Abstract
Cardiovascular morbidity has been associated with particulate matter (PM) air pollution, although the relation between pollutants and sudden death from cardiac arrest has not been established. This study examined associations between out-of-hospital cardiac arrests and fine PM (of aerodynamic diameter ≤2.5 μm, or PM2.5), ozone, nitrogen dioxide, sulfur dioxide, and carbon monoxide in New York City. The authors analyzed 8,216 out-of-hospital cardiac arrests of primary cardiac etiology during the years 2002–2006. Time-series and case-crossover analyses were conducted, controlling for season, day-of-week, same-day, and delayed/apparent temperature. An increased risk of cardiac arrest in time-series (relative risk (RR) = 1.06, 95% confidence interval (CI): 1.02, 1.10) and case-crossover (RR = 1.04, 95% CI: 0.99, 1.08) analysis for a PM2.5 increase of 10 μg/m3 in the average of 0- and 1-day lags was found. The association was significant in the warm season (RR = 1.09, 95% CI: 1.03, 1.15) but not the cold season (RR = 1.01, 95% CI: 0.95, 1.07). Associations of cardiac arrest with other pollutants were weaker. These findings, consistent with studies implicating acute cardiovascular effects of PM, support a link between PM2.5 and out-of-hospital cardiac arrests. Since few individuals survive an arrest, air pollution control may help prevent future cardiovascular mortality.
Keywords: air pollution; death, sudden, cardiac; emergency medical services; particulate matter
Fine particulate matter (PM; particles with an aerodynamic diameter of ≤2.5 μm, or PM2.5) consists of a mixture of chemical constituents generated by various combustion sources, including traffic and coal-fired power plants. Fine PM constituents vary by region and by season, depending on the relative strength of sources (1). It is estimated that in 2007 1 in 3 people in the United States lived in areas where PM2.5 reached levels that are considered unhealthy (2). A number of reports have demonstrated a relation between ambient PM and cardiovascular morbidity and mortality (3, 4), including an increased risk of hospitalizations for ischemic heart disease, heart failure, and ventricular dysrhythmias after short-term exposure to fine particles (5). Reviews of time-series, case-crossover, and other study designs find no evidence of a safe threshold of fine particulate air pollution, and the exposure-response relation with cardiovascular health effects is near linear across reported ranges of exposure (6).
Sudden cardiac arrest accounts for more than half of all deaths of primary cardiac etiology in the United States (7), and more than 95% of sudden cardiac arrest victims do not survive to hospital discharge (8). A time-series study in New York City found an association between PM and overall cardiovascular mortality, although the study did not specifically address out-of-hospital cardiac arrests (9). Since acute ischemic events and ventricular dysrhythmias predispose to sudden death from cardiac arrests, it is hypothesized that ambient PM is a risk factor for out-of-hospital cardiac arrest. To date, however, studies that examined associations between PM and out-of-hospital cardiac arrest have shown conflicting results (10–13).
In this study we utilized a large, multiyear data set to examine the association between air pollution and out-of-hospital cardiac arrest in New York City. Our main interest was the effect of ambient PM2.5, but we also examined ozone because of reports linking it with overall cardiovascular mortality (14). Since nitrogen dioxide is a marker of air pollution from local combustion sources (primarily motor vehicle traffic), and because recent multicity studies from Canada and Europe have suggested short-term total mortality effects (15, 16), we also evaluated the association of nitrogen dioxide with cardiac arrest. Sulfur dioxide and carbon monoxide have been shown to be more spatially variable (i.e., with an expected large exposure misclassification error) than PM2.5, ozone, and nitrogen dioxide in the New York City metropolitan area (17); interpreting sulfur dioxide and carbon monoxide levels requires caution, but we analyzed these pollutants for reference purposes.
MATERIALS AND METHODS
Air pollution and weather data
Data from 33 PM2.5, 16 ozone, 15 nitrogen dioxide, 14 sulfur dioxide, and 19 carbon monoxide monitors within the 20-mile (32-km) radius of the geographic center of New York City were retrieved from the US Environmental Protection Agency's Air Quality System. We computed the average of multiple monitors’ data, using the 24-hour average values for PM2.5, nitrogen dioxide, sulfur dioxide, and carbon monoxide and the daily 8-hour maximum values for ozone. The 24-hour average weather variables for LaGuardia Airport (New York) were obtained from the National Climatic Data Center.
