Abstract
Background Microbial water-quality indicators, in high concentrations in sewage, are used to determine whether water is safe for recreational purposes. Recently, the use of these indicators to regulate recreational water bodies, particularly in sub/tropical recreational marine waters without known sources of sewage, has been questioned. The objectives of this study were to evaluate the risk to humans from exposure to subtropical recreational marine waters with no known point source, and the possible relationship between microbe densities and reported symptoms in human subjects with random-exposure assignment and intensive individual microbial monitoring in this environment.
Methods A total of 1303 adult regular bathers were randomly assigned to bather and non-bather groups, with subsequent follow-up for reported illness, in conjunction with extensive environmental sampling of indicator organisms (enterococci).
Results Bathers were 1.76 times more likely to report gastrointestinal illness [95% confidence interval (CI) 0.94–3.30; P = 0.07]; 4.46 times more likely to report acute febrile respiratory illness (95% CI 0.99–20.90; P = 0.051) and 5.91 times more likely to report a skin illness (95% CI 2.76–12.63; P < 0.0001) relative to non-bathers. Evidence of a dose–response relationship was found between skin illnesses and increasing enterococci exposure among bathers [1.46 times (95% CI 0.97–2.21; P = 0.07) per increasing log10 unit of enterococci exposure], but not for gastrointestinal or respiratory illnesses.
Conclusions This study indicated that bathers may be at increased risk of several illnesses relative to non-bathers, even in the absence of any known source of domestic sewage impacting the recreational marine waters. There was no dose–response relationship between gastroenteritis and increasing exposure to enterococci, even though many current water-monitoring standards use gastroenteritis as the major outcome illness.
Keywords: Gastrointestinal illness, respiratory illness, skin illness, indicator organisms, enterococci, recreational water quality
Introduction
Traditionally, analogous to drinking water, monitoring of microbial water quality for coastal waters used for recreational purposes has been regulated by measuring the concentrations of indicator microbes. These microbes are those typically found in human faeces in high concentrations, and are not pathogenic. Thus, an elevated concentration of these indicator microbes in coastal waters should indicate that these waters have been contaminated by human sewage, and are unsafe for recreational use.1–5
Recently, the use of indicator microbes to regulate the recreational use of coastal waters has come into question, particularly in subtropical marine environments characterized by no known source of human sewage (i.e. non-point source). Studies conducted in subtropical areas have shown collectively that, in the absence of any known sources of human faecal materials, indicator microbes are consistently present, and may be recovered in high concentrations in the environment.6–14 It is thought that indicator microbes may re-grow due to climate conditions conducive for re-growth, and thus may not be representative of any pathogens that might be present. However, the regulator is presented with a perplexing situation where the possibility of micro-organisms or pathogens within urban run-off might be responsible for an increased risk of illness among bathers. In addition, within subtropical waters, it remains unclear which indicator microbe(s) should be utilized, and once the data are obtained, how these data should be interpreted.5,15
Several epidemiological studies have found that bathing in temperate recreational waters with known point sources of faecal contamination (such as domestic sewage or storm-drain runoff) has been associated with an increased risk for transmission of infectious diseases (including gastroenteritis, and febrile respiratory, skin, eye and ear illnesses).3,16–26 The few epidemiological studies conducted in subtropical environments have shown no statistically significant relationship between human health and current US Environmental Protection Agency (EPA) recommended indicator microbes for evaluating beach water quality in subtropical regions.2,8,12,27 The objective of this study, the first prospective randomized exposure study in non-point source subtropical recreational marine waters, was to evaluate the risks to human health in recreational marine environment with no known source of domestic sewage, and the use of indicator organisms to assess these exposures and risks.
Methods
The design of the epidemiological study was based on past studies at the study site and on the work of Fleisher et al.11,14,16–19,28–30 The investigators conducted a prospective randomized exposure study with participant exposure randomly assigned to either marine recreational waters or to beach-only exposure. The epidemiological study data-collection activities were conducted over 15 individual study days beginning 15 December 2007 and ending 21 June 2008.
Epidemiological study protocol
Local adult residents (≥18 years of age) who reported regular bathing in recreational marine waters were recruited to participate using word of mouth, email and local publicity as recruitment tools. After recruitment, the participants were screened for current illnesses and symptoms, possible alternative risk factors for the study illnesses, and a brief health history; they were then given an appointment for the study beach-exposure day. Members of the same household or family members were recruited, but subject to the same randomization procedures as all other study participants. Possible secondary spread of infection among family members was ascertained by asking ‘Did any member of your family become ill before or after recruitment into the study?’, with control in subsequent statistical analyses.
