA2-associated ovarian cancer are not yet clear; however, these observations provide important insights into how germ cell–stromal cell interactions in the ovary might be related to cancer susceptibility.
Materials and Methods
Zebrafish Maintenance.
All of the experiments with zebrafish were approved by the National Cancer Institute's Animal Care and Use Committee.
WISH of Zebrafish Embryos.
Embryos from incrosses of AB* zebrafish were used for analyses of WT brca2 expression. Embryos from incrosses of brca2Q658X heterozygous zebrafish were used for analyses of vasa expression. Embryos were processed routinely for WISH experiments (see SI Materials and Methods).
In Situ Hybridization of Adult Zebrafish Gonads.
Adult AB* zebrafish were processed routinely for analysis of WT brca2 expression by in situ hybridization (see SI Materials and Methods).
Identification and Establishment of brca2Q658X Mutant Zebrafish Line.
A previously established ENU-mutagenized library (38) was screened by PCR and sequencing with overlapping primer pairs spanning zebrafish brca2 exon 11. The zebrafish brca2Q658X mutant line was identified by analysis of the sequence data using PolyPhred and recovered by in vitro fertilization as described previously (38). In the Zebrafish Model Organism Database (ZFIN), the mutant allele designation for this zebrafish line is brca2hg5.
Histological Analyses.
Zebrafish were euthanized with tricaine methanesulfonate and fixed in 4% paraformaldehyde at 4 °C for a minimum of 24 h before being transferred to 70% ethanol. Specimens were processed routinely for paraffin embedding and preparation of 5-μm H&E-stained sections (Histoserv).
Histological Analyses of Zebrafish Testes by TUNEL Assay.
Testes from 10 adult males (5.5 mo of age) of each genotype were dissected and grouped by genotype for fixation as described above. Sections were processed routinely for TUNEL labeling (see SI Materials and Methods).
Histological Analyses of Zebrafish Testes by Immunohistochemistry for Cleaved Caspase-3.
Testes from 10 adult males (5.5 mo of age) of each genotype were dissected and grouped by genotype for fixation as described above. Sections were processed routinely for immunohistochemistry (see SI Materials and Methods).
Introduction of tp53M214K Mutation.
Zebrafish embryos homozygous for the tp53M214K mutation were acquired from the Zebrafish International Resource Center. Adult tp53M214K homozygotes were crossed with brca2Q658X heterozygotes to establish a cohort of brca2Q658X heterozygous;tp53M214K heterozygous zebrafish, which were subsequently incrossed.
Supplementary Material
Acknowledgments
We thank the sequencing team at Beijing Genomics Institute for help with PCR analyses and sequencing of the ENU-mutagenized library, Kevin Bishop for help with recovery of the mutant line, and Jennifer Edwards for assistance with immunohistochemistry. This research was supported by the National Institutes of Health's Intramural Research Program, the National Cancer Institute, the Center for Cancer Research, and the National Human Genome Research Institute.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011630107/-/DCSupplemental.
References
- 1.Wooster R, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994;265:2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- 2.King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 3.Thompson D, Easton D, Breast Cancer Linkage Consortium Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410–419. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gayther SA, et al. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997;15:103–105. doi: 10.1038/ng0197-103. [DOI] [PubMed] [Google Scholar]
- 5.Gudmundsson J, et al. Different tumor types from BRCA2 carriers show wild-type chromosome deletions on 13q12-q13. Cancer Res. 1995;55:4830–4832. [PubMed] [Google Scholar]
- 6.Thorslund T, West SC. BRCA2: A universal recombinase regulator. Oncogene. 2007;26:7720–7730. doi: 10.1038/sj.onc.1210870. [DOI] [PubMed] [Google Scholar]
- 7.Venkitaraman AR. Functions of BRCA1 and BRCA2 in the biological response to DNA damage. J Cell Sci. 2001;114:3591–3598. doi: 10.1242/jcs.114.20.3591. [DOI] [PubMed] [Google Scholar]
- 8.Yu VP, et al. Gross chromosomal rearrangements and genetic exchange between nonhomologous chromosomes following BRCA2 inactivation. Genes Dev. 2000;14:1400–1406. [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki A, et al. Brca2 is required for embryonic cellular proliferation in the mouse. Genes Dev. 1997;11:1242–1252. doi: 10.1101/gad.11.10.1242. [DOI] [PubMed] [Google Scholar]
- 10.Evers B, Jonkers J. Mouse models of BRCA1 and BRCA2 deficiency: Past lessons, current understanding and future prospects. Oncogene. 2006;25:5885–5897. doi: 10.1038/sj.onc.1209871. [DOI] [PubMed] [Google Scholar]
