A remarkable trademark of most tumors is their ability to break down glucose by glycolysis at a vastly higher rate than in normal tissues, even when oxygen is copious. This phenomenon, known as the Warburg effect, enables rapidly dividing tumor cells to generate essential biosynthetic building blocks such as nucleic acids, amino acids, and lipids from glycolytic intermediates to permit growth and duplication of cellular components during division (1). An assumption dominating research in this area is that the Warburg effect is specific to cancer. Thus, much of the focus has been on uncovering mechanisms by which cancer-causing mutations influence metabolism to stimulate glycolysis. This has lead to many exciting discoveries. For example, the p53 tumor suppressor can suppress glycolysis through its ability to control expression of key metabolic genes, such as phosphoglycerate mutase (2), synthesis of cytochrome C oxidase-2 (3), and TP53-induced glycolysis and apoptosis regulator (TIGAR) (4). Many cancer-causing mutations lead to activation of the Akt and mammalian target of rapamycin (mTOR) pathway that profoundly influences metabolism and expression of metabolic enzymes to promote glycolysis (5). Strikingly, all cancer cells but not nontransformed cells express a specific splice variant of pyruvate kinase, termed M2-PK, that is less active, leading to the build up of phosphoenolpyruvate (6). Recent work has revealed that reduced activity of M2-PK promotes a unique glycolytic pathway in which phosphoenolpyruvate is converted to pyruvate by a histidine-dependent phosphorylation of phosphoglycerate mutase, promoting assimilation of glycolytic products into biomass (7). However, despite these observations, one might imagine that the Warburg effect need not be specific for cancer and that any normal cell would need to stimulate glycolysis to generate sufficient biosynthetic materials to fuel expansion and division. Recent work by Salvador Moncada's group published in PNAS (8) and other recent work from the same group (9, 10) provides exciting evidence supporting the idea that the Warburg effect is also required for the proliferation of noncancer cells.
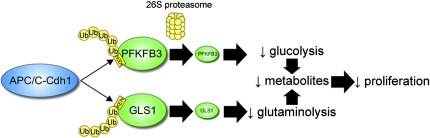
The key discovery was that the anaphase-promoting complex/cyclosome-Cdh1 (APC/C-Cdh1), a master regulator of the transition of G1 to S phase of the cell cycle, inhibits glycolysis in proliferating noncancer cells by mediating the degradation of two key metabolic enzymes, namely 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 (PFKFB3) (9, 10) and glutaminase-1 (Fig. 1) (8). PFKFB3 potently stimulates glycolysis by catalyzing the formation of fructose-2,6-bisphosphate, the allosteric activator of 6-phosphofructo-1-kinase (11). Glutaminase-1 is the first enzyme in glutaminolysis, converting glutamine to lactate, yielding biosynthetic intermediates required for cell proliferation (12).
Fig. 1.
Mechanism by which APC/C-Cdh1 inhibits glycolysis and glutaminolysis to suppress cell proliferation. APC/C-Cdh1 E3 ligase recognizes KEN-box–containing metabolic enzymes, such as PFKFB3 and glutaminase-1 (GLS1), and ubiquitinates and targets them for proteasomal degradation. This inhibits glycolysis and glutaminolysis, leading to decrease in metabolites that can be assimilated into biomass, thereby suppressing proliferation.
APC/C is a cell cycle-regulated E3 ubiquitin ligase that promotes ubiquitination of a distinct set of cell cycle proteins containing either a D-box (destruction box) or a KEN-box, named after the essential Lys-Glu-Asn motif required for APC recognition (13). Among its well-known substrates are crucial cell cycle proteins, such as cyclin B1, securin, and Plk1. By ubiquitinating and targeting its substrates to 26S proteasome-mediated degradation, APC/C regulates processes in late mitotic stage, exit from mitosis, and several events in G1 (14). The Cdh1 subunit is the KEN-box binding adaptor of the APC/C ligase and is essential for G1/S transition. Importantly, APC/C-Cdh1 is inactivated at the initiation of the S-phase of the cell cycle when DNA and cellular organelles are replicated at the time of the greatest need for generation of biosynthetic materials. APC/C-Cdh1 is reactivated later at the mitosis/G1 phase of the cell cycle when there is a lower requirement for biomass generation.
Both PFKFB3 (9, 10) and glutaminase-1 (8) possess a KEN-box and are rapidly degraded in nonneoplastic lymphocytes during the cell cycle when APC/C-Cdh1 is active. Consistent with destruction being mediated by APC-C-Cdh1, ablation of the KEN-box prevents degradation of PFKFB3 (9, 10) and glutaminase-1 (8). Inhibiting the proteasomal-dependent degradation with the MG132 inhibitor markedly increases levels of ubiquitinated PFKFB3 and glutaminase-1 (8). Moreover, overexpression of Cdh1 to activate APC/C-Cdh1 decreases levels of PFKFB3 as well as glutmaninase-1 and concomitantly inhibited glycolysis, as judged by decrease in lactate production. This effect is also observed when cells were treated with a glutaminase-1 inhibitor (6-diazo-5-oxo-l-norleucine) (8). The final evidence supporting the authors’ hypothesis is that proliferation and glycolysis is inhibited after shRNA-mediated silencing of either PFKFB3 or glutaminase-1 (8).