On the study beach-exposure day, participants read and signed the consent form. At this point, participants were again interviewed briefly about any current illnesses and symptoms, food consumption and beach exposure since the baseline recruitment interview. Study subjects were then randomly assigned to either (exposed) bather group or (unexposed) non-bather group. The bathers were assigned to the bathing station where staff members supervised the exposure activity of each bather, including the time, location, unusual activities and duration each individual bather spent in the water. Bathers were required to spend 15 min in knee-deep water (due to the relative shallowness of the study site), and to immerse their head three times completely under water. Using ropes, a 30–40-m stretch of beach was subdivided into 5-m intervals forming six to eight bathing exposure zones, with exposure of any individual bather restricted to their own individual swim zone that was 5 m wide. Each subject was instructed to take their own water sample at 5-min intervals near the surface before their head immersion, as well as provided with an appropriate individual water-sampling container. Staff members instructed participants to thoroughly rinse the collection container before filling completely with the marine water, as well as the avoidance of microbial contamination of the collection container by the participant. When the subjects left the water, they gave their individual water samples to the environmental research study staff for microbial analysis processing (described below). No bather was allowed to enter the water more than once during the actual study exposure. The participants in the randomized non-bather group were restricted to sitting on chairs on plastic sheeting in a covered roped-off area distant from water and sand exposure for 15 min.
After participating in the beach-day protocol, all participants were given a US$50 gift card, and an appointment to complete an extensive follow-up phone questionnaire 7 days after the beach visit. After completing the follow-up questionnaire, study participants received an additional US $25 gift card to compensate them for participating in the study.
Informed consent documents and study questionnaires were provided in English and Spanish, and approved by the Florida Department of Health and University of Miami Human Subjects Committees. The questionnaires (as well as the study design) were adapted from those of Dr David Kay and Dr Jay M. Fleisher from the prospective randomized exposure studies in the UK and Europe.16–19,22 Adaptations included some language changes to ‘American’ English, as well as logistical changes (in particular, instead of follow-up at 7 days and 3 weeks, due to resources and logistics, these follow-ups were combined into a single follow-up questionnaire given 7 days post exposure via telephone).
Disease endpoint criteria
The disease endpoints of interest in this study were self-reported symptoms consistent with gastrointestinal illness, acute febrile respiratory illness [i.e. International Classification of Diseases 9: 461–466, 480], eye or ear infections and skin infections occurring within 7 days of beach exposure.
The following definitions were used to derive disease endpoint categories prior to data analysis based on the results of the seventh-day follow-up questionnaire. ‘Gastrointestinal illness’ was defined as a report of the following symptoms: all cases of vomiting or diarrhoea, or all reported cases of indigestion or nausea accompanied by a fever; ‘diarrhoea’ defined as having three or more runny stools within a 24-h period; ‘acute febrile respiratory illness’ defined as report of at least one of each of the symptoms listed in each of the three categories: (i) fever; (ii) headache and/or body-aches and/or unusual fatigue and/or loss of appetite; and (iii) sore throat and/or runny nose and/or dry or productive cough; ‘skin illness’ defined as report of at least one of the following symptoms: (i) skin rash; (ii) skin ulcer/sore; or (iii) itching/irritation; ‘ear illness’ defined as report of an ear infection (sore/discharge); ‘eye illness’ as report of an eye infection on the follow-up questionnaire. These illness definitions were compatible with past studies carried out by the investigators in Europe, and are roughly compatible with the ‘highly creditable illness’ definitions used in the majority of the US recreational water cohort studies.3,16–19,22
Environmental sampling and microbial assays
The study site had been well characterized by the investigators previously in terms of the indicator organisms in the water and sediments as being a non-point source subtropical beach with periodic use by people, dogs and birds as well as seasonal heavy rains.14,28–33
As described above, while each study participant was in the water, each subject collected their own environmental water sample from the demarcated bathing lanes. All samples were collected in the morning between 8 am and 12 pm on each sampling day concomitant with the epidemiological study. As described above, water samples were collected in knee-deep water by each study participant using 5 litres sterilized plastic containers; sampling points from the shoreline ranged from 10 to 40 m depending on tidal stage corresponding with the knee height.
