Abstract
Hepatitis E virus (HEV) is the most common cause of acute viral hepatitis in the world. Most of South Asia is HEV endemic, with frequent seasonal epidemics of hepatitis E and continuous sporadic cases. This author group's epidemiologic work and clinical reports suggest that Bangladesh is HEV endemic, but there have been few population-based studies of this country's HEV burden. The authors calculated HEV infection rates, over an 18-month interval between 2003 and 2005, by following a randomly selected cohort of 1,134 subjects between the ages of 1 and 88 years, representative of rural communities in southern Bangladesh. Baseline prevalence of antibody to hepatitis E virus (anti-HEV) was 22.5%. Seroincidence was 60.3 per 1,000 person-years during the first 12 months and 72.4 per 1,000 person-years from >12 to 18 months (during the monsoon season), peaking by age 50 years and with low rates during childhood. Few of the seroconverting subjects reported hepatitis-like illness. Overall incidence was calculated to be 64 per 1,000 person-years, with 1,172 person-years followed. No significant associations were found between anti-HEV incidence and demographic or socioeconomic factors for which data were available. This is the first study to document annual HEV infection rates among “healthy” and very young to elderly subjects in a rural Bangladeshi population.
Keywords: Asia, Bangladesh, hepatitis E, hepatitis E virus, incidence, prevalence, seroepidemiologic studies
Hepatitis E virus (HEV), an emerging pathogen (1, 2), causes significant disease in endemic countries and is the leading cause of enterically transmitted viral hepatitis illness globally (3). Large annual epidemics are attributed to HEV (3, 4), and studies suggest that HEV is etiologically responsible for 10%–95% of admitted cases of hepatitis across South Asia (5–11). Globally, prevalence rates of antibody to hepatitis E virus (anti-HEV) vary by region, population, and circulating genotypes of HEV, with unexpectedly high seropositivity in some developed settings (3, 12–15).
HEV researchers struggle to explain several perplexing phenomena. The low rate of infection in children under 15 years of age is unusual for an enteric pathogen in environments of poor sanitation (16, 17). The high mortality rate (up to 20%) in infected pregnant women remains unexplained (18–20). HEV immunopathogenesis and protection after infection are unclear (21–23).
Given the challenges involved in following large populations over time to quantify infection rates, there have been few longitudinal studies of HEV. A Nepalese police/army cohort (n = 757), followed for ∼19 months, estimated an incidence of 64 per 1,000 person-years, with ∼31 illnesses per 100 cases (17). In contrast, Stoszek et al. (24) reported an ∼11 month follow-up of ∼1,900 Egyptian villagers, revealing an incidence of 42 per 1,000 person-years, with little to no clinical illness.
Our study followed a randomly selected, age-representative cohort of rural Bangladeshi volunteers for a total of 18 months to calculate age-specific population incidence rates of HEV infection and disease under endemic, nonoutbreak conditions. We included 2 follow-up timepoints to specifically look at the impact of rainy monsoon seasons and to calculate period-specific incidence density.
MATERIALS AND METHODS
Participants were randomly selected for 12- and 18-month follow-up from a longitudinally followed population cohort of the Matlab Health Research Program of the International Center for Diarrheal Disease Research, Bangladesh, consisting of 110,000 people inhabiting 67 villages (25). This agrarian population of southern Bangladesh has been enumerated under the Matlab Health and Demographic Surveillance System (26).
A random list of 1,300 individuals was generated from the 2003 census of the Matlab Health and Demographic Surveillance System, excluding children <1 year of age. Two teams, each consisting of an interviewer and a phlebotomist, visited individuals up to 4 times. Consenting subjects were interviewed to collect socioeconomic data, enteric risk factors, and recent morbidity history. The 3½-month baseline enrollment period began on December 23, 2003, and ended April 8, 2004. Participants were tested for antibodies to HEV to identify susceptibles. The results of the baseline survey are described elsewhere (27).
Study teams attempted to revisit the 1,134 participants 12 and 18 months after the baseline visit for up to 5 times each to minimize attrition. Follow-up visits were scheduled exactly 12 and, later, 18 months from baseline. The 12–18-month period intentionally spanned a second monsoon (rainy) season to measure the specific effect, if any, of annual flooding from heavy rains on infection and illness rates. Field team performance and refusal rates were tracked daily, and a study supervisor visited refusals to respond to any participant concerns. Individuals not met or refusing participation at 12 months were contacted again at 18 months and invited to rejoin the cohort, to contribute person-time to the overall incidence calculations between baseline and 18 months.
At both time points, teams collected blood and administered a short questionnaire to assess changes in exposures and to record self-reported recent morbidity. Prior to follow-up serosurveys, the field teams conducted community-priming advocacy visits to reduce attrition and lower refusal rates.
A ∼350-μL fingerstick blood specimen was collected by using a capillary system (Safe-T-Fill; RAM Scientific, Needham, Massachusetts) following the manufacturer's guidelines. Specimens were transferred on ice to the laboratory within 4 hours of collection. Microtubes were centrifuged at 4,000 × g for 10 minutes, and serum was transferred by aliquots into two 200-μL eppendorf-type tubes. Aliquots were stored at −20°C until shipment on dry ice to the Armed Forces Research Institute of Medical Sciences (Bangkok, Thailand), a regional hepatitis reference laboratory.
Baseline specimens were tested for anti-HEV total immunoglobulin and antibodies to other hepatitis viruses (27). As available commercial anti-HEV assays were considered inappropriate for epidemiologic study use because of widely varying sensitivities and specificities (28), the quantitative anti-HEV total immunoglobulin enzyme immunoassay developed by the Walter Reed Army Institute of Research (WRAIR; Silver Spring, Maryland) was used; this assay is recognized as a highly sensitive (96%) and specific (98%) test for HEV infection in populations (29, 30). The immunoassay reports a quantitative total for human immunoglobulin antibodies to recombinant ORF2 proteins of the Pakistani Sar-55 HEV strain, and it reports antibody concentrations in Walter Reed antibody units (WR-U)/mL. -Cutoffs of ≥20 WR-U/mL and ≥500 WR-U/mL are used by WRAIR to identify “definite past infections” and “definite acute infections,” respectively (21). Individuals with titers below 20 WR-U/mL were considered susceptible or “seronegative.” This assay is described elsewhere in detail (29, 30).
