Abstract
The authors’ objective in this study was to estimate the changes in serum folate and homocysteine concentration that resulted from 6 weeks of supplementation with folic acid. A randomized, double-blind, placebo-controlled, dose-response trial with a parallel-group design was conducted. A total of 133 participants aged 60–90 years (70% female, 19% nonwhite) were assigned to receive 0, 100, 400, 1,000, or 2,000 μg/day of folic acid for 6 weeks. Data were collected in the United States between June and September 1996. At baseline, median serum folate and plasma homocysteine concentrations were 5.7 ng/mL (interquartile range (25th–75th percentiles), 4.1–7.8) and 8.3 μmol/L (interquartile range, 7.1–10.0), respectively. As the folic acid dose increased, serum folate levels increased (P-trend < 0.001). There was no dose-response relation with homocysteine level among all participants. In analyses restricted to persons with the lowest serum folate concentration (<4.5 ng/mL) at baseline, there was a trend (P = 0.06) toward decreased homocysteine levels with increasing folic acid dose. In healthy, older adults with adequate folate status, folic acid supplementation is not beneficial for homocysteine reduction. However, for older adults with low serum folate levels, supplementation will improve folate status and may be beneficial for lowering homocysteine concentrations.
Keywords: adult, dietary supplements, folic acid, homocysteine
Low folate concentration is associated with increased homocysteine concentration (1, 2), and homocysteine concentration increases with age (3, 4). By lowering homocysteine concentration, folic acid supplementation may prevent colorectal cancer (5, 6), cognitive decline (7–9), vascular dementia (10), and possibly cardiovascular disease (11–13), although trial results related to cardiovascular disease prevention have been disappointing (14–22). The importance of folic acid supplementation in disease prevention remains widely debated.
Folic acid supplementation may be particularly important in ensuring adequate folate status in vulnerable populations such as older adults. Folate deficiency in older adults has been documented, with estimates as high as 40% in the United States (3) and 30%–50% in the Netherlands, Belgium, and Germany (23). Age-related increases in homocysteine levels are related to suboptimal intake and absorption of key vitamin B cofactors or substrates for homocysteine metabolism (3), reduced activity of homocysteine-metabolizing enzymes (24), and declining kidney function (25). Moreover, older adults with elevated homocysteine levels (≥14.3 μmol/L) have increased rates of all-cause and cardiovascular disease mortality (4). Pharmacologic doses of >2,000 μg/day of folic acid lower homocysteine concentration in older adults, but few dose-response studies have been conducted in this population using low-to-moderate doses (23, 26).
Our aim in this placebo-controlled dose-response trial was to estimate the changes in serum folate and plasma homocysteine concentration that result from supplementation with a wide range of doses of folic acid (0, 100, 400, 1,000, and 2,000 μg/day) in older adults in the United States.
MATERIALS AND METHODS
This folic acid supplementation study was a randomized, double-blind, placebo-controlled dose-response trial with a parallel-group design. The trial was conducted at the Johns Hopkins Medical Institutions in Baltimore, Maryland, in 1996. The study protocol was approved by the institutional review board of the Johns Hopkins Medical Institutions. Each participant provided written informed consent.
Participants
Healthy, community-dwelling adults aged 60–90 years who were not taking multivitamins or B vitamins were enrolled. To participate, prior supplement users had to discontinue use of multivitamins, folic acid, and pyridoxine for at least 8 weeks before enrollment. Other exclusion criteria were: 1) intramuscular use of vitamin B12; 2) seizure disorder; 3) pernicious anemia; or 4) chronic use of antifolate drugs (e.g., methotrexate, sulfa antibiotics). Recruitment was completed between June and August of 1996, and follow-up ended in September 1996. Participants were recruited from a local Baltimore retirement community and from lists of prior study participants and screenees.