Samples taken by each study participant were assayed for enterococci by membrane filtration using the method recommended by the US EPA.34,35
Randomization, epidemiological study database and statistical analyses
Block randomization into bather and non-bather groups was used to maximize the probability of achieving an equal number of study participants in the two groups under study. Block size consisted of a random ordering of blocks of two, four and six study participants per individual block. It should be noted that the random ordering of block size eliminates any chance of study personnel discovering the blocking pattern. Participants were only informed of their randomized status after completing the pre-exposure day interview at the beach. The sample size was calculated to obtain a difference in gastrointestinal illness between bathers and non-bathers of 5/100 at α = 0.05 and β = 0.80.
The statistical software packages Statistical Analysis Software (SAS), and where necessary StatXact, were used to perform all statistical analyses. Initial analyses evaluated the effects of randomization for all demographic variables to insure equal distribution of demographic risk factors among exposed (i.e. bathers) and the unexposed (i.e. non-bathers). Subsequent basic analyses were geared to answer two questions: (i) ‘Was there an excess of the illnesses under study among bathers vs non-bathers?’; and (ii) ‘Was there a dose–response relationship between indicator organism density and the incidence of the outcome illnesses studied among bathers?’. Participants reporting a particular illness on the study day were excluded from the statistical analysis of that particular illness. Statistical analyses included univariate analysis using chi-square and, where expected cell size was <5, the Fisher’s exact test (two-tailed). Unconditional logistic regression modelling was then used to control for possible confounders/non-water-related risk factors among bathers vs non-bathers. Non-water-related risk factors or possible confounders for all outcome illness under analysis were evaluated separately in univariate analyses (Table 1). Inclusion criterion for the potential confounders was P < 0.15, with a model retention criterion of P < 0.05. A backward stepwise elimination strategy was employed.
Table 1.
Non-water-related risk factors and possible confounders evaluated for both bathers and non-bathers
| Risk factor/possible confounder |
|---|
| • Age |
| • Gender |
| • History of significant illness: gastrointestinal, respiratory, skin, eye, ear, migraines, stress |
| • Use of medications within 4 weeks of exposure day |
| • Illness within 4 weeks of exposure day |
| • Consumption of the following foods from 3 days before to 7 days after exposure day: ethanol, mayonnaise, chicken, eggs, ice cream, salad, hamburgers, hot dogs, raw milk, meat pies, seafood, purchased sandwiches |
| • Illness in household after exposure day |
| • Additional bathing after exposure day |
| • Various measures of risk perception |
Results
Of 1341 subjects who participated in the 15 beach study days, 38 (2.9%) were lost to follow-up, resulting in a final total study cohort of 1303 with a follow-up of 97.1%. Of note, 80% of the 1303 subjects were successfully followed up within 7 days of exposure, and the remaining 20% within 3 weeks. After randomization, there were 652 bathers and 651 non-bathers. Randomization of subjects into bather and non-bather exposure for the purposes of the study was successful in terms of equal distribution of demographics with no significant differences (Table 2).
Table 2.
Demographics of the study population
| Variable | Bather | Non-bather |
|---|---|---|
| Bather status, N (%) | 652 (50.04) | 651 (49.96) |
| Mean age (±SE) | 32.20 ± 12.64 | 32.50 ± 13.40 |
| Female, N (%) | 370 (49.27) | 381 (50.73) |
| Hispanic ethnicity, N (%) | 243 (48.70) | 256 (51.30) |
| Occupation, N (%) | ||
| Office | 141 (47.80) | 154 (52.20) |
| Catering/leisure | 9 (64.29) | 5 (35.71) |
| Agriculture | 5 (50) | 5 (50) |
| Student/school/university | 285 (49.83) | 287 (50.17) |
| Factory | 7 (87.50) | 1 (12.50) |
| Caring for others | 26 (41.94) | 36 (58.06) |
| Building/construction | 23 (62.16) | 14 (37.84) |
| Other | 154 (51.16) | 147 (48.84) |
SE, standard error.