Cardiac arrest data
We extracted medical records from a database of out-of-hospital cardiac arrest cases collected by the Emergency Medical Service of the Fire Department of New York City for the years 2002–2006. Study physicians evaluated the records to categorize the presumptive etiology of cardiac arrest utilizing the Utstein criteria for standardization of event reporting (18). We included only cardiac arrests presumed to be of primary cardiac etiology where resuscitation was attempted by paramedics in the prehospital setting.
Statistical analysis
Both time-series and case-crossover analyses were conducted to address the sensitivity of results to model uncertainty. The main difference between the 2 methods is that in the case-crossover analysis with a time-stratified referent sampling, the day-of-week and seasonal trends are adjusted by design, whereas in the time-series design these factors “compete” with the exposure variables in the model. An a priori exposure window averaging 0- and 1-day lagged air pollution levels was used (for each pollutant separately) for the main analysis, and we examined individual lag days 0 through 3 as a sensitivity analysis. Since the exposure conditions and compositions of PM2.5 change across seasons, analyses were also conducted by season (warm season: April–September; cold season: October–March). Risk estimates were computed for an interquartile-range increment of air pollutants, which provides the magnitude-of-risk estimates that are comparable across the pollutants. Data were analyzed using the R statistical package (19).
For the time-series analysis, a Poisson generalized linear model was used to estimate the impact of air pollutants, adjusting for weather effects, seasonal/temporal trends, and day of week and accommodating overdispersion. We used natural cubic splines of days to adjust for potentially confounding temporal trends and seasonal cycles. The choice of the degrees of freedom for this temporal adjustment term (7 degrees of freedom per year) was based on the examination of the sum of absolute values of partial autocorrelation function of residuals (20). To adjust for immediate and delayed nonlinear temperature effects, natural cubic splines of the same-day and the average of past 2- and 3-day apparent temperatures were included in the model with 3 degrees of freedom over the range for each term. Apparent temperature was computed from temperature and dewpoint using the equation given by Steadman (21) to model the body's perceived temperature incorporating both temperature and humidity.
For the case-crossover analysis, we used a conditional logistic regression model with the time-stratified referent day sampling scheme in which a case was matched with control days from the same month of the year and the same day of the week (22). We included the same apparent temperature smoothing terms as in the time-series model described above. All estimated risks are expressed as relative risk rather than odds ratio, even for the case-crossover result. This is because of the equivalence of the time-series design and case-crossover design in a large population context (23), with the assumption that exposure for a given day is similar for all persons in the city.
RESULTS
A total of 8,216 cardiac arrest cases met inclusion criteria and were used in the analysis. The average age was 65.6 years, with slightly more men than women (Table 1). Table 2 shows the distribution of air pollution variables for the entire year and by season during the study period. As expected, ozone levels were much higher in the warm season than in the cold season, whereas PM2.5 and nitrogen dioxide levels showed little seasonal differences. Sulfur dioxide levels were consistently higher in the cold season. Table 3 shows correlations among air pollutants and apparent temperature by season. PM2.5 was moderately correlated with nitrogen dioxide, ozone, and apparent temperature in the warm season. During the cold season, ozone was negatively correlated with PM2.5, nitrogen dioxide, sulfur dioxide, and carbon dioxide.
Table 1.
Characteristics of the Study Population with Out-of-Hospital Cardiac Arrests in New York City, 2002–2006
| No. | % | |
| All | 8,216 | 100 |
| Sex | ||
| Male | 4,261 | 51.9 |
| Female | 3,955 | 48.1 |
| Age, years | ||
| <40 | 484 | 5.9 |
| 40–69 | 3,293 | 40.1 |
| ≥70 | 4,439 | 54.0 |
Table 2.
Distribution of Air Pollution Variables (Warm Season: April–September; Cold Season: October–March) in New York City, 2002–2006
| 5% | 25% | 50% | 75% | 95% | |
| PM2.5, μg/m3 | |||||
| All year | 5 | 8 | 12 | 18 | 30 |
| Warm | 5 | 8 | 12 | 19 | 31 |
| Cold | 5 | 7 | 12 | 17 | 28 |
| O3, ppb | |||||
| All year | 7 | 18 | 28 | 40 | 67 |
| Warm | 20 | 31 | 40 | 51 | 75 |
| Cold | 4 | 12 | 19 | 26 | 35 |
| NO2, ppb | |||||
| All year | 15 | 21 | 27 | 32 | 43 |
| Warm | 14 | 20 | 26 | 31 | 42 |
| Cold | 17 | 23 | 28 | 34 | 44 |
| SO2, ppb | |||||
| All year | 2.0 | 3.8 | 6.3 | 9.6 | 18.0 |
| Warm | 1.8 | 3.0 | 4.2 | 6.0 | 8.8 |
| Cold | 3.6 | 6.6 | 9.3 | 13.2 | 21.6 |
| CO, ppm | |||||
| All year | 0.6 | 0.7 | 0.9 | 1.0 | 1.5 |
| Warm | 0.6 | 0.7 | 0.8 | 1.0 | 1.3 |
| Cold | 0.6 | 0.7 | 0.9 | 1.1 | 1.6 |
Abbreviations: CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; PM2.5, particulate matter of aerodynamic diameter <2.5 μm; SO2, sulfur dioxide.