Data were entered by using customized screens, incorporating range and consistency checks, created with Visual FoxPro (Microsoft Corp., Seattle, Washington). Statistical analyses were performed by using STATA, version 9.0, software (StataCorp LP, College Station, Texas). Seroprevalence rates were calculated on the basis of the number of specimens exceeding the manufacturer's cutoffs for commercial assays for total antibody to hepatitis B core antigen (anti-HBc), anti-HCV, or total anti-HAV (Abbott Laboratories, Abbott Park, Illinois) or the WRAIR-recommended cutoff, over the total number tested.
An enrollment diagram of the population followed through 12 and 18 months illustrates cause-specific attrition at each time point (Figure 1). Seroconversions throughout the study period are described in a second diagram (Figure 2). Baseline characteristics of losses to follow-up were compared with those remaining in the cohort by χ2 tests or Student's t tests, where appropriate.
Figure 1.
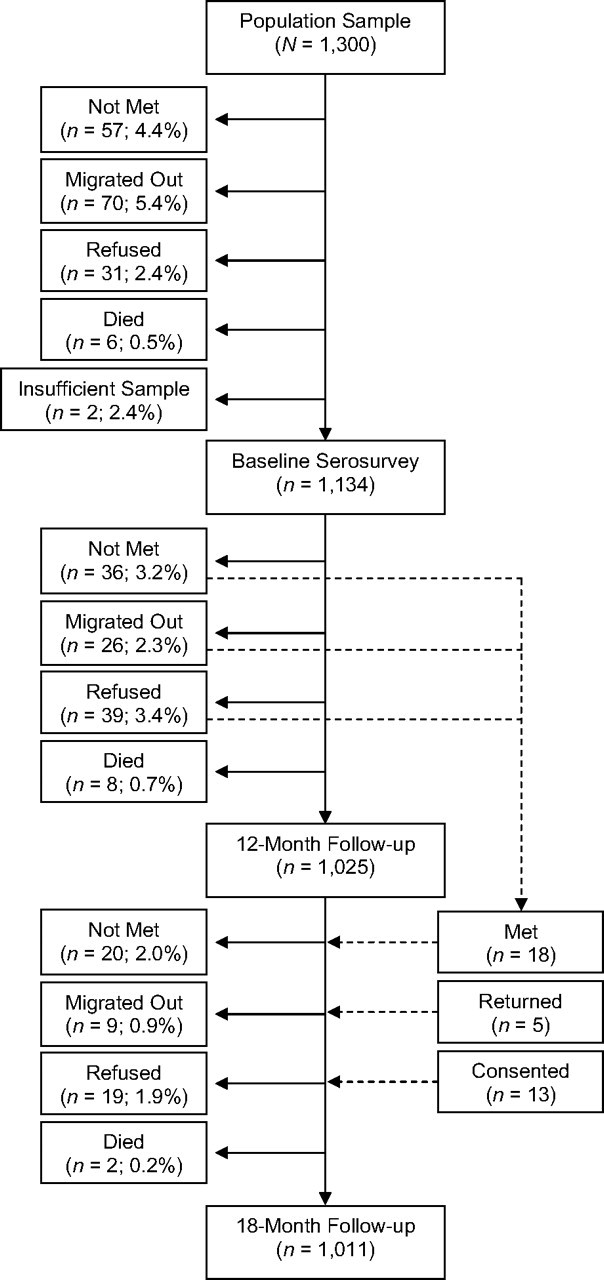
Diagram of enrollment and losses to follow-up of the baseline cohort to estimate seroprevalence and incidence of antibodies to hepatitis E virus, Bangladesh, 2003–2005.
Figure 2.
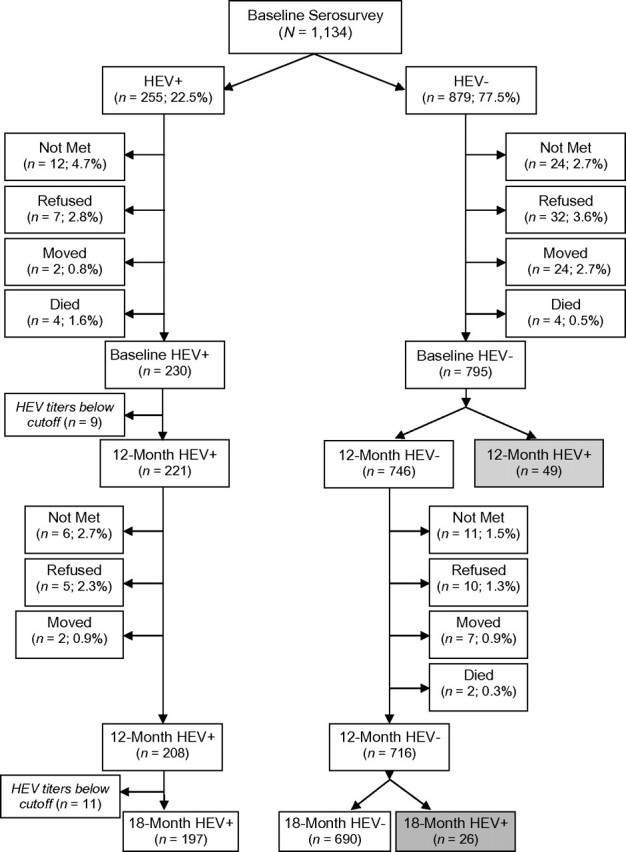
Follow-up of baseline susceptibles by outcome at 12 months and 18 months, Bangladesh, 2003–2005. “HEV+” denotes individuals who tested seropositive for antibodies to human E virus (HEV) by the WRAIR assay and, likewise, “HEV–” denotes individuals who tested seronegative. Gray-shaded boxes highlight the seroconversion events used in the incidence density calculations. WRAIR, Walter Reed Army Institute of Research.