These results are interesting, because unlike most recent work in this area, Colombo et al. (8) link the Warburg effect to the machinery of the cell cycle that is present in all cells rather than to cancer-driving mutations. Further work is required to properly define the overall importance of this pathway, which has thus far only been studied in a limited number of cells. It would also be of value to undertake a more detailed analysis of how the rate of glycolysis and other metabolic pathways vary during the cell cycle of normal and cancer cells, by quantitatively assessing the concentration of key metabolites at different stages of the cell cycle. Strikingly, several other metabolic enzymes involved in biomass generation possess putative KEN-box motifs, namely pyruvate carboxylase, malate dehydrogenase-1, and acetyl-CoA carboxylase-1 (8). It would also be interesting to determine whether expression and ubiquitination of these enzymes is also regulated by APC/C-Cdh1. It would also be worthwhile to investigate whether any mutations reported in human cancers disrupt the KEN-boxes of PFKFB3, glutaminase-1, or any of the other enzymes mentioned above, because this might be expected to enhance glycolysis and proliferation. It would also be appealing to search for mutations that affect the ability of APC/C-Cdh1 to recognize the KEN-box of PFKFB3/glutaminase-1 and/or ubiquitinate these targets. It would also be interesting to analyze the mechanism by which APC/C-Cdh1 is regulated and whether activity and/or levels of this complex are controlled by signaling pathways disrupted in cancer, such as the Akt–mTOR or p53 pathways. Interestingly, Cdh1 is reportedly phosphorylated in vivo on serine and threonine, as well as tyrosine residues on at least 15 residues (http://www.phosphosite.org/). The relevance of these modifications or upstream protein kinases that phosphorylate these sites is unknown.
Nonproliferating neurons have high levels of APC/C-Cdh1 activity, thereby suppressing PFKFB3 expression and maintaining low fructose-2,6-bisphosphate levels (10). Inappropriate stimulation of glycolysis in neuronal cells by overexpression of PFKFB3 or reducing expression of Cdh1 resulted in marked oxidative stress and apoptosis (10). This was attributed to the activation of glycolysis reducing the pool of glucose available to be oxidized through the pentose phosphate pathway, resulting in insufficient regeneration of reduced glutathione (10). These findings imply that in nonproliferating cells such as neurons, high APC/C-Cdh1 activity preserves sufficient glucose levels to maintain antioxidant status at the expense of its utilization for biosynthetic purposes.
As mentioned above, one of the key ways that the p53 tumor suppressor inhibits glycolysis is to promote the expression of the TIGAR enzyme that breaks down
Colombo et al. link the Warburg effect to the machinery of the cell cycle that is present in all cells.
fructose-2,6-bisphosphate (4). Loss of p53 in tumors results in reduced TIGAR levels, thereby elevating fructose-2,6-bisphosphate and stimulating glycolysis. PFKFB isoforms can also be directly activated by phosphorylation by kinases, such as Akt, that are inappropriately activated in many cancers (15). Taken together with the findings that PFKFB3 is a key regulator of glycolysis, these more recent studies provide valuable insights into how fructose-2,6-bisphosphate levels are controlled. They also emphasize the importance of fructose-2,6-bisphosphate in regulating flux through the glycolytic pathway, a phenomena that has been appreciated for many decades (11).
Firmer genetic data validating the importance of the different mechanisms by which glycolysis can be stimulated in cancer and normal cells are required. In the case of PFKFB3 and glutaminase-1, the best way to address this issue would be to generate knockin models, in which the KEN-boxes of PFKFB3 and glutaminase-1 are disrupted, to prevent recognition and ubiquitination by APC/C-Cdh1. The expectation is that glycolysis should be stimulated in the knockin models. It would also be fascinating to define how these KEN-box–ablating mutations impacted on other metabolic and survival pathways and whether they predispose to development of cancer. Similarly, it would be interesting to perform analogous studies with other metabolic knockin/knockout models, for example, those that lack expression of PFKFB3, TIGAR, or PK-M2 or that express a mutant or phosphoglycerate mutase incapable of catalyzing the conversion of phosphoenolpyruvate to pyruvate. Such experiments would provide much needed data to define the relative importance of these different pathways in regulating glycolysis in normal as well as cancer cells.
This knowledge could be exploited to gain better insights into how drugs might be developed that counteract the effects of cancer-causing mutations on stimulating glycolysis. It would be desirable to develop compounds that inhibit the Warburg effect in cancer cells that do not influence the metabolism of normal tissues. If the Warburg effect in normal proliferating cells is mainly mediated through the effects of APC/C-Cdh1 on regulating expression of metabolic enzymes, this may provide hope that it would be possible to develop drugs that suppress glycolysis in cancer cells without interfering with the ability of the APC/C-Cdh1 pathway to promote the Warburg effect in normal proliferating cells.
Footnotes
The authors declare no conflict of interest.
See companion article on page 18868 in issue 44 of volume 107.
References
- 1.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Kondoh H, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–185. [PubMed] [Google Scholar]
- 3.Matoba S, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 4.Bensaad K, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Robey RB, Hay N. Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 7.Vander Heiden MG, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo SL, et al. Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S phase in human T lymphocytes. Proc Natl Acad Sci USA. 2010;107:18868–18873. doi: 10.1073/pnas.1012362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida A, Bolaños JP, Moncada S. E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proc Natl Acad Sci USA. 2010;107:738–741. doi: 10.1073/pnas.0913668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrero-Mendez A, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 11.Hue L, Rider MH. Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues. Biochem J. 1987;245:313–324. doi: 10.1042/bj2450313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBerardinis RJ, Cheng T. Q's next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfleger CM, Kirschner MW. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 14.Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- 15.Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]