Data collection
Data were collected at 3 clinic visits—screening, baseline, and a follow-up visit 6 weeks after baseline. At the screening visit, eligibility was determined, and informed consent was obtained. If the person was eligible, a questionnaire on medical history and sociodemographic factors was self-administered before the baseline visit, along with a validated food frequency questionnaire (27). Height and weight were also measured. At the baseline visit, randomization was completed and a fasting venous blood sample was obtained. At the follow-up visit, another fasting venous blood sample was obtained. Participants were asked about vitamin use to ensure that folic acid and other B vitamins were not taken during the intervention period. Plasma homocysteine and serum folate, vitamin B12, and creatinine concentrations were measured after an overnight fast. Samples collected for determination of plasma homocysteine level were drawn into tubes containing ethylenediaminetetraacetic acid, immediately placed on ice, and centrifuged within 90 minutes. Blood samples for determination of serum folate level were drawn and kept at room temperature for at least 15 minutes and allowed to clot before centrifugation. All aliquots of plasma and serum were frozen at −70°C until analysis. Dietary intake of folate was determined from the Block food frequency questionnaire. Body mass index was calculated as weight (kilograms) divided by height squared (meters squared). We calculated estimated glomerular filtration rate (eGFR) as a marker of kidney function using the 4-variable simplified Modification of Diet in Renal Disease Study equation (28). eGFR is expressed in mL/minute/1.73 m2. The equation for eGFR is 186 × (PCr)−1.154 × (age)−0.203 × (0.742 if female) × (1.210 if black).
Randomization and intervention
At the baseline visit, simple randomization was carried out, with no restrictions. Participants were assigned on an individual basis to an intervention dose in blocks of 10. The allocation scheduled was concealed, and the study coordinator was unaware of the next assignment in the sequence. Participants were randomized to one of 5 dose groups of daily folic acid supplementation. Doses used were 0, 100, 400, 1,000, and 2,000 μg and were comparable to the reference category (0 μg) and 1) the anticipated increase in dietary folic acid to be achieved by folic acid fortification (100 μg); 2) the typical dose of folic acid in multivitamin supplements (400 μg); 3) the upper limit of the Institute of Medicine Dietary Reference Intakes (1,000 μg) (29); and 4) a pharmacologic dose (2,000 μg), respectively. The 2,000-μg dose is twice the upper limit of the 1,000 μg/day recommended by the Institute of Medicine Dietary Reference Intakes (29). This upper limit is set because of concerns about masking vitamin B12 deficiency and its neurologic consequences, but this dose is currently used pharmacologically. Serum vitamin B12 was measured at baseline, and no participants were found to have inadequate vitamin B12 status (170–250 pg/mL). At the baseline clinic visit, participants were given a 6-week supply of pills and instructions on pill-taking. Participants remained on the same dose throughout the entire study. Study pills were provided by Whitehall-Robins Healthcare (Madison, New Jersey). Participant compliance in taking the study capsules was measured by pill count. Blood folate levels are also a marker of folic acid intake.
Laboratory procedures
Folate and homocysteine assays were conducted at the Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University in Boston, Massachusetts, in 1996. Serum folate concentration was measured by means of radio-protein binding Quantaphase II (Bio-Rad Laboratories, Hercules, California). Serum samples were analyzed together in a single run. Intraassay variability for folate using the Quantaphase II was 11%. Intraassay variability for vitamin B12 was 11%.
Plasma homocysteine concentration was measured by high-performance liquid chromatography with fluorometric detection. Samples were assayed over the course of 3 runs with well-accepted variability. The intraassay variability was 4%, and the interassay variabilities were 7%, 12%, and 6%.
Statistical analysis
Statistical analyses were performed using SAS software (version 9.1; SAS Institute, Inc., Cary, North Carolina) and Stata software (release 8.0l; Stata Corporation, College Station, Texas). Changes in serum folate and homocysteine concentrations between baseline and the follow-up visits were the primary variables of interest. Homocysteine values were not normally distributed. To minimize the potential influence of outliers, we display median values with interquartile ranges (25th–75th percentiles). Change in homocysteine level from baseline to follow-up was calculated. Median changes in homocysteine concentration are presented for each dose group. Nonparametric tests were used. We tested the hypothesis that there was a difference in change in homocysteine level among the 5 dose groups (SAS npar1way command) using the Kruskal-Wallis test. The Kruskal-Wallis test is the nonparametric analog of the 1-way analysis of variance test. Pairwise comparisons between active dose groups and the placebo group were examined (SAS npar1way command) using the exact Wilcoxon rank-sum test. The rank-sum test is the nonparametric analog of the independent 2-sample t test. Tests for a trend in homocysteine reduction across the 5 dose groups were also conducted (Stata nptrend command). Statistical significance was set at P < 0.05. To detect a ≥2.5-μmol/L difference in homocysteine concentration between the placebo group and each active dose group, a sample size of 25 people was needed for each of the 5 study groups at α = 0.05 (2-sided) with 80% power.