Table 3.
Correlation Matrix Among Air Pollutants and Apparent Temperature by Season in New York City, 2002–2006
| PM2.5 | O3 | NO2 | SO2 | CO | AT | |
| PM2.5 | 1.00 | −0.43 | 0.77 | 0.66 | 0.67 | 0.02 |
| O3 | 0.61 | 1.00 | −0.38 | −0.49 | −0.44 | 0.12 |
| NO2 | 0.54 | 0.34 | 1.00 | 0.68 | 0.68 | −0.05 |
| SO2 | 0.51 | 0.43 | 0.68 | 1.00 | 0.45 | −0.41 |
| CO | 0.40 | 0.03 | 0.61 | 0.35 | 1.00 | 0.09 |
| AT | 0.51 | 0.46 | 0.05 | −0.04 | 0.11 | 1.00 |
Abbreviations: AT, apparent temperature; CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; PM2.5, particulate matter of aerodynamic diameter <2.5 μm; SO2, sulfur dioxide.
Upper diagonal shows correlation during cold season; lower diagonal shows correlation during warm season.
Table 4 shows relative risks of cardiac arrest for PM2.5 estimated from both time-series and case-crossover analyses. The risk of a cardiac arrest was significantly higher when PM2.5 levels were elevated. For a PM2.5 increase of 10 μg/m3 in the average of 0- and 1-day lags, the increased relative risk for a cardiac arrest was 1.06 (95% confidence interval: 1.02, 1.10) using a time-series design and 1.04 (95% confidence interval: 0.99, 1.08) using a case-crossover design. These associations between cardiac arrest and PM2.5 were found only in the warm-weather seasons, and there was no apparent PM2.5-related increase in arrests during the cold season (Table 4). There were no differences in risk between men and women or between older (ages 70 years) and younger (ages 40–69 years) adults in the all-year or seasonal analyses. These patterns of associations were similar between the time-series and case-crossover analyses, though the risk estimates obtained using the case-crossover method were generally slightly smaller than those from the time-series method.
Table 4.
Relative Risk of Cardiac Arrest per Interquartile-Range Increment Increase (10 μg/m3) of the Average of 0- and 1-Day PM2.5 in New York City, 2002–2006
| Category and Period | Relative Risk Estimated Using Time-Series Analysis | 95% CI | Relative Risk Estimated Using Case-Crossover Analysis | 95% CI |
| All ages | ||||
| All year | 1.06 | 1.02, 1.10 | 1.04 | 0.99, 1.08 |
| Warm | 1.09 | 1.03, 1.15 | 1.08 | 1.02, 1.15 |
| Cold | 1.01 | 0.95, 1.07 | 0.99 | 0.93, 1.06 |
| Male, all ages | ||||
| All year | 1.06 | 1.01, 1.13 | 1.04 | 0.98, 1.10 |
| Warm | 1.10 | 1.02, 1.18 | 1.09 | 1.01, 1.18 |
| Cold | 1.02 | 0.94, 1.11 | 0.99 | 0.90, 1.08 |
| Female, all ages | ||||
| All year | 1.05 | 0.99, 1.11 | 1.03 | 0.97, 1.10 |
| Warm | 1.07 | 0.98, 1.16 | 1.05 | 0.96, 1.14 |
| Cold | 1.00 | 0.92, 1.09 | 1.01 | 0.92, 1.11 |
| Aged 40–69 years | ||||
| All year | 1.04 | 0.97, 1.10 | 1.02 | 0.95, 1.10 |
| Warm | 1.06 | 0.97, 1.17 | 1.05 | 0.95, 1.15 |
| Cold | 1.00 | 0.91, 1.09 | 1.00 | 0.90, 1.11 |
| Aged ≥70 years | ||||
| All year | 1.06 | 1.00, 1.12 | 1.03 | 0.97, 1.09 |
| Warm | 1.09 | 1.01, 1.18 | 1.06 | 0.98, 1.15 |
| Cold | 1.00 | 0.92, 1.09 | 0.99 | 0.91, 1.08 |
Abbreviations: CI, confidence interval; PM2.5, particulate matter of aerodynamic diameter <2.5 μm.