The distribution of enterococci exposure among bathers was: for all 668 bathers, the mean enterococci exposure was 71 enterococci/100 ml [standard deviation (SD) 244]; the median = 19 enterococci/100 ml; the minimum exposure below the detection limit of 2 enterococci/100 ml; and the maximum enterococci exposure = 3320 enterococci/100 ml. We recorded the number of days between the exposure study day for each individual participant and the day of onset of their symptoms. For gastrointestinal illnesses, the average time for bathers to the onset of symptoms was 6.10 (SD 2.81) days and for non-bathers 7.78 (SD 3.15) days (P = 0.16); for acute febrile illness, bathers 5.18 (SD 2.44) days and for non-bathers 12 (SD 2.82) days (P = 0.004]; for skin illnesses, bathers 4.36 (SD 2.56) days and for non-bathers 5.55 (SD 2.17) (P = 0.19); for eye illnesses, bathers 5.75 (SD 2.56) days and for non-bathers 9.20 (SD 7.19) days, (P = 0.41); and for ear illnesses, bathers 3.67 (SD 1.75) days and for non-bathers 9.67 (SD 0.58) days, (P = 0.0008). For every illness outcome, the un-exposed non-bathers reported a longer time to onset than the exposed bathers.
Bathers vs non-bathers
Bathers reported more gastrointestinal illness (P = 0.08), respiratory illness (P = 0.04) and skin illness (P = 0.0001) relative to non-bathers (Table 3). After controlling for non-water-related risk factors/possible confounders for each of these illnesses using multiple logistic regression analysis, subsequent to 7 days of follow-up from beach exposure, bathers were 1.79 times [odds ratio 1.79; 95% confidence interval (CI) 0.94–3.43; P = 0.07] more likely to report gastrointestinal illness relative to non-bathers; bathers were 4.46 times (95% CI 0.99–20.97; P = 0.051) more likely to report acute febrile respiratory illness; and bathers were 5.31 times (95% CI 2.58–10.96; P = 0.0001) more likely to report skin illness. No substantial confounding was observed between any of these three illnesses and the non-water-related risk factor/confounders (Table 4). No differences between bathers and non-bathers for ear and eye illnesses were observed (Table 3).
Table 3.
Crude rates of the outcome illnesses assessed by bather vs non-bather groups
| Total analyseda | Bathers, N (%) | Non-bathers, N (%) | P-valueb | |
|---|---|---|---|---|
| Gastroenteritis | 1239 | 31 (4.75) | 18 (2.90) | 0.08 |
| Acute febrile respiratory illness | 1240 | 12 (1.94) | 4 (0.64) | 0.04 |
| Skin | 1253 | 47 (7.47) | 9 (1.44) | 0.0001 |
| Eye | 1299 | 4 (0.61) | 5 (0.77) | 0.75 |
| Ear | 1300 | 6 (0.92) | 3 (0.46) | 0.51 |
aNumbers vary due to exclusion of participants reporting particular symptoms on exposure day.
bStatistical testing by chi-square, or (where any expected cell size was <5), Fisher’s exact test.
Table 4.
Results of unconditional multiple logistic regression analyses by reported illness: bathers vs non-bathers
| Risk factor | Chi-square | P-value | Odds ratio (95% CI) |
|---|---|---|---|
| Gastrointestinal illness | |||
| Gastrointestinal symptoms | 3.17 | 0.07 | 1.79 (0.94–3.43) |
| Excessive tiredness 3 weeks prior to initial interview, lasting <24 h | 7.21 | 0.007 | 4.65 (1.51–14.27) |
| Eat salad after exposure day | 4.54 | 0.03 | 0.75 (0.57–0.98) |
| Illness in household within 7 days of beach exposure | 7.91 | 0.005 | 0.49 (0.30–0.81) |
| Eat ice-cream since exposure day | 3.50 | 0.06 | 0.79 (0.62–1.01) |
| Respiratory illness | |||
| Report of respiratory symptoms | 3.80 | 0.051 | 4.46 (0.99–20.97) |
| Illness in household within 7 days of beach exposure | 7.81 | 0.005 | 2.03 (1.24–3.34) |
| Skin illness | |||
| Skin symptoms | 20.96 | 0.0001 | 5.31 (2.58–10.96) |
| Illness in household within 7 days of beach exposure | 8.33 | 0.004 | 0.49 (0.30–0.80) |
Expressed in terms of attributable risk, the excess risk for gastroenteritis was 3.17/100 (P = 0.01); for acute febrile respiratory illness, the excess risk was 1.28/100 (P = 0.04); and for skin illness, the excess risk was 6.03/100 (P < 0.0001).
Bathers only
Enterococci by membrane filtration showed a dose–response relationship with skin illness. Unconditional multiple logistic regression modelling was used to compute the probability of bathers acquiring a skin illness with increasing enterococci exposure rose while controlling for any of the non-water-related risk factors/confounders shown in Table 1. Covariates in which the P-value was <0.15 in the univariate analysis comparing bathers reporting skin illnesses with bathers who did not report such illness were included in the model, while model retention was set at P < 0.05.