RESULTS
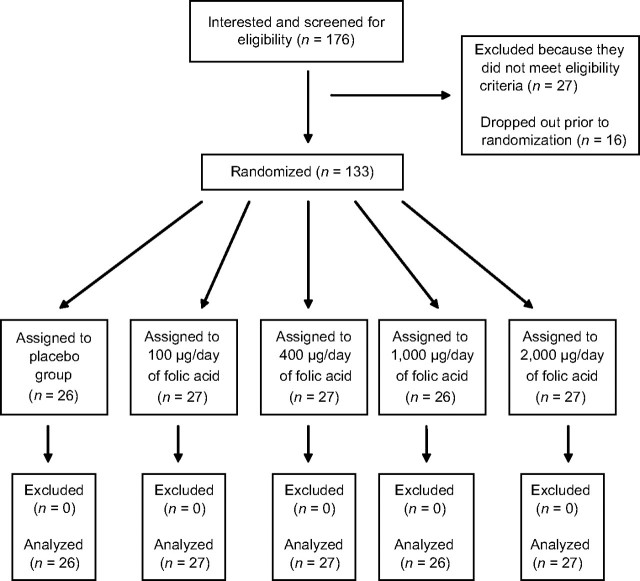
Of the 176 screened participants, 133 were randomized (Figure 1). The follow-up rate was 100%, and analyses were conducted using all randomized participants. As shown in Table 1, participants were mostly female, white, nonsmokers, nondrinkers, and nonusers of vitamins. The median age was 76 years. There were slightly more African Americans in the 100-μg and 400-μg dose groups than in the other 3 groups. All participants were compliant on the basis of having missed no more than 1 study capsule per week. Blood folate levels also increased in persons in the active dose groups, corroborating compliance findings obtained by pill count.
Figure 1.
Participant flow through each stage of a 6-week trial of folic acid supplementation and change in serum folate and plasma homocysteine concentrations among adults aged 60–90 years, Baltimore, Maryland, 1996.
Table 1.
Baseline Demographic and Clinical Characteristics of Adults Aged 60–90 Years in a 6-Week Trial of Folic Acid Supplementation and Change in Serum Folate and Plasma Homocysteine Concentrations, by Folic Acid Dose Group, Baltimore, Maryland, 1996a
| All Participants (n = 133) |
Placebo Group (0 μg/day) (n = 26) |
Folic Acid Supplement Dose, μg/day |
||||||||||||||||
| 100 (n = 27) |
400 (n = 27) |
1,000 (n = 26) |
2,000 (n = 27) |
|||||||||||||||
| No. | % | Median (IQRb) | No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | |
| Demographic factors | ||||||||||||||||||
| Female sex | 93 | 70 | 21 | 81 | 21 | 78 | 17 | 63 | 17 | 65 | 17 | 63 | ||||||
| Black race | 20 | 15 | 2 | 8 | 6 | 22 | 6 | 22 | 4 | 15 | 2 | 7 | ||||||
| Current smoker | 9 | 7 | 3 | 12 | 0 | 0 | 2 | 7 | 1 | 4 | 3 | 11 | ||||||
| Prior multivitamin user | 41 | 31 | 7 | 27 | 7 | 26 | 8 | 30 | 9 | 35 | 10 | 37 | ||||||
| Age, years | 76 (71–81) | 75.5 (73–84) | 75 (69–80) | 76 (69–82) | 75.5 (71–81) | 77 (72–80) | ||||||||||||
| Clinical characteristics | ||||||||||||||||||
| Body mass indexc | 27.1 (24.2–30.1) | 26.0 (22.5–27.8) | 27.7 (24.8–31.1) | 27.1 (24.2–31.3) | 27.4 (25.4–30.8) | 27.4 (24.0–31.6) | ||||||||||||
| Dietary folate intake, μg/day | 282 (221–349) | 310 (248–417) | 244 (195–309) | 289 (221–341) | 281 (206–364) | 302 (184–388) | ||||||||||||
| Alcohol consumption, drinks/week | 0 (0–1) | 0 (0–1) | 0 (0–2) | 0 (0–1) | 0 (0–2) | 0 (0–2) | ||||||||||||
| Serum vitamin B12 concentration, pg/mL | 407 (308–480) | 425 (294–477) | 393 (308–526) | 423 (315–475) | 356 (305–493) | 424 (357–471) | ||||||||||||
| Serum creatinine concentration, mg/dL | 1.0 (0.8–1.2) | 1.0 (0.8–1.0) | 0.9 (0.8–1.1) | 1.1 (0.9–1.2) | 1.0 (0.9–1.2) | 0.9 (0.8–1.1) | ||||||||||||
| Estimated glomerular filtration rated, mL/minute/1.73 m2 | 65 (58–75) | 65 (57–75) | 73 (57–81) | 65 (57–76) | 64 (57–72) | 72 (59–79) | ||||||||||||