Two periods of surveillance for HEV seroincidence were defined: from baseline to 12 months and from >12 to 18 months. All individuals, regardless of baseline status, were eligible for testing at each follow-up. At the end of each period, individuals who tested negative at baseline and whose titers increased to ≥20 WR-U/mL were considered seroconverters. Individuals who seroconverted or were lost to follow-up during an interval were allowed to contribute half the interval's complete follow-up person-time, assuming that they dropped out of the cohort or seroconverted, on average, at the midpoint of the interval. Incidence density was calculated by dividing the number of seroconversions by the observed person-years in a given interval, converted to rates per 1,000 person-years for each period; stratified analyses by gender and by 10-year age category were performed. Confidence intervals for incidence density estimates were calculated by using an exact approach based on the Poisson distribution.
The characteristics of all susceptible persons at baseline were compared with those of seroconverters in each of the 2 surveillance periods by using univariate χ2 or log-rank tests. Differences between age-specific incidence rates were explored by log-rank analysis. The relative risk between the lowest age category and subsequent age categories was assessed by using a Cox proportional hazards model in which a “failure” event was defined as an incident HEV infection.
To explore possible characteristics associated with incidence, we used log-rank tests for differences between categories (by religion, gender, household size, employment type, place of work, education, household head's education, income, recent morbidity, recent contact with sick or jaundiced individuals, history of recent travel, history of injection use, traditional healer use, adult malnutrition (mid-upper arm circumference, <22.5 cm), injectable contraceptive use (women only), and pregnancy status at baseline or follow-up (women only). Hazard ratios were calculated by using univariate and multivariate Cox proportional hazard models, adjusting for age when necessary. Only individuals eligible for an exposure were included in that model: For example, stratified analysis “by marital status” excluded individuals <15 years of age, “by employment status” excluded students and young children, and “by <7 years of age” excluded persons by level of education. P < 0.05 was considered statistically significant for each analysis.
At each follow-up time point, participants were asked about a history of hepatitis-associated symptoms (severe weakness, yellow eyes or skin, dark urine, clay/ash-colored stools, upper-right quadrant abdominal pain, anorexia, fever, nausea/vomiting) during the previous 3 months. Data were used to estimate possible hepatitis E among seroconverters.
The proportion of individuals reporting morbidity was compared between seroconverters and nonseroconverters by using χ2 tests. Specific symptoms that occurred more frequently in the seroconverter group were used to define clinical illness unique to HEV seroconverters. The disease ratio was calculated as the proportion of clinical cases per 100 incident HEV infections (seroconversions).
As near-ubiquitous hepatitis A virus (HAV) infection and subsequent immunity occur very early in this population (27, 31, 32), possible misattribution of HAV illness as HEV disease was unlikely. When serum was sufficient, specimens were tested for anti-HBc, to account for possible hepatitis caused by hepatitis B virus (HBV). Hepatitis C virus (HCV) seroconversion is usually rare in this population (27).
Study procedures were approved by the Committee on Human Research at the Johns Hopkins Bloomberg School of Public Health and by the International Center for Diarrheal Disease Research, Bangladesh, Ethical Review Committee. Informed consent was obtained from all adults; parental consent was sought for children, accompanied by age-appropriate assent. Participants testing positive for antibodies to HEV, HBV, or HCV were counseled by using ethics committee-approved messages.
RESULTS
Initially, a total of 1,300 individuals were randomly selected from the Matlab Health and Demographic Surveillance System database. Between December 23, 2003, and April 23, 2004, a total of 1,136 (87.4%) of the selected individuals consented to be enrolled. Of the 164 not enrolled in the baseline cohort, 57 (4.4%) were not met, 70 (5.4%) had migrated out of the study area since the census, 31 (2.4%) refused to participate, and 6 (0.5%) had died. Only 2 specimens were inadequate for analysis (Figure 1).
The 12-month follow-up was conducted between December 23, 2004, and April 28, 2005. Of the initial 1,134 enrolled, 1,025 (90.4%) were successfully visited, with 109 (9.6%) lost to follow-up (Figure 1). At 18 months, between June 23, 2005, and November 25, 2005, 1,011 (89.2%) of the 1,134 baseline participants were revisited; overall, 975 (86.0%) subjects contributed a blood specimen at both follow-up time points.
At baseline, 255 (22.5%) individuals had an anti-HEV titer of ≥20 WR-U (Table 1), leaving 879 individuals as HEV susceptible. Causes of attrition by 12 and 18 months are detailed in Figure 1. Subjects lost to follow-up (n = 84) were no different in age from those who remained in the sample (χ2 test, P > 0.2), but they were more likely to be male (57.1% vs. 42.9%; χ2 test, P < 0.05).
Table 1.
Seroprevalence of Viral Hepatitis Infections in Rural Bangladesh at 3 Time Points, 2003–2005
| Enzyme Immunoassay | December 2003–April 2004 Baseline Seroprevalence |
December 2004–April 2005 12-Month Follow-up Seroprevalence |
June 2005–November 2005 18-Month Follow-up Seroprevalence |
|||
| No. Positive/ No. Tested | % | No. Positive/ No. Tested | % | No. Positive/ No. Tested | % | |
| Anti-HEV (total immunoglobulin)a | 255/1,134 | 22.5 | 270/1,025 | 26.3 | 247/1,011 | 24.4 |
| Anti-HBc (total immunoglobulin)b | 380/1,080 | 35.2 | 369/960 | 38.4 | 357/1,011 | 35.3 |
| Anti-HCV (IgG)b | 14/917 | 1.5 | —c | —c | ||
| Anti-HAV (total immunoglobulin)b | 116/124 | 93.5 | —d | —d | ||
Abbreviations: anti-HAV, antibody to hepatitis A virus; anti-HBc, antibody to hepatitis B core antigen; anti-HCV, antibody to hepatitis C virus; anti-HEV, antibody to hepatitis E virus; IgG, immunoglobulin G; WR-U, Walter Reed antibody units.
“Positive” defined as ≥20 WR-U/mL, with no significant differences between groups by Fisher's exact test.
Abbott commercial assay (Abbott Laboratories, Abbott Park, Illinois), with no significant differences between groups by Fisher's exact test.
—, not tested because of limited serum volumes and low baseline seroprevalence.
—, only tested for less than 10 years because of limited serum volumes and high baseline seroprevalence.