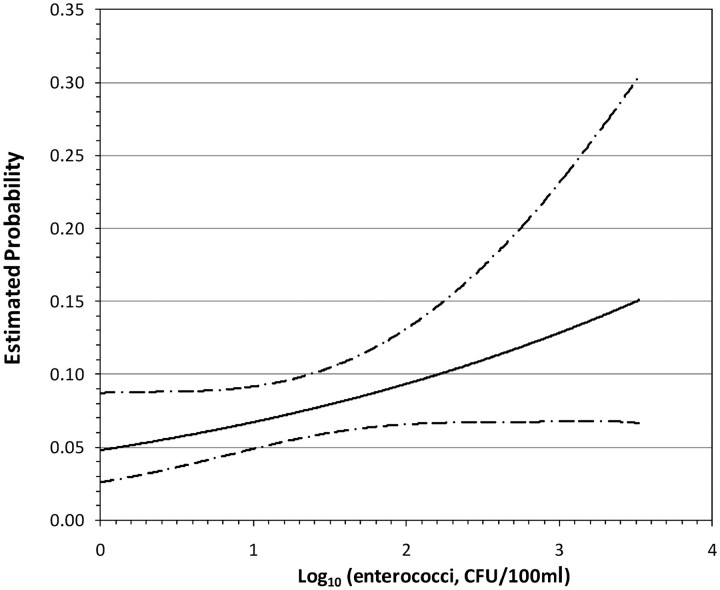
Enterococci exposure was modelled as a continuous variable (data not shown). Enterococci exposures were transformed via log10 prior to inclusion into the model. The results showed that the risk of a skin illness increased by 1.46 times (0.97–2.21; P = 0.07) per increasing log10 unit of enterococci exposure, while controlling for gender and for the question ‘Have you heard anything about chemical pollution and bathing waters?’ as a measure of risk perception (P = 0.04 and 0.02, respectively). A plot of the probability of bathers contracting skin illness with increasing enterococci exposure (after log10 transformation) with 95% CIs is illustrated in Figure 1. With regard to gastroenteritis, the same method of analysis was used, but demonstrated no relationship with the increasing levels of enterococci (1.39 per increasing log10 unit of enterococci exposure (0.74–2.61; P = 0.31), and thus no dose–response relationship).
Figure 1.
Dose–response (with 95% CIs) between the probability of skin illnesses and increasing enterococci levels (bathers only). Maximum enterococci measured = 3.52 log. CFU, colony forming units
Discussion
This study was the first randomized exposure study of bathers with individual exposure assessment in subtropical recreational marine waters with no known source of domestic sewage. The results demonstrated bathers to be at increased risk of reported gastrointestinal, acute febrile respiratory and skin illnesses relative to non-bathers. In addition, there was evidence for a dose–response relationship between bather exposure to increasing levels of enterococci and an increased risk of reporting skin illness.
The fact that we found no dose–response relationship between respiratory or gastrointestinal illnesses and enterococci exposure remains unexplained, and may be a result of the study sample size. Based on our prior studies, some contribution to each individual participant’s enterococci exposure may have come from their own bacterial shedding; however, our prior research has indicated that this is not the major source of enterococci exposure in non-point source subtropical recreational marine waters.29,32,33 It should be noted that the actual pathogens responsible for all of these illnesses remain unknown; they may vary from study site to study site, and thus, the apparent lack of correlation with enterococci exposure reported herein. Although ‘urban runoff’ is a probable source of pathogen(s), we cannot rule out other micro-organisms that are naturally present in subtropical marine waters and not the result of contamination by man and other animals. If this is the case, we would not expect a correlation with any indicator organism. Since this study was the first randomized prospective exposure of bathers performed in subtropical recreational marine waters with no known point source, additional studies need to be performed to confirm and explore our results further.