- 11.Ramus SJ, et al. Contribution of BRCA1 and BRCA2 mutations to inherited ovarian cancer. Hum Mutat. 2007;28:1207–1215. doi: 10.1002/humu.20599. [DOI] [PubMed] [Google Scholar]
- 12.Titus TA, et al. The Fanconi anemia gene network is conserved from zebrafish to human. Gene. 2006;371:211–223. doi: 10.1016/j.gene.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 13.Pelegri F. Maternal factors in zebrafish development. Dev Dyn. 2003;228:535–554. doi: 10.1002/dvdy.10390. [DOI] [PubMed] [Google Scholar]
- 14.Titus TA, et al. The Fanconi anemia/BRCA gene network in zebrafish: Embryonic expression and comparative genomics. Mutat Res. 2009;668:117–132. doi: 10.1016/j.mrfmmm.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maack G, Segner H. Morphological development of the gonads in zebrafish. J Fish Biol. 2003;62:895–906. [Google Scholar]
- 16.Takahashi H. Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bull Fac Fish Hokkaido Univ. 1977;28:57–65. [Google Scholar]
- 17.Weidinger G, et al. dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol. 2003;13:1429–1434. doi: 10.1016/s0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- 18.Siegfried KR, Nüsslein-Volhard C. Germ line control of female sex determination in zebrafish. Dev Biol. 2008;324:277–287. doi: 10.1016/j.ydbio.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Berghmans S, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane DA, Kimmel CB. The zebrafish midblastula transition. Development. 1993;119:447–456. doi: 10.1242/dev.119.2.447. [DOI] [PubMed] [Google Scholar]
- 21.Marcon E, Moens PB. The evolution of meiosis: Recruitment and modification of somatic DNA-repair proteins. Bioessays. 2005;27:795–808. doi: 10.1002/bies.20264. [DOI] [PubMed] [Google Scholar]
- 22.Sharan SK, et al. BRCA2 deficiency in mice leads to meiotic impairment and infertility. Development. 2004;131:131–142. doi: 10.1242/dev.00888. [DOI] [PubMed] [Google Scholar]
- 23.Schulz RW, et al. Spermatogenesis in fish. Gen Comp Endocrinol. 2010;165:390–411. doi: 10.1016/j.ygcen.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Howlett NG, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- 25.Giri N, Batista DL, Alter BP, Stratakis CA. Endocrine abnormalities in patients with Fanconi anemia. J Clin Endocrinol Metab. 2007;92:2624–2631. doi: 10.1210/jc.2007-0135. [DOI] [PubMed] [Google Scholar]
- 26.Wong JC, et al. Targeted disruption of exons 1 to 6 of the Fanconi anemia group A gene leads to growth retardation, strain-specific microphthalmia, meiotic defects and primordial germ cell hypoplasia. Hum Mol Genet. 2003;12:2063–2076. doi: 10.1093/hmg/ddg219. [DOI] [PubMed] [Google Scholar]
- 27.Uchida D, Yamashita M, Kitano T, Iguchi T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol. 2002;205:711–718. doi: 10.1242/jeb.205.6.711. [DOI] [PubMed] [Google Scholar]
- 28.McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- 29.Page AW, Orr-Weaver TL. Stopping and starting the meiotic cell cycle. Curr Opin Genet Dev. 1997;7:23–31. doi: 10.1016/s0959-437x(97)80105-0. [DOI] [PubMed] [Google Scholar]
- 30.Daniels MJ, Wang Y, Lee M, Venkitaraman AR. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science. 2004;306:876–879. doi: 10.1126/science.1102574. [DOI] [PubMed] [Google Scholar]
- 31.Lekomtsev S, Guizetti J, Pozniakovsky A, Gerlich DW, Petronczki M. Evidence that the tumor-suppressor protein BRCA2 does not regulate cytokinesis in human cells. J Cell Sci. 2010;123:1395–1400. doi: 10.1242/jcs.068015. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Marí A, et al. Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 2010;6:e1001034. doi: 10.1371/journal.pgen.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feitsma H, Leal MC, Moens PB, Cuppen E, Schulz RW. Mlh1 deficiency in zebrafish results in male sterility and aneuploid as well as triploid progeny in females. Genetics. 2007;175:1561–1569. doi: 10.1534/genetics.106.068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meetei AR, et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003;35:165–170. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 35.Baker SM, et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- 36.Leal MC, Feitsma H, Cuppen E, França LR, Schulz RW. Completion of meiosis in male zebrafish (Danio rerio) despite lack of DNA mismatch repair gene mlh1. Cell Tissue Res. 2008;332:133–139. doi: 10.1007/s00441-007-0550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guigon CJ, Magre S. Contribution of germ cells to the differentiation and maturation of the ovary: Insights from models of germ cell depletion. Biol Reprod. 2006;74:450–458. doi: 10.1095/biolreprod.105.047134. [DOI] [PubMed] [Google Scholar]
- 38.Sood R, et al. Methods for reverse genetic screening in zebrafish by resequencing and TILLING. Methods. 2006;39:220–227. doi: 10.1016/j.ymeth.2006.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.