Among 795 individuals with complete 12-month follow-up, we observed 49 seroconversions. With the 84 losses and seroconverters contributing a half-year of person-time, 813 person-years were accrued over 12 months. This yielded an overall incidence density of 60.3 (95% confidence interval (CI): 44.6, 79.7) infections per 1,000 person-years (Table 2).
Table 2.
Age-specific Anti-HEV Seroincidence in Rural Bangladesh Over 2 Surveillance Periods and Overall, 2003–2005a,b
| Age Group, years | No. | % | Baseline Anti-HEV Prevalence, % | 0–12 Months’ Seroincidence Estimates* |
>12–18 Months’ Seroincidence Estimates |
Overall Rates |
|||||||
| No. Susceptible | Losses, no. | Infections, no. | Rate/1,000 Person-Years | No. Susceptible | Losses, no. | Infections, no. | Rate/1,000 Person-Years | Total Infections/ Person-Years | Rate/1,000 Person-Years | ||||
| 1–10 | 265 | 23.4 | 3.8 | 255 | 30 | 3 | 12.6 | 222 | 7 | 7 | 65.1 | 10/346 | 28.9 |
| 11–20 | 247 | 21.8 | 19.0 | 200 | 22 | 4 | 21.4 | 174 | 4 | 7 | 83.1 | 11/271 | 40.6 |
| 21–30 | 177 | 15.6 | 28.8 | 126 | 14 | 6 | 51.7 | 106 | 7 | 5 | 100.0 | 11/166 | 66.3 |
| 31–40 | 159 | 14.0 | 41.5 | 93 | 6 | 9 | 105.3 | 78 | 3 | 3 | 80.0 | 12/123 | 97.6 |
| 41–50 | 118 | 10.4 | 27.1 | 86 | 6 | 16 | 213.3 | 64 | 5 | 0 | 0.0 | 16/106 | 151.3 |
| 51–60 | 74 | 6.5 | 29.7 | 52 | 1 | 5 | 102.0 | 46 | 1 | 4 | 183.9 | 9/71 | 127.2 |
| ≥61 | 94 | 8.3 | 28.7 | 67 | 5 | 6 | 97.6 | 56 | 3 | 0 | 0.0 | 6/89 | 67.6 |
| Total | 1,134 | 22.5 | 879 | 84 | 49 | 60.3 | 746 | 30 | 26 | 72.4 | 75/1,172 | 64.0 | |
Abbreviations: anti-HEV, antibody to hepatitis E virus; WR-U, Walter Reed antibody units.
* P < 0.001 (log-rank test comparing infection rates between age categories).
The incidence density is calculated as events per person-years: ((no. of infections)/(no. of nonevents + 0.5(no. of infections + no. of losses)) × 1,000. Nonevents: (no. susceptible − (no. of losses + no. of infections)).
Seronegative individual experiencing an increase in total anti-HEV immunoglobulin to ≥20 WR-U/mL.
The 746 individuals still seronegative at 12 months were followed for an additional 6 months. After all losses (Figure 1), 716 eligible subjects completed their 18-month follow-up. Within this rainy season interval, 26 seroconversions were observed over 359 person-years, yielding an incidence density of 72.4 (95% CI: 47.3, 106.1) per 1,000 person-years (Table 2), only 12.1 seroconversions more than during the prior period alone. An overall incidence density of 63.9 (95% CI: 50.3, 80.1) per 1,000 person-years was calculated, combining both periods (Table 2).
Differences in characteristics between seroconverters and the overall cohort of susceptible individuals at baseline are described in Table 3. The age-stratified incidence density between baseline and 12 months increased from 12.6 per 1,000 person-years in those aged 1–10 years to a peak of 213.3 per 1,000 person-years in those aged 41–50 years and to 97.6 per 1,000 person-years among those aged ≥61 years (Table 2). A log-rank test of HEV incidence between 10-year age categories, between baseline and 12 months was significant (P < 0.001). The hazard ratio of infection by age strata, comparing each stratum with the youngest category (0–10 years), revealed significantly higher risks of infection in each age category, with the hazard ratios (not shown) peaking at 15.81 (95% CI: 4.61, 54.27) in those aged 41–50 years at baseline.
Table 3.
Characteristics of Susceptible Participants at Baseline and Seroconverters at 12 and 18 Months of Follow-up, Bangladesh, 2003–2005
| Demographic Characteristics | Baseline Susceptibles |
Seroconverters at 0–12 Months |
Seroconverters at >12–18 Months |
|||
| No. Susceptible/Total | % | No. Susceptible/Total | % | No. Susceptible/Total | % | |
| Age, years* | ||||||
| 1–10 | 255/879 | 29.0 | 3/49 | 6.1 | 7/26 | 26.9 |
| 11–20 | 200/879 | 22.8 | 4/49 | 8.2 | 7/26 | 26.9 |
| 21–30 | 126/879 | 14.3 | 6/49 | 12.2 | 5/26 | 19.2 |
| 31–40 | 93/879 | 10.6 | 9/49 | 18.4 | 3/26 | 11.5 |
| 41–50 | 86/879 | 9.8 | 16/49 | 32.7 | 0/26 | 0 |
| 51–60 | 52/879 | 5.9 | 5/49 | 10.2 | 4/26 | 15.4 |
| ≥61 | 67/879 | 7.6 | 6/49 | 12.2 | 0/26 | 0 |
| Gender | ||||||
| Male | 386/879 | 43.9 | 21/49 | 42.9 | 9/26 | 34.6 |
| Female | 493/879 | 56.1 | 28/49 | 57.1 | 17/26 | 65.4 |
| Nutritional statusa | ||||||
| Male | 22/188 | 11.7 | 1/16 | 6.3 | 0/4 | 0 |
| Female | 77/315 | 24.4 | 7/27 | 25.9 | 1/11 | 9.1 |
| Religion | ||||||
| Muslim | 739/879 | 84.1 | 41/49 | 83.7 | 21/26 | 80.8 |
| Hindu | 140/879 | 15.9 | 8/49 | 16.3 | 5/26 | 19.2 |
| Marital statusb | ||||||
| Single (≤15 years) | 376/879 | 42.8 | 6/49 | 12.2 | 11/26 | 42.3 |
| Single | 97/879 | 11.0 | 3/49 | 6.1 | 1/26 | 3.9 |
| Married | 351/879 | 39.9 | 36/49 | 73.5 | 14/26 | 53.9 |
| Divorced | 6/879 | 0.7 | 0/49 | 0 | 0/26 | 0 |
| Widowed | 49/879 | 5.6 | 4/49 | 8.2 | 0/26 | 0 |
| Educationc | ||||||
| None (<7 years) | 170/879 | 19.3 | 2/49 | 4.1 | 7/26 | 26.9 |
| None | 185/879 | 21.1 | 17/49 | 34.7 | 6/26 | 23.1 |
| Classes 1–5 | 255/879 | 29.0 | 12/49 | 24.5 | 5/26 | 19.2 |
| Classes 6–11 | 232/879 | 26.4 | 17/49 | 34.7 | 6/26 | 23.1 |