This is the only study of the association between concentrations of microbial water contamination and subsequent illnesses in which the bathers collected their own water samples at the same time they were being ‘exposed’. This should provide the most accurate estimate of individual exposure to water-borne microbial indicators, including enterococci, to date. This should aid to minimize the misclassification of exposures associated with non-randomized cohort designs, which would likely be non-differential, and thus result in an underestimation of the risk. It must, however, be emphasized that the bather cohort used in our study consisted of regular adult bathers. Care must therefore be used when interpreting these findings, especially with respect to susceptible subpopulations such as small children or anyone with a compromised immune system.15,36
In addition to individual exposure assessment, another strength of our study was that, unlike the designs of almost all other published epidemiological studies which used a prospective cohort design, the basic unit of measurement was the individual bather rather than the average rates of illness among many bathers on different study days. This use of an aggregated measure of individual exposures (i.e. rates or rate differences) as the basic unit of measurement in all previous prospective cohort designs would lead to significant misclassification of exposure bias.16 The design of the study reported herein would minimize this source of misclassification of exposure bias. In addition, the randomization of individual study participants who reported bathing regularly in recreational marine waters into exposed and unexposed groups solely for the purposes of the study further avoided another bias possibly inherent in the prospective cohort design: the hypothesized phenomena that persons who report regular non-bathing might constitute a less healthy group relative to regular bathers, leading to a self-selection bias.17,37,38 In the presence of such selection bias (unavoidable in previous prospective cohort studies), the comparison of bathers with non-bathers might lead to results of questionable validity.
In a regulatory context, the results we report can be transformed into the probability of illness, attributable risk and other measures of risk, thus avoiding the use of rates as the basic unit of measurement published in previous prospective cohort designs, which inevitably introduce misclassification of exposure. Thus, the methods used in this study will supply the regulator with more accurate estimates of risk upon which to build criteria in the future.
The implications of the findings of this study are far reaching. Our findings suggest that there is an increased risk to bathers even when using marine recreational waters with no known source of domestic sewage. We also observed a correlational dose–response association between skin illnesses and increasing enterococci exposure. These findings might indicate that microbes from non-point sources are causing increased risk from pathogens introduced by urban run-off, and thus from a regulatory viewpoint,20 the notion of zero additional risk to bathers could be challenged.15 Further epidemiological and laboratory studies will be necessary to confirm this hypothesis.
Funding
National Center for Environmental Health (NCEH), Centers for Disease Control and Prevention (CDC); Florida Dept of Health (FL DOH) through monies from the Florida Dept of Environmental Protection (FL DEP); the EPA Internship Program; the National Science Foundation (NSF) and the National Institute of Environmental Health Sciences (NIEHS) Oceans and Human Health Center at the University of Miami Rosenstiel School (NSF 0CE0432368) and (NIEHS P50 ES12736) and NSF REU in Oceans and Human Health and the NSF SGER (NSF SGER 0743987) in Oceans and Human Health.
Acknowledgements
The researchers would like to dedicate this research to the memory of Ms Seana Campbell, a very talented, hardworking and creative young researcher who enriched all people whose lives she touched, and who died too young. The researchers would like to thank the following collaborators at the following Institutions: NOAA Southeast Miami Lab; Miami-Dade County Department of Health; Miami SeaAquarium, University of Florida; BCS Laboratories; University of South Florida; NOAA Charleston Hollings Laboratory; Woods Hole Oceanographic Institute and the University of Missouri. The researchers would also like to thank the many students of University of Miami and Florida International University, other student researchers and other researchers for their assistance in the performance of this study.
Conflict of interest: None declared.
KEY MESSAGES.
The objectives of this study were to evaluate the risk to humans from exposure to subtropical recreational marine waters with no known point source of microbial pollution.
This study indicated that bathers may be at increased risk of several illnesses relative to non-bathers, even in the absence of any known source of domestic sewage impacting the recreational marine waters.
Interestingly, no dose–response relationship between gastroenteritis and increasing exposure to enterococci was detected, given that many current water monitoring standards use gastroenteritis as the major outcome illness and enterococcus as the recommended indicator organism for monitoring purposes.