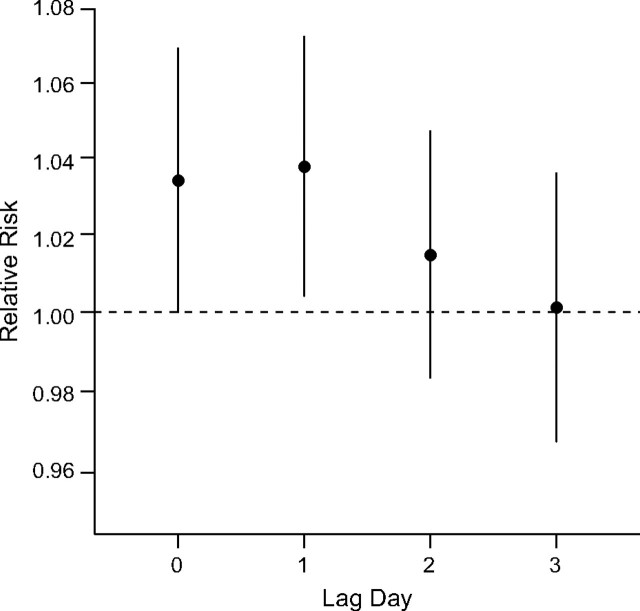
In the sensitivity analysis of lagged associations using single-day lags, we found that PM2.5 was positively associated at lag 0 and 1 days. The association decreased at lag day 2 and fell to null at lag day 3 (Figure 1). The estimated lag day 1 risk for cardiac arrests was similar to the lag day 0, indicating that elevated pollution levels closely preceding the event had the stronger associations. A similar pattern of lagged associations was found in the warm season, but, in the cold season, only the same-day PM2.5 showed a positive association (results not shown).
Figure 1.
Lagged associations between particulate matter of aerodynamic diameter <2.5 μm (PM2.5) and cardiac arrests. The days 0–3 lagged associations between PM2.5 (per interquartile-range increase, 10 μg/m3) and cardiac arrests for all year using time-series design, New York City, 2002–2006.
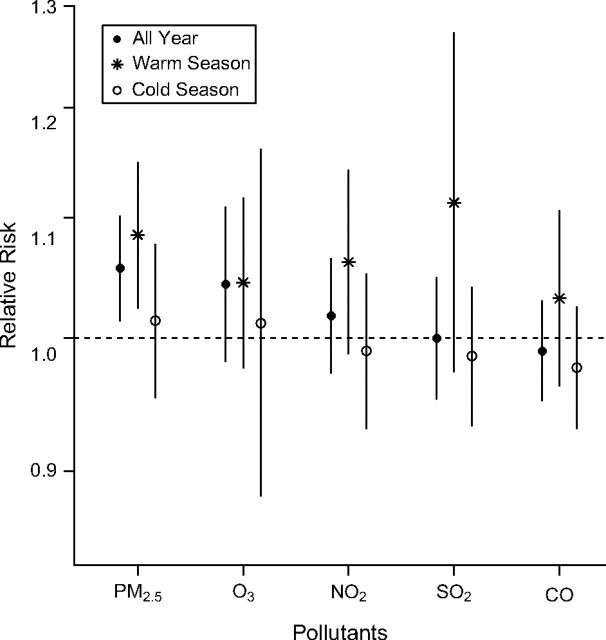
Among the all-year time-series analysis data for the 5 pollutants, only PM2.5 had statistically significant associations with cardiac arrests (Figure 2). The associations of each pollutant with cardiac arrest appeared stronger in warm-weather months, although, even in the warm weather, only PM2.5 had a statistically significant association.
Figure 2.
Seasonal cardiac arrest relative risk for air pollutants. Relative risk of cardiac arrest per interquartile-range (IQR) increase in the average of 0- and 1-day lagged air pollutants using Poisson time-series design, New York City, 2002–2006. The IQR increments are 10 μg/m3 for particulate matter of aerodynamic diameter ≤2.5 μm (PM2.5), 22 parts per billion (ppb) for ozone (O3), 11 ppb for nitrogen dioxide (NO2), 6 ppb for sulfur dioxide (SO2), and 0.3 parts per million for carbon monoxide (CO). Warm season is April through September. Cold season is October through March.