| Classes ≥12 | 37/879 | 4.2 | 1/49 | 2.0 | 2/26 | 7.7 |
| Monthly household income, Bangladeshi taka | ||||||
| <3,000 (<US $45) | 222/879 | 25.3 | 12/49 | 24.5 | 7/26 | 26.9 |
| 3,000–4,999 (US $45–$75) | 311/879 | 35.4 | 20/49 | 40.8 | 11/26 | 42.3 |
| ≥5,000 (>US $75) | 346/879 | 39.4 | 17/49 | 34.7 | 8/26 | 30.8 |
| Primary employment location | ||||||
| Indoors | 399/879 | 45.4 | 28/49 | 57.1 | 13/26 | 50.0 |
| Outdoors | 477/879 | 54.3 | 21/49 | 42.9 | 13/26 | 50.0 |
| Primary employmentd | ||||||
| None/housework | 263/455 | 57.8 | 26/42 | 61.9 | 11/16 | 68.8 |
| Farming/fishing/labor | 91/455 | 20.0 | 6/42 | 14.3 | 1/16 | 6.3 |
| Own business/rickshaw | 40/455 | 8.8 | 8/42 | 19.1 | 2/16 | 12.5 |
| Office-based service | 27/455 | 5.9 | 0/42 | 0 | 2/16 | 12.5 |
| Other | 34/455 | 7.5 | 2/42 | 4.8 | 0/16 | 0 |
Abbreviation: MUAC, mid-upper arm circumference.
* P < 0.001 (χ2 between incident cases and nonseroconverters significant only between 0 and 12 months; the log-rank test of incidence between age categories was also significant (P < 0.001)).
Percent malnourished defined by MUAC <22.5 cm. MUAC was restricted to participants >15 years of age. There was no significant difference between incident cases and nonseroconverters.
Comparison of groups excluded participants ≤15 years of age and therefore ineligible to be married. No significant difference was shown.
Comparison of groups excluded participants <7 years of age and therefore ineligible for school. No significant difference was shown.
Comparison excluded participants <15 years of age because of nonsignificant employment prior to this age. No significant difference was shown.
Stratified analysis by gender found no significant difference in seroconversion rates between males and females between baseline and 12 months, after age adjustment. The incidence density among male participants was 59.7 (95% CI: 37.0, 91.3) per 1,000 person-years, whereas for female participants it was 60.7 (95% CI: 40.4, 87.8) per 1,000 person-years.
No significant difference between incidence by religion, household size, location of primary employment, or indicators of socioeconomic status (education, income, and employment type) was detectable in this study. Recent travel to a town or self-reported contact with a “jaundiced” patient was not significant. Gross malnutrition among individuals over 15 years of age was not associated with seroconversion. Among women, incidence was not different by history of injectable contraception or active pregnancy at baseline or 12 months (Table 3).
When a comparison was made between the incidence among individuals whose secondary employment was farm work and that among those who worked at home or were unemployed, a significant 3.13-fold (95% CI: 1.40, 7.00) increased hazard ratio was found. Recent (past 3 months) complaints of yellow eyes or skin were significantly associated with infection (hazard ratio = 2.38, 95% CI: 1.01, 5.60; P < 0.05). Only 1 of the 6 seroconverters complaining of recent yellow eyes or skin had a high anti-HEV titer (1,486 WR-U/mL) consistent with recent infection.
Of the 49 seroconverters between baseline and 12 months, 33 individuals recalled experiencing at least 1 symptom consistent with viral hepatitis. Nonspecific symptoms were common in this population (Table 4). Among all seroconverters and nonseroconverters, at least 40% complained of anorexia, 27% of weakness, ∼28% of nausea, ∼26% of fever, and ∼27% of upper-right quadrant pain, none of these being significantly different by seroconversion status (Table 4). Dark urine and ash-colored stools were less common but not different between seroconverters and nonseroconverters. Only icterus (conjunctival or overt jaundice) was reported more often by seroconverters (P < 0.05), with 6 cases in the 3-month recall period. Assuming constant rates of infection and illness over the whole year (6 cases every 3-month interval), we extrapolate 24 cases among the 49 seroconversions, suggesting a disease to infection rate of 49.0% (95% CI: 31.4, 72.9). Among nonseroconverters, the rate of yellow eyes/skin was reported at around 5.1%. Upon subtraction of this background rate of icterus among “healthy” individuals from the seroconverter group, an adjusted illness rate of ∼28.4% can be estimated. (The risk of illness being caused by HBV was low, as only 25 of 577 HBV-susceptible individuals were HBV infected over 18 months, with only 1 dual HBV-HEV coinfection.)
Table 4.
Self-reported Morbidities in the Past 3 Months Among Anti-HEV Seroconverters and Nonseroconverters Between Baseline and 12 Months of Follow-up, Bangladesh, 2003–2005
| Symptom | Nonseroconverters (n = 746) |
Seroconverters (n = 49) |
||
| No. | % | No. | % | |
| Anorexia | 307 | 41.2 | 21 | 42.9 |
| Nausea/vomiting | 209 | 28.0 | 15 | 30.6 |
| Severe weakness | 208 | 27.9 | 18 | 36.7 |
| Yellow eyes/skin* | 38 | 5.1 | 6 | 12.2 |
| Fever | 274 | 36.7 | 13 | 26.5 |
| Clay-colored stools | 49 | 6.6 | 1 | 2.0 |
| Dark urine | 57 | 7.6 | 4 | 8.2 |
| Upper right quadrant/liver pain | 206 | 27.6 | 18 | 36.7 |
Abbreviation: anti-HEV, antibody to hepatitis E virus.