Abbreviation: IQR, interquartile range.
There were no statistically significant differences among groups at baseline with regard to demographic factors, clinical characteristics, or outcome variables (P > 0.05).
25th–75th percentiles.
Weight (kg)/height (m)2.
Calculated using the Modification of Diet in Renal Disease Study (28) 4-variable simple equation variables (P > 0.05).
Table 2 displays serum folate and homocysteine concentrations before and after the 6-week folic acid intervention, within-group changes by folic acid intervention group, and between-group differences (active doses vs. placebo). At baseline, there were no significant differences among the groups in serum folate or homocysteine concentrations. Differences were seen among the 5 dose groups for change in serum folate level (P < 0.001, Kruskal-Wallis test) but not for change in homocysteine level (P = 0.42, Kruskal-Wallis test). For pairwise comparisons of active dose groups versus placebo, except for the comparison of 100 μg/day vs. 0 μg/day, there were differences in change in serum folate level. In contrast, there were no differences in change in homocysteine level for pairwise comparisons of groups with active doses versus placebo. There was a significant trend toward increased serum folate with increasing folic acid dose (P < 0.001), but there was no trend in homocysteine reduction for increasing doses of folic acid. The correlation of change in homocysteine with change in serum folate was not significant (Pearson's correlation coefficient: r = 0.05; P = 0.61).
Table 2.
Homocysteine and Serum Folate Concentrationsa at Baseline and at the End of a 6-Week Intervention Period Involving Folic Acid Supplementation Among Adults Aged 60–90 Years, Within-Group Changes by Folic Acid Dose Group, and Between-Group Comparisons (Active Dose vs. Placebo), Baltimore, Maryland, 1996
| All Participants (n = 133) |
Placebo Group (0 μg/day) (n = 26) |
Folic Acid Supplement Dose, μg/day |
P-Trend | ||||||||||
| 100 (n = 27) |
400 (n = 27) |
1,000 (n = 26) |
2,000 (n = 27) |
||||||||||
| Median | IQRb | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| Week 0 | |||||||||||||
| Folate RIA, ng/mL | 5.7 | 4.1–7.8 | 5.2 | 4.1–7.4 | 4.8 | 3.7–6.7 | 6.7 | 3.7–8.1 | 6.0 | 4.1–7.7 | 6.6 | 5.4–7.9 | |
| Homocysteine, μmol/L | 8.3 | 7.1–10.0 | 9.1 | 7.1–11.6 | 7.8 | 7.2–9.2 | 8.1 | 7.4–9.9 | 9.2 | 7.0–10.6 | 7.9 | 6.9–9.1 | |
| Week 6 | |||||||||||||
| Folate RIA, ng/mL | 5.4 | 3.8–6.2 | 7.0 | 5.4–9.4 | 12.4 | 9.6–16.7 | 16.1 | 12.4–32.6 | 16.4 | 10.7–65.5 | |||
| Homocysteine, μmol/L | 8.0 | 6.9–10.4 | 7.7 | 6.9–9.0 | 8.5 | 7.5–9.7 | 7.8 | 6.6–9.4 | 7.7 | 6.4–8.9 | |||
| Within-group change postintervention | |||||||||||||
| Folate RIA, ng/mL | −0.6 | −1.2 to 1.0 | 2.2 | 0.9 to 3.2 | 6.0 | 3.3 to 11.6 | 10.8 | 6.3 to 27.3 | 8.2 | 2.6 to 60.5 | <0.001 | ||