DISCUSSION
We found an association between ambient fine particle concentrations and out-of-hospital cardiac arrests in New York City. For an increase of 10 μg/m3 in PM2.5, there was a 4%–10% increase in the number of arrests. This study indicates that the consequences of air pollution can be acute and severe. Since an out-of-hospital arrest is usually fatal, with approximately 5% of patients surviving to hospital discharge, an increase in cardiac arrests can be considered an increase in cardiac fatalities. The likelihood of being resuscitated from an out-of-hospital cardiac arrest in large metropolitan areas is among the lowest, with survival ranging from about 1% to 3% (24, 25).
A number of previous studies have linked short-term exposure to PM with the development of acute ischemic heart disease and dysrhythmia. The possible pathobiologic mechanisms include increased platelet aggregation and clotting potential (26), inflammatory mediators, and C-reactive protein (27). PM is associated with dysregulation of the cardiac autonomic nervous system, including increased heart rate and reduced heart rate variability (28). Among individuals at high risk for sudden death with implanted defibrillators, an increased frequency of recorded ventricular dysrhythmias occurs with increasing ambient pollution (29, 30). Short-term exposure to PM is also associated with myocardial infarction, and the risk for an acute infarction increases with greater exposure (31, 32).
The data that implicate PM as a risk factor for acute coronary ischemia support our findings of PM2.5 as a risk for cardiac arrest, since sudden cardiac arrest most typically occurs in individuals with underlying ischemic heart disease (33). Those with known coronary artery disease, as well as elderly individuals, are more likely to have ST depression when stressed on higher pollution days (PM2.5 or black carbon), suggesting that those most vulnerable to developing ischemia are impacted more by pollution (34). Additional evidence comes from a controlled study in which men with established coronary heart disease developed acute cardiac ischemia after a brief exposure to dilute diesel exhaust (35).
There are conflicting findings in the literature on whether pollution increases the risk of sudden death. Similar to our findings, an analysis in Rome, Italy, of 5,144 cases found associations between PM indices (estimated particle number concentration and particles with an aerodynamic diameter of ≤10 μm, or PM10) and out-of-hospital cardiac deaths (12). The link between pollution and particle number concentration was stronger in the elderly. While the particle number concentration was indirectly estimated in that study, it is considered a good surrogate of ultrafine particles (which are produced mostly by local traffic) (36). Additional support comes from 2 other studies where deaths that occurred outside of hospitals (all-cause mortality) were more strongly associated with air pollution than deaths in hospitals (37, 38). A study of sudden death from Indianapolis, Indiana, did not find associations between daily PM2.5 and cases (n = 1,374) but did report associations between the hourly data of PM2.5 exposure and the cardiac arrests witnessed by bystanders (n = 511) (13). Since both cardiac arrest and PM2.5 likely have diurnal patterns, such analysis may have more accurate exposure characterization but at the same time may also reflect temporal confounding not present in the daily data.
In contrast, 2 studies of 362 and 1,206 cases from the Seattle, Washington, area that examined out-of-hospital sudden death showed no associations between fine particles (as measured by nephelometer) and cardiac arrests (10, 11). Associations between fine particles and the onset of acute myocardial infarction (5,793 cases) were evaluated in King County, Washington (39), and, in contrast to other reports evaluating acute myocardial infarction, no association was found (31, 32). Since health effects are known to vary by PM composition (and therefore by region), a possible explanation for the lack of association between fine particles and cardiac arrest in the Seattle area may be the local PM composition, which is lower in sulfate and transition metal content than northeast US cities (11). Further support for region-specific health effects is found in a study of PM2.5-mortality associations in 27 US cities (40), where PM2.5 was associated with all-cause mortality in Manhattan (i.e., New York County, the only New York City county analyzed in that study) but not in Seattle.
We found that significant associations between fine particles and out-of-hospital cardiac arrests appear to be found mainly in the warm season. This is consistent with several analyses of PM and all-cause mortality in which the strongest associations were found in summer (40–43). The reasons are not known, but there are several possible explanations. The stronger association in the warm season may be related to the higher penetration rate of outdoor PM into indoors (44). In addition, among studies of specific PM components and mortality in Phoenix, Arizona (45), and Washington, DC (46), sulfate-related PM showed the strongest associations among the source-apportioned PM components. One possible explanation is that sulfate particles are more harmful; another potential explanation is that a higher warm-weather rate of photochemical conversion from sulfur dioxide to sulfuric acid may make transition metals more water soluble and therefore more bioavailable to cells (47).