* P < 0.05 (χ2 test for differences between groups).
DISCUSSION
Our finding of 22.5% baseline seroprevalence to HEV in this enteric-disease–prone population of rural Bangladesh is similar to that of neighboring South Asian countries (27). Other studies in Nepal and India have found seroprevalence rates of between 4% and 64%, with an average near 20% (3, 33), although seldom with representative population samples. Our study represents one of the largest longitudinal populations followed for HEV infection; the low attrition rate is noteworthy, as over 89% of those enrolled were met at 18 months. The significant loss to follow-up of males and of those working in farming and fishing is worth consideration. Our sex- or gender-specific HEV incidence might be underestimated if losses were at increased risk of infection due to age-, gender-, or occupation-specific exposures. We previously reported that males in this population were more likely to be anti-HEV seropositive than females, across all age categories (27). As age remains of critical interest in HEV epidemiology, it is important to note that our cohort's age distribution was identical to that of the Matlab population (data not shown), with over 40% under the age of 20 years (26), enabling confident calculation of the low rate of pediatric HEV incidence in this population (Table 2).
The incidence density of 60.3 per 1,000 person-years during our study's first year represents a high rate of infection, nearly identical to a Nepalese cohort followed between 1992 and 1993 (64/1,000 person-years) (17). This similarity was unexpected, given that near-annual epidemics attributable to HEV have been reported for decades in Nepal whereas, in rural Bangladesh, HEV has only recently been recognized as an etiologic agent of sporadic or hospitalized cases (16). As the paucity of HEV data from Bangladesh may be due to underreporting or surveillance bias, our additional 12–18-month surveillance round was conducted to capture a second annual monsoon rainy season, in the event of a rainy-season HEV outbreak, as frequently seen in Nepal (17). The estimate of seroincidence was slightly, albeit nonsignificantly, higher in this period, at 72.4/1,000 person-years (Table 2).
Studies of HEV seroepidemiology in endemic populations, especially in South Asia, report perplexingly low anti-HEV frequencies in children (19, 34), in sharp contrast with exposure to HAV that is often ubiquitous by the age of 10 years (5, 19). Low numbers of pediatric cases seen during HEV outbreaks further strengthen the hypothesis that children are, for unidentified reasons, less likely to be infected than adults (35). The age-specific HEV seroincidence seen in our cohort confirms the predilection of HEV to infect proportionately more individuals beginning in early adulthood.
Except for the important influence of age on incidence, no other individual characteristics were found to be significantly associated with HEV infection (Table 3). This is not surprising given the limited number of infections observed in this cohort and subsequent low statistical power to detect any differences. The household-level monthly surveillance, of 20% of the cohort, did not capture any definitive hepatitis-like illness in the interim period between baseline and 18 months. Among the self-reported recent illnesses, only yellow eyes emerged as clearly associated with seroconversion (Table 4), leading to an adjusted disease rate estimate near 28%, or roughly one-third of those infected; these data are consistent with those of prior studies (17).
We may have misestimated the disease rate by extrapolating from the 3-month period of symptom recall that we used to minimize the potential for recall bias, as reported by others (17). The assumptions that infections were constant throughout the year and that at least 5% of nonseroconverters reported scleral icterus/jaundice suggest a bias toward disease rate overestimation. There was no evidence of a seasonal outbreak of hepatitis despite concurrent community-based hepatitis surveillance of over 23,500 individuals during the year in which this seroincidence study was conducted (data not shown). Stoszek et al. (24) reported a slightly lower incidence in rural Egypt (∼42/1,000 person-years) but also did not identify hepatitis illness among the 34 seroconverters followed for nearly a year. Individuals in this population report high rates of fever, anorexia, weakness, and nausea (Table 4). This finding is not surprising, given the elevated rate of malnutrition and known infectious disease burden in rural Bangladesh, but it may have confounded our ability to detect cases of hepatitis illness. It is also possible that the epidemiologic profile of HEV in this community may be one of mild or subclinical infections.
Rapid declines in circulating antibodies to HEV may help to explain why seasonal epidemics occur in endemic populations such as Nepal or parts of India, despite a backdrop of continuous, albeit varying intensities of environmental HEV exposure. A study of acute-HEV patients (identified by viremia, fecal shedding, and symptoms) in Nepal was unable to detect anti-HEV in ∼20% of cases, leading to a suggestion that some infections may not trigger a substantial or immediate antibody response, or that reinfections of previously infected individuals may be possible, resulting in immunoglobulin M-negative cases of clinical hepatitis E (17, 36).
Several researchers have proposed that hepatitis E itself is immunopathologic (19, 37). In animal models of HEV, the production of disease can require challenge doses over a thousand times greater than that required for infection (38). Perhaps, in human populations, we see clinical illness only when the infective dose increases sufficiently compared with background levels of HEV. In the riverine, flood-prone ecology of rural Bangladesh, continuous, low-level HEV exposures should be common. This is true for other common enteric pathogens (e.g., HAV) that cause widespread infection and immunity early in life. Understanding how HEV differs from other enteric pathogens in terms of host response, transmission, and pathogenesis has implications for HEV vaccination and estimation of populations at risk. If HEV infection is not sufficient to protect individuals from future infection and illness as suggested by some (39, 40), long-term follow-up of incident infections and HEV immunity is warranted.
Finally, because of the fact that both antibody seroprevalence (27) and infection rates are low in this cohort's children, a hypothesis of infection early in infancy, followed by rapid antibody decline below the threshold of antibody detection, is difficult to support (4). It is more likely that 1) the dose of HEV to which young children are exposed is insufficient to trigger a detectable adaptive immune response and/or illness, or 2) the increase in age-specific incidence is due to a dramatic increase in exposures coincident with early adulthood. The role of genotypic and subgenotypic variation also deserves closer attention, and presently genomic analysis of HEV (genotype 1) isolated from this cohort is ongoing and may shed additional light on these epidemiologic observations.