| Homocysteine, μmol/L | −0.5 | −1.3 to 0.4 | −0.2 | −1.2 to 0.5 | 0.1 | −1.0 to 1.0 | −1.0 | −1.7 to −0.1 | −0.5 | −1.1 to 0.9 | 0.75 | ||
| Between-group change postintervention (active dose vs. placebo) | Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | |||||
| Folate RIA, ng/mL | 2.8 | 0.63, 4.93 | 6.6 | 4.45, 8.74 | 12.5 | 10.3, 14.6 | 8.8 | 6.6, 11.0 | |||||
| Homocysteine, μmol/L | 0.4 | −0.47, 1.21 | 0.7 | 0.17, 1.51 | −0.4 | −1.28, 0.42 | 0.1 | −2.9, 0.39 | |||||
Abbreviations: CI, confidence interval; IQR, interquartile range; RIA, radioimmunoassay.
To convert ng/mL to nmol/L, multiply by 2.27.
25th–75th percentiles.
Table 3 shows results from analyses restricted to persons in the lowest tertile of serum folate concentration at baseline (<4.5 ng/mL). Similar to the overall group, differences were seen in this subgroup among the 5 dose groups for change in serum folate level (P < 0.001, Kruskal-Wallis test) but not for change in homocysteine level (P = 0.34, Kruskal-Wallis test). There were significant differences in serum folate concentration for all active dose groups versus placebo, except for the 100-μg/day versus 0-μg/day comparison. However, there were no significant differences in homocysteine concentration for pairwise comparisons of active doses versus placebo. Serum folate concentrations significantly increased as folic acid dose increased (P-trend < 0.001). In contrast to the findings in the entire study population, there was a borderline-significant trend in homocysteine reduction with increasing doses of folic acid.
Table 3.
Homocysteine and Serum Folate Concentrationsa Before and After a 6-Week Intervention Period Involving Folic Acid Supplementation Among Adults Aged 60–90 Years and Median Changes After Intervention by Intervention Group for Persons in the Lowest Tertile (≤4.5 ng/L) of Baseline Serum Folate Concentration, Baltimore, Maryland, 1996
| All Participants (n = 43) |
Placebo Group (0 μg/day) (n = 9) |
Folic Acid Supplement Dose, μg/day |
P-Trend | ||||||||||
| 100 (n = 11) |
400 (n = 10) |
1,000 (n = 10) |
2,000 (n = 3) |
||||||||||
| Median | IQRb | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| Week 0 | |||||||||||||
| Folate RIA, ng/mL | 3.7 | 3.0–4.1 | 3.7 | 3.0–4.1 | 3.7 | 2.1–3.9 | 3.2 | 2.5–3.9 | 3.5 | 3.1–4.3 | 3.1 | 2.9–4.5 | |
| Homocysteine, μmol/L | 11.0 | 8.9–12.8 | 11.0 | 8.9–12.8 | 8.9 | 7.5–10.8 | 9.2 | 7.8–12.6 | 10.3 | 9.2–11.2 | 9.1 | 8.5–22.2 | |
| Week 6 | |||||||||||||
| Folate RIA, ng/mL | 4.2 | 3.6–5.6 | 5.6 | 4.4–8.0 | 8.6 | 6.6–12.4 | 16.5 | 12.4–25.7 | 84.2 | 7.0–105.5 | |||
| Homocysteine, μmol/L | 10.1 | 7.2–12.0 | 8.5 | 6.9–9.8 | 8.8 | 7.7–9.5 | 9.1 | 7.7–9.7 | 6.4 | 5.3–20.7 | |||
| Within-group change postintervention | |||||||||||||
| Folate RIA, ng/mL | −1.0 | −0.1 to −1.8 | 2.5 | 2.0 to 3.5 | 5.6 | 3.3 to 8.5 | 13.0 | 9.7 to 22.6 | 80.6 | 2.4 to 102.4 | <0.001 | ||