We also analyzed cardiac arrests relative to changes in ozone, nitrogen dioxide, sulfur dioxide, and carbon monoxide but found that these gaseous pollutants’ associations with cardiac arrest were weaker than that for PM2.5. In our study, ozone was not significantly associated with out-of-hospital cardiac arrest. This contrasts with the positive and significant association other studies found with ozone for all-cause or circulatory mortality in New York City (9, 48). One possible explanation is that our study is far smaller in terms of daily counts (∼4.4 cardiac arrest/day) compared with daily mortality for all-cause (∼180/day) or circulatory mortality (∼87/day), limiting its statistical power. Nitrogen dioxide was also not significantly associated with cardiac arrest in our analysis, but the magnitude of risk estimate for the warm season was similar to the trend of increased risk found in Rome (12). In the Rome study, carbon monoxide was significantly associated with cardiac deaths but in our study was not. The lack of associations for carbon monoxide and sulfur dioxide in our study may be due in part to the expected larger exposure misclassification for these pollutants compared with PM2.5, ozone, or nitrogen dioxide (17), as representativeness of exposures relevant to the residents for these local combustion sources may vary from city to city.
In using both time-series and case-crossover designs, the risk estimates in our study results from case-crossover analysis were slightly less significant, which was expected. The time-stratified referent day sampling scheme (the same day of week in the same month/year) in the case-crossover method we applied effectively uses 12 degrees of freedom/year, as compared with 7 degrees of freedom/year for time-series design, to adjust for season/temporal trends and may explain the slightly smaller risk estimates in the case-crossover result. A recent study compared time-series, case-crossover, and extended Cox regression designs in an analysis of a cohort of myocardial infarction survivors in 5 European cities to assess the probability of recurrent hospitalizations (49). These methods gave similar results, and findings suggested that time-series analysis might also be most practical. Since time-series analysis involves selection of degrees of freedom for temporal adjustment, case-crossover analysis provides an alternative to ensure that the result is not dependent on model specifications. However, a recent study that discussed the equivalency of these 2 methods pointed out that the case-crossover analysis does not account for overdispersion of the Poisson variance (23). Thus, while the 2 methods may produce generally similar results, they may be used as model-checking methods for each other.
Among study limitations, the derivation of this data set comes from only cases treated by paramedics of the Fire Department of New York City and not by voluntary ambulance crews. Though the data set represents the majority of calls within the 5 boroughs, the New York City 911 system comprises not only the Fire Department of New York City but also a number of voluntary hospital ambulance systems, representing 65% and 35% of the available paramedic units, respectively. We do not suspect the data would be biased based on whether a municipal or a voluntary ambulance crew responded to the call, especially since our endpoint was cardiac arrest and not death. In addition, we did not follow patients to either admission to or discharge from the hospital. For large cities, however, because an out-of-hospital cardiac arrest almost always results in death, the arrest endpoint is nearly equivalent and, regardless, represents a substantial cardiac event.
In conclusion, fine particles are associated with an increased risk of sudden out-of-hospital cardiac arrests. These observations are supported by previous data linking ischemic heart disease and dysrthymias with PM. Since few individuals survive an out-of-hospital cardiac arrest, controlling air pollution may be a preventable means to decrease cardiovascular mortality.
Acknowledgments
Author affiliations: Department of Emergency Medicine, NS-LIJ Health System, New York, New York (Robert A. Silverman, Brad J. Kaufman, Dannilyn De Claro); the Feinstein Institute for Medical Research, Manhasset, New York (Robert A. Silverman); the Nelson Institute of Environmental Medicine, New York University School of Medicine, Tuxedo, New York (Kazuhiko Ito); and the New York City Fire Department, New York, New York (John Freese, Brad J Kaufman, James Braun, David J Prezant).
This work was supported by National Institutes of Health grants R01ES014387 and NIEHS ES00260.
The investigators thank the Fire Department of the City of New York for allowing use of the Emergency Medical Services data, and Joshua Rubin Morey and Liza Mishan for assistance in extracting the data.
Conflict of interest: none declared.