Although there is little evidence of common long-term sequelae of HEV genotype 1 infections, the fatality associated with hepatitis E in pregnancy and the economic burden of this disease require that resources be committed to improving our understanding of a virus that has been “emerging” for nearly 3 decades (1, 41, 42). Our data begin to shed light on the population burden of HEV in rural Bangladesh; considering its ∼150 million population, ∼70% of whom are rural inhabitants, it is important to recognize the true potential burden of hepatitis E, especially as the means to control this infection are within our grasp (43).
Acknowledgments
Author affiliations: Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Alain B. Labrique); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Kenrad E. Nelson); International Center for Diarrheal Disease Research, Bangladesh, Dhaka, Bangladesh (K. Zaman, Zahid Hossain, Parimalendu Saha, Mohammad Yunus, Anowar Hossain); and National Security Technology Department, Applied Physics Laboratory, Johns Hopkins University, Laurel, Maryland (John R. Ticehurst).
This study was conducted under an R01 grant AI51/31/2004 from the National Institutes of Health. Additional support was provided in kind (HAV, HBV, HCV, and HEV antibody testing of study specimens) by the Armed Forces Research Institute of Medical Sciences, a special foreign activity of the Walter Reed Army Institute of Research in Bangkok, Thailand.
The authors would like to express their thanks to Dr. Tim Endy, Dr. Mammen P. Mammen, Jr., Dr. Khin Saw Aye Myint, and the laboratory team (Kittinun Hussem and Permpanich Jib Pattama) of the Armed Forces Research Institute of Medical Sciences for their generous hepatitis testing support. They would also like to acknowledge the dedication and hard work of the HEV-Matlab field team (Bashiruddin Ahmad, S. R. Paul, A. B. M. Borhan Uddin, Nasrin Akter, Nurunnahar Lina, and Sabina Akhter), as well as the International Center for Diarrheal Disease Research, Bangladesh, and Matlab Health Research Center administration and staff for their support.
Conflict of interest: none declared.
Glossary
Abbreviations
- anti-HAV
antibody to hepatitis A virus (anti-HCV and anti-HEV defined similarly)
- anti-HBc
antibody to hepatitis B core antigen
- CI
confidence interval
- HAV
hepatitis A virus (HBV, HCV, and HEV defined similarly)
- WRAIR
Walter Reed Army Institute of Research
- WR-U
Walter Reed antibody units
References
- 1.Balayan MS, Andjaparidze AG, Savinskaya SS, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20(1):23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 2.Reyes GR, Purdy MA, Kim JP, et al. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247(4948):1335–1339. doi: 10.1126/science.2107574. [DOI] [PubMed] [Google Scholar]
- 3.Labrique AB, Thomas DL, Stoszek SK, et al. Hepatitis E: an emerging infectious disease. Epidemiol Rev. 1999;21(2):162–179. doi: 10.1093/oxfordjournals.epirev.a017994. [DOI] [PubMed] [Google Scholar]
- 4.Melnick JL. A water-borne urban epidemic of hepatitis. In: Hartman FW, LoGrippo GA, Mateer JG, editors. Hepatitis Frontiers. 1st ed. Boston, MA: Little, Brown and Company; 1957. pp. 211–225. [Google Scholar]
- 5.Sheikh A, Sugitani M, Kinukawa N, et al. Hepatitis E virus infection in fulminant hepatitis patients and an apparently healthy population in Bangladesh. Am J Trop Med Hyg. 2002;66(6):721–724. doi: 10.4269/ajtmh.2002.66.721. [DOI] [PubMed] [Google Scholar]
- 6.Bansal J, He J, Yarbough PO, et al. Hepatitis E virus infection in eastern India. Am J Trop Med Hyg. 1998;59(2):258–260. doi: 10.4269/ajtmh.1998.59.258. [DOI] [PubMed] [Google Scholar]
- 7.John R, Abraham P, Kurien G, et al. Sporadic hepatitis E in southern India [short report] Trans R Soc Trop Med Hyg. 1997;91(4):392. doi: 10.1016/s0035-9203(97)90252-2. [DOI] [PubMed] [Google Scholar]
- 8.Clayson ET, Myint KS, Snitbhan R, et al. Viremia, fecal shedding, and IgM and IgG responses in patients with hepatitis E. J Infect Dis. 1995;172(4):927–933. doi: 10.1093/infdis/172.4.927. [DOI] [PubMed] [Google Scholar]
- 9.Khuroo MS, Rustgi VK, Dawson GJ, et al. Spectrum of hepatitis E virus infection in India. J Med Virol. 1994;43(3):281–286. doi: 10.1002/jmv.1890430316. [DOI] [PubMed] [Google Scholar]
- 10.Acharya SK, Panda SK, Saxena A, et al. Acute hepatic failure in India: a perspective from the East. J Gastroenterol Hepatol. 2000;15(5):473–479. doi: 10.1046/j.1440-1746.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- 11.Shrestha SM. Enteric Non-A, Non-B Hepatitis in Nepal: Clinical and Epidemiological Observations. Amsterdam, the Netherlands: Elsevier Science Publishers BV; 1991. pp. 265–275. [Google Scholar]
- 12.Focaccia R, da Conceição OJ, Sette H, Jr, et al. Estimated prevalence of viral hepatitis in the general population of the municipality of São Paulo, measured by a serologic survey of a stratified, randomized and residence-based population. Braz J Infect Dis. 1998;2(6):269–284. [PubMed] [Google Scholar]
- 13.Mast EE, Kuramoto IK, Favorov MO, et al. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in northern California. J Infect Dis. 1997;176(1):34–40. doi: 10.1086/514037. [DOI] [PubMed] [Google Scholar]
- 14.Abe K, Li TC, Ding X, et al. International collaborative survey on epidemiology of hepatitis E virus in 11 countries. Southeast Asian J Trop Med Public Health. 2006;37(1):90–95. [PubMed] [Google Scholar]
- 15.Kuniholm MH, Purcell RH, McQuillan GM, et al. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988–1994. J Infect Dis. 2009;200(1):48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longer CF, Shrestha MP, MacArthy PO. Surrey, United Kingdom: Springer-Verlag; 1994. Epidemiology of Hepatitis E Virus (HEV): A Cohort Study in Kathmandu, Nepal; pp. 409–411. [Google Scholar]