References
- 1.Cabelli VJ, Dufour AP, McCabe LJ, Levin MA. Swimming associated gastroenteritis and water quality. Am J Epidemiol. 1982;115:606–16. doi: 10.1093/oxfordjournals.aje.a113342. [DOI] [PubMed] [Google Scholar]
- 2.Fujioka RS, Byappanahalli MN. Draft of Final Report, Tropical Indicator Workshop. Washington, DC: USEPA Office of Water; 2001. [Google Scholar]
- 3.Wade TJ, Pai N, Eisenberg JN, Colford JM., Jr Do U.S. Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ Health Perspect. 2003;111:1102–9. doi: 10.1289/ehp.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craun GF, Calderon RL, Wade TJ. Assessing waterborne risks: an introduction. J Water Health. 2004;4:3–18. doi: 10.2166/wh.2006.015. [DOI] [PubMed] [Google Scholar]
- 5.Boehm AB, Ashbolt NJ, Colford JM, et al. A sea change ahead for recreational water quality criteria. J Water Health. 2009;7:9–20. doi: 10.2166/wh.2009.122. [DOI] [PubMed] [Google Scholar]
- 6.Toranzos GA. Current and possible alternate indicators of fecal contamination in tropical waters: a short review. Environ Toxicol Water Quality. 1991;6:121–30. [Google Scholar]
- 7.Calderon RL, Mood EW, Dufour AP. Health effects of swimmers and nonpoint sources of contaminated water. Int J Environ Health Res. 1991;1:21–31. doi: 10.1080/09603129109356701. [DOI] [PubMed] [Google Scholar]
- 8.Dwight RH, Baker DB, Semenza JC, Olson BH. Health effects associated with recreational coastal water use: urban versus rural California. Am J Public Health. 2004;94:565–67. doi: 10.2105/ajph.94.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solo-Gabriele HM, Wolfert MA, Desmarais TR, Palmer CJ. Sources of Escherichia coli in a coastal subtropical environment. Applied Environ Microbiol. 2000;66:230–37. doi: 10.1128/aem.66.1.230-237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmarais TR, Solo-Gabriele HM, Palmer CJ. Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Applied Environ Microbiol. 2002;68:1165–72. doi: 10.1128/AEM.68.3.1165-1172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibata T, Solo Gabriele HM, Fleming LE, Elmir S. Monitoring marine recreational water quality using multiple microbial indicators in an urban tropical environment. Water Res. 2004;38:3119–31. doi: 10.1016/j.watres.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colford JM, Jr, Wade TJ, Schiff KC, et al. Water quality indicators and the risk of illness at beaches with nonpoint sources of fecal contamination. Epidemiology. 2007;18:27–35. doi: 10.1097/01.ede.0000249425.32990.b9. [DOI] [PubMed] [Google Scholar]
- 13.Yamahara KM, Layton BA, Santoro AE, Boehm AB. Beach sands along the California coast are diffuse sources of fecal bacteria to coastal waters. Environ Sci Technol. 2007;41:4515–21. doi: 10.1021/es062822n. [DOI] [PubMed] [Google Scholar]
- 14.Wright ME. Evaluation of enterococci, an indicator microbe, and the sources that impact the water quality at a subtropical non-point source recreational beach. Masters Thesis in Engineering. Coral Gables, FL: University of Miami; 2008. [Google Scholar]
- 15.US Environmental Protection Agency (US EPA) Report of the Experts Scientific Workshop on Critical Research Needs for the Development of New or Revised Recreational Water Quality Criteria. Washington, DC: EPA 823-R-07-006, http://www.epa.gov/waterscience/criteria/recreation/, 2007 (1 May 2010, date last accessed) [Google Scholar]
- 16.Fleisher JM. A re-analysis of the data supporting U.S. federal bacteriological water quality criteria governing marine recreational waters. Water Pollution Control Federation J. 1991;63:259–65. [Google Scholar]
- 17.Fleisher JM, Jones F, Kay D, Stanwell-Smith R, Wyer M, Morano R. Water and non-water risk factors for gastroenteritis among bathers exposed to sewage-contaminated marine waters. Int J Epidemiol. 1993;22:698–708. doi: 10.1093/ije/22.4.698. [DOI] [PubMed] [Google Scholar]
- 18.Fleisher JM, Kay D, Salmon RL, Jones F, Wyer MD, Godfree AF. Marine waters contaminated with domestic sewage: nonenteric illness associated with bather exposure in the United Kingdom. Am J Public Health. 1996;86:1228–34. doi: 10.2105/ajph.86.9.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleisher JM, Kay D, Wyer MD, Godfree AF. Estimates of the severity of illnesses associated with bathing in marine recreational waters contaminated with domestic sewage. Int J Epidemiol. 1998;27:722–26. doi: 10.1093/ije/27.4.722. [DOI] [PubMed] [Google Scholar]