Glossary
Abbreviations
- PM
particulate matter
- PM2.5
particulate matter of aerodynamic diameter ≤2.5 μm
References
- 1.US Environmental Protection Agency, National Center for Environmental Assessment. Air Quality Criteria for Particulate Matter. Research Triangle Park, NC: RTP Office, Office of Research and Development; 2004. [Google Scholar]
- 2.American Lung Association. Washington, DC: American Lung Association; 2008. State of the Air 2008. ( http://www.lungusa2.org/sota/SOTA2008.pdf). (Accessed August 3, 2010) [Google Scholar]
- 3.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 4.Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol. 2008;52(9):719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(1):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 7.Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 8.American Heart Association. Dallas, TX: American Heart Association; 2008. CPR facts and stats. ( http://www.americanheart.org/presenter.jhtml?identifier=3034352). (Accessed August 3, 2010) [Google Scholar]
- 9.De Leon SF, Thurston GD, Ito K. Contribution of respiratory disease to nonrespiratory mortality associations with air pollution. Am J Respir Crit Care Med. 2003;167(8):1117–1123. doi: 10.1164/rccm.200205-409OC. [DOI] [PubMed] [Google Scholar]
- 10.Levy D, Sheppard L, Checkoway H, et al. A case-crossover analysis of particulate matter air pollution and out-of-hospital primary cardiac arrest. Epidemiology. 2001;12(2):193–199. [PubMed] [Google Scholar]
- 11.Sullivan J, Ishikawa N, Sheppard L, et al. Exposure to ambient fine particulate matter and primary cardiac arrest among persons with and without clinically recognized heart disease. Am J Epidemiol. 2003;157(6):501–509. doi: 10.1093/aje/kwg015. [DOI] [PubMed] [Google Scholar]
- 12.Forastiere F, Stafoggia M, Picciotto S, et al. A case-crossover analysis of out-of-hospital coronary deaths and air pollution in Rome, Italy. Am J Respir Crit Care Med. 2005;172(12):1549–1555. doi: 10.1164/rccm.200412-1726OC. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal FS, Carney JP, Olinger ML. Out-of-hospital cardiac arrest and airborne fine particulate matter: a case-crossover analysis of emergency medical services data in Indianapolis, Indiana. Environ Health Perspect. 2008;116(5):631–636. doi: 10.1289/ehp.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell ML, McDermott A, Zeger SL, et al. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA. 2004;292(19):2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnett RT, Stieb D, Brook JR, et al. Associations between short-term changes in nitrogen dioxide and mortality in Canadian cities. Arch Environ Health. 2004;59(5):228–236. doi: 10.3200/AEOH.59.5.228-236. [DOI] [PubMed] [Google Scholar]
- 16.Samoli E, Aga E, Touloumi G, et al. Short-term effects of nitrogen dioxide on mortality: an analysis within the APHEA project. Eur Respir J. 2006;27(6):1129–1138. doi: 10.1183/09031936.06.00143905. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Thurston GD, Silverman RA. Characterization of PM2.5, gaseous pollutants, and meteorological interactions in the context of time-series health effects models. J Expo Sci Environ Epidemiol. 2007;17(suppl 2):S45–S60. doi: 10.1038/sj.jes.7500627. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110(21):3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 19.R Development Core Team. 2008. R: A Language and Environment for Statistical Computing. ( http://cran.r-project.org/doc/manuals/refman.pdf). (Accessed August 3, 2010) [Google Scholar]
- 20.Touloumi G, Samoli E, Pipikou M, et al. Seasonal confounding in air pollution and health time-series studies: effect on air pollution effect estimates. APHEA-2 Project Group. Stat Med. 2006;25(24):4164–4178. doi: 10.1002/sim.2681. [DOI] [PubMed] [Google Scholar]
- 21.Steadman RG. The assessment of sultriness. Part I: a temperature-humidity index based on human physiology and clothing science. J Appl Meteorol. 1979;18(7):861–873. [Google Scholar]
- 22.Janes H, Sheppard L, Lumley T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. 2005;16(6):717–726. doi: 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Zeger SL. On the equivalence of case-crossover and time series methods in environmental epidemiology. Biostatistics. 2007;8(2):337–344. doi: 10.1093/biostatistics/kxl013. [DOI] [PubMed] [Google Scholar]
- 24.Lombardi G, Gallagher J, Gennis P. Outcome of out-of-hospital cardiac arrest in New York City. The Pre-Hospital Arrest Survival Evaluation (PHASE) Study. JAMA. 1994;271(9):678–683. [PubMed] [Google Scholar]
- 25.Becker LB, Ostrander MP, Barrett J, et al. Outcome of CPR in a large metropolitan area—where are the survivors? Ann Emerg Med. 1991;20(5):355–361. doi: 10.1016/s0196-0644(05)81654-3. [DOI] [PubMed] [Google Scholar]