- 17.Clayson ET, Shrestha MP, Vaughn DW, et al. Rates of hepatitis E virus infection and disease among adolescents and adults in Kathmandu Nepal. J Infect Dis. 1997;176(3):763–766. doi: 10.1086/517296. [DOI] [PubMed] [Google Scholar]
- 18.Jameel S. Molecular biology and pathogenesis of hepatitis E virus. Expert Rev Mol Med. 1999;1(18):1–16. doi: 10.1017/S1462399499001271. [DOI] [PubMed] [Google Scholar]
- 19.Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13(3):145–154. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- 20.Banait VS, Sandur V, Parikh F, et al. Outcome of acute liver failure due to acute hepatitis E in pregnant women. Indian J Gastroenterol. 2007;26(1):6–10. [PubMed] [Google Scholar]
- 21.Myint KS, Endy TP, Shrestha MP, et al. Hepatitis E antibody kinetics in Nepalese patients. Trans R Soc Trop Med Hyg. 2006;100(10):938–941. doi: 10.1016/j.trstmh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Arankalle VA, Favorov MO, Chadha MS, et al. Rhesus monkeys infected with hepatitis E virus (HEV) from the former USSR are immune to subsequent challenge with an Indian strain of HEV. Acta Virol. 1993;37(6):515–518. [PubMed] [Google Scholar]
- 23.Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28(9):1190–1199. doi: 10.1111/j.1478-3231.2008.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoszek SK, Engle RE, Abdel-Hamid M, et al. Hepatitis E antibody seroconversion without disease in highly endemic rural Egyptian communities. Trans R Soc Trop Med Hyg. 2006;100(2):89–94. doi: 10.1016/j.trstmh.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 25.van Ginniken J, Bairagi R, de Francisco A. Dhaka, Bangladesh: Public Health Sciences Division, International Center for Diarrhoeal Disease Research; 1998. Health and Demographic Surveillance in Matlab: Past, Present and Future. [Google Scholar]
- 26.Matlab: Women, Children and Health. Dhaka, Bangladesh: International Center for Diarrhoeal Disease Research, Bangladesh; 1994. [Google Scholar]
- 27.Labrique AB, Zaman K, Hossain Z, et al. Population seroprevalence of hepatitis E virus antibodies in rural Bangladesh. Am J Trop Med Hyg. 2009;81(5):875–881. doi: 10.4269/ajtmh.2009.09-0352. [DOI] [PubMed] [Google Scholar]
- 28.Mast EE, Alter MJ, Holland PV, et al. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatitis E Virus Antibody Serum Panel Evaluation Group. Hepatology. 1998;27(3):857–861. doi: 10.1002/hep.510270331. [DOI] [PubMed] [Google Scholar]
- 29.Innis BL, Seriwatana J, Robinson RA, et al. Quantitation of immunoglobulin to hepatitis E virus by enzyme immunoassay. Clin Diagn Lab Immunol. 2002;9(3):639–648. doi: 10.1128/CDLI.9.3.639-648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seriwatana J, Shrestha MP, Scott RM, et al. Clinical and epidemiological relevance of quantitating hepatitis E virus-specific immunoglobulin M. Clin Diagn Lab Immunol. 2002;9(5):1072–1078. doi: 10.1128/CDLI.9.5.1072-1078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed M, Munshi SU, Nessa A, et al. High prevalence of hepatitis A virus antibody among Bangladeshi children and young adults warrants pre-immunization screening of antibody in HAV vaccination strategy. Indian J Med Microbiol. 2009;27(1):48–50. [PubMed] [Google Scholar]
- 32.Saha SK, Saha S, Shakur S, et al. Community-based cross-sectional seroprevalence study of hepatitis A in Bangladesh. World J Gastroenterol. 2009;15(39):4932–4937. doi: 10.3748/wjg.15.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arankalle VA, Tsarev SA, Chadha MS, et al. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171(2):447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 34.Purcell RH, Emerson SU. Hepatitis E virus infection [letter] Lancet. 2000;355(9203):578. doi: 10.1016/S0140-6736(05)73231-1. [DOI] [PubMed] [Google Scholar]
- 35.Naik SR, Aggarwal R, Salunke PN, et al. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70(5):597–604. [PMC free article] [PubMed] [Google Scholar]
- 36.Clayson ET, Innis BL, Myint KS, et al. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am J Trop Med Hyg. 1995;53(3):228–232. doi: 10.4269/ajtmh.1995.53.228. [DOI] [PubMed] [Google Scholar]
- 37.Ticehurst JR. Hepatitis E virus. In: Murray P, Baron E, Pfaller M, editors. Manual of Clinical Microbiology. 7th ed. Washington, DC: ASM Press; 1999. pp. 1057–1069. [Google Scholar]
- 38.Tsarev SA, Tsareva TS, Emerson SU, et al. Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. J Med Virol. 1994;43(2):135–142. doi: 10.1002/jmv.1890430207. [DOI] [PubMed] [Google Scholar]
- 39.Bryan JP, Tsarev SA, Iqbal M, et al. Epidemic hepatitis E in Pakistan: patterns of serologic response and evidence that antibody to hepatitis E virus protects against disease. J Infect Dis. 1994;170(3):517–521. doi: 10.1093/infdis/170.3.517. [DOI] [PubMed] [Google Scholar]
- 40.Tsarev SA, Tsareva TS, Emerson SU, et al. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci U S A. 1994;91(21):10198–10202. doi: 10.1073/pnas.91.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emerson SU, Purcell RH. Running like water—the omnipresence of hepatitis E. N Engl J Med. 2004;351(23):2367–2368. doi: 10.1056/NEJMp048285. [DOI] [PubMed] [Google Scholar]
- 42.Clark KL, Howell RM, Scott RM, et al. The socioeconomic impact of hepatitis E in Nepal. Am J Trop Med Hyg. 1999;61(3):505–510. doi: 10.4269/ajtmh.1999.61.505. [DOI] [PubMed] [Google Scholar]
- 43.Teo CG. Subduing the hepatitis E python. Epidemiol Infect. 2009;137(4):480–484. doi: 10.1017/S0950268808001295. [DOI] [PubMed] [Google Scholar]