- 20.Fujioka RS, Roll K, Morens D. A Pilot Epidemiological Study of Health Risks Associated with Swimming at Kuhio Beach. Honolulu, Hawaii: Hawaii Water Resources Research Center; 1994. [Google Scholar]
- 21.Haile RW, Witte JS, Gold M, et al. The health effects of swimming in ocean water contaminated by storm drain runoff. Epidemiology. 1999;10:355–63. [PubMed] [Google Scholar]
- 22.Kay D, Fleisher JM, Salmon RL, et al. Predicting likelihood of gastroenteritis from sea bathing: results from randomised exposure. Lancet. 1994;344:905–9. doi: 10.1016/s0140-6736(94)92267-5. [DOI] [PubMed] [Google Scholar]
- 23.Kueh CS, Tam TY, Lee T. Epidemiological study of swimming associated illnesses relating to bathing beach water quality. Water SciTech. 1995;31:1–4. [Google Scholar]
- 24.Prieto MD, Lopez B, Juanes JA, Revilla JA, Llorca J, Delgado Rodriguez M. Recreation in coastal waters: health risks associated with bathing in sea water. J Epidemiol Community Health. 2001;55:442–47. doi: 10.1136/jech.55.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruss A. Review of epidemiological studies on health effects from exposure to recreational water. Int J Epidemiol. 1998;27:1–9. doi: 10.1093/ije/27.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Shuval H. Estimating the global burden of thalassogenic diseases: human infectious diseases caused by wastewater pollution of the marine environment. J Water Health. 2003;1:53–64. [PubMed] [Google Scholar]
- 27.Fleming LE, Solo Gabriele H, Elmir S, et al. A pilot study of microbial contamination of subtropical recreational waters. Fl J Env Health. 2004:29–33. [PMC free article] [PubMed] [Google Scholar]
- 28.Elmir SM. Development of a water quality model which incorporates non-point microbial sources. PhD Thesis. Coral Gables, FL: University of Miami; 2006. [Google Scholar]
- 29.Elmir SM, Wright ME, Solo-Gabriele HM, et al. Quantitative evaluation of bacteria released by bathers in marine water. Water Res. 2007;41:3–10. doi: 10.1016/j.watres.2006.10.005. (with supplement available online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleming LE, Solo Gabriel HM, Fleisher JM, et al. Final Report on the Pilot Epidemiologic Assessment of Microbial Indicators for Monitoring Recreational Water Quality in Marine Sub/Tropical Environments. Miami, FL: The NSF NIEHS Oceans and Human Health Center, Rosenstiel School of Marine and Atmospheric Sciences, University of Miami; 2008. [Google Scholar]
- 31.Solo Gabriele HM, Abdelzaher A, Wright M, et al. Pathogen Monitoring of a South Florida Beach Which Has Been Characterized by Elevated Microbe Levels. Tallahassee, FL: Final Report to the Florida Department of Health; 2008. [Google Scholar]
- 32.Elmir SM, Shibata T, Solo Gabriele HM, et al. Quantitative evaluation of enterococci and Bacteroidales released by adults and toddlers in marine water. Water Research. 2009;43:4610–16. doi: 10.1016/j.watres.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright ME, Solo Gabriele HM, Elmir S, Fleming LE. Microbial load from animal feces at a recreational beach. Mar Pollut Bull. 2009;58:1649–56. doi: 10.1016/j.marpolbul.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinigalliano CD, Fleisher JH, Gidley ML, et al. Traditional and molecular analyses for fecal indicator Bacteria in non-point source subtropical recreational marine waters. Water Research. doi: 10.1016/j.watres.2010.04.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Environmental Protection Agency (US EPA) Improved Enumeration Methods for the Recreational Water Quality Indicators: Enterococci and Escherichia coli. U.S. Environmental Protection Agency. Washington, DC: Office of Science and Technology; 2000. [Google Scholar]
- 36.Wade TJ, Calderon RL, Brenner KP, et al. High sensitivity of children to swimming-associated gastrointestinal illness: results using a rapid assay of recreational water quality. Epidemiology. 2008;19:375–83. doi: 10.1097/EDE.0b013e318169cc87. [DOI] [PubMed] [Google Scholar]
- 37.Fleisher JM, Kay D. Risk perception bias, self-reporting of illness, and the validity of reported results in an epidemiologic study of recreational water associated illnesses. Mar Pollut Bull. 2006;52:264–68. doi: 10.1016/j.marpolbul.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kay D, Bartram J, Prüss A, et al. Derivation of numerical values for the World Health Organization guidelines for recreational waters. Water Res. 2004;38:1296–304. doi: 10.1016/j.watres.2003.11.032. [DOI] [PubMed] [Google Scholar]