- 26.Peters A, Döring A, Wichmann HE, et al. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997;349(9065):1582–1587. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- 27.Riediker M, Cascio WE, Griggs TR, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169(8):934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- 28.Gold DR, Litonjua A, Schwartz J, et al. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 29.Peters A, Liu E, Verrier RL, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11(1):11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Dockery DW, Luttmann-Gibson H, Rich DQ, et al. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005;113(6):670–674. doi: 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters A, Dockery DW, Muller JE, et al. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 32.Peters A, von Klot S, Heier M, et al. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351(17):1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 33.Soo LH, Gray D, Hampton JR. Pathological features of witnessed out-of-hospital cardiac arrest presenting with ventricular fibrillation. Resuscitation. 2001;51(3):257–264. doi: 10.1016/s0300-9572(01)00417-8. [DOI] [PubMed] [Google Scholar]
- 34.Pekkanen J, Peters A, Hoek G, et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: the Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) Study. Circulation. 2002;106(8):933–938. doi: 10.1161/01.cir.0000027561.41736.3c. [DOI] [PubMed] [Google Scholar]
- 35.Mills NL, Törnqvist H, Gonzalez MC, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357(11):1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 36.Paatero P, Aalto P, Picciotto S, et al. Estimating time series of aerosol particle number concentrations in the five HEAPSS cities on the basis of measured air pollution and meteorological variables. Atmos Environ. 2005;39(12):2261–2273. [Google Scholar]
- 37.Schwartz J. What are people dying of on high air pollution days? Environ Res. 1994;64(1):26–35. doi: 10.1006/enrs.1994.1004. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz J. Assessing confounding, effect modification, and thresholds in the association between ambient particles and daily deaths. Environ Health Perspect. 2000;108(6):563–568. doi: 10.1289/ehp.00108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan J, Sheppard L, Schreuder A, et al. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology. 2005;16(1):41–48. doi: 10.1097/01.ede.0000147116.34813.56. [DOI] [PubMed] [Google Scholar]
- 40.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17(3):279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 41.Peng RD, Dominici F, Pastor-Barriuso R, et al. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161(6):585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- 42.Nawrot TS, Torfs R, Fierens F, et al. Stronger associations between daily mortality and fine particulate air pollution in summer than in winter: evidence from a heavily polluted region in Western Europe. J Epidemiol Community Health. 2007;61(2):146–149. doi: 10.1136/jech.2005.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stafoggia M, Schwartz J, Forastiere F, et al. Does temperature modify the association between air pollution and mortality? A multicity case-crossover analysis in Italy. SISTI Group. Am J Epidemiol. 2008;167(12):1476–1485. doi: 10.1093/aje/kwn074. [DOI] [PubMed] [Google Scholar]
- 44.Sarnat JA, Brown KW, Schwartz J, et al. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology. 2005;16(3):385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- 45.Mar TF, Ito K, Koenig JQ, et al. PM source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions of PM2.5 and daily mortality in Phoenix, AZ. J Expo Sci Environ Epidemiol. 2006;16(4):311–320. doi: 10.1038/sj.jea.7500465. [DOI] [PubMed] [Google Scholar]
- 46.Ito K, Christensen WF, Eatough DJ, et al. PM source apportionment and health effects: 2. An investigation of intermethod variability in associations between source-apportioned fine particle mass and daily mortality in Washington, DC. J Expo Sci Environ Epidemiol. 2006;16(4):300–310. doi: 10.1038/sj.jea.7500464. [DOI] [PubMed] [Google Scholar]
- 47.Gavett SH, Madison SL, Dreher KL, et al. Metal and sulfate composition of residual oil fly ash determines airway hyperreactivity and lung injury in rats. Environ Res. 1997;72(2):162–172. doi: 10.1006/enrs.1997.3732. [DOI] [PubMed] [Google Scholar]
- 48.Ito K, De Leon SF, Lippmann M. Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology. 2005;16(4):446–457. doi: 10.1097/01.ede.0000165821.90114.7f. [DOI] [PubMed] [Google Scholar]
- 49.Peters A, von Klot S, Berglind N, et al. Comparison of different methods in analyzing short-term air pollution effects in a cohort study of susceptible individuals. Epidemiol Perspect Innov. 2006;3:10. doi: 10.1186/1742-5573-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]