| Homocysteine, μmol/L | −0.8 | −1.4 to −0.3 | −0.4 | −2.3 to 0.7 | −1.2 | −3.2 to −0.5 | −1.6 | −2.0 to −1.0 | −2.1 | −3.8 to −1.5 | 0.06 | ||
| Between-group change postintervention (active dose vs. placebo) | Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | |||||
| Folate RIA, ng/mL | 1.6 | −0.4, 3.6 | 4.8 | 1.7, 8.0 | 13.1 | 9.1, 17.0 | 79.6 | 75.8, 83.4 | |||||
| Homocysteine, μmol/L | 0.4 | −1.7, 2.5 | −0.6 | −3.0, 1.8 | −0.7 | −2.0, 0.6 | −1.3 | −3.9, 1.3 | |||||
Abbreviations: CI, confidence interval; IQR, interquartile range; RIA, radioimmunoassay.
To convert ng/mL to nmol/L, multiply by 2.27.
25th–75th percentiles.
Table 4 shows results from analyses restricted to persons in the highest tertile of plasma homocysteine concentration at baseline. The change in serum folate level was different among the 5 dose groups (P < 0.001), while there was no difference in change in homocysteine level (P = 0.50). The change in serum folate level was different for active-dose groups versus placebo, except between the 100-μg/day group and the placebo group, but there were no differences in change in homocysteine for active doses versus placebo. There was a significant trend toward an increased serum folate concentration with increasing folic acid dose (P < 0.001). There was no trend in homocysteine reduction for increasing doses of folic acid. Note that baseline kidney function in this subgroup, as determined by eGFR, was similar to that in the entire study population. The median eGFR concentration among all participants was 65 mL/minute/1.73 m2 (interquartile range, 58–75) as compared with 64 mL/minute/1.73 m2 (interquartile range, 52–73) among persons in the highest tertile of plasma homocysteine.
Table 4.
Homocysteine and Serum Folate Concentrationsa Before and After a 6-Week Intervention Period Involving Folic Acid Supplementation Among Adults Aged 60–90 Years and Median Changes After Intervention by Intervention Group for Persons in the Highest Tertile (≥9.19 mmol/L) of Baseline Plasma Homocysteine Concentration, Baltimore, Maryland, 1996
| All Participants (n = 45) |
Placebo Group (0 μg/day) (n = 12) |
Folic Acid Supplement Dose, μg/day |
P-Trend | ||||||||||
| 100 (n = 6) |
400 (n = 9) |
1,000 (n = 12) |
2,000 (n = 6) |
||||||||||
| Median | IQRb | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||
| Week 0 | |||||||||||||
| Folate RIA, ng/mL | 4.4 | 3.3–6.4 | 4.6 | 3.8–6.4 | 4.1 | 2.1–4.5 | 3.7 | 2.5–8.7 | 4.4 | 3.5–6.3 | 5.8 | 4.9–7.6 | |
| Homocysteine, μmol/L | 11.0 | 9.9–12.6 | 11.7 | 10.5–12.7 | 11.5 | 10.4–13.2 | 11.2 | 9.9–12.6 | 10.8 | 9.5–11.1 | 11.5 | 9.6–18.5 | |
| Week 6 | |||||||||||||
| Folate RIA, ng/mL | 3.8 | 3.3–5.5 | 6.8 | 4.9–10.0 | 10.8 | 6.6–15.4 | 16.5 | 12.3–32.9 | 61.5 | 11.6–105.8 | |||
| Homocysteine, μmol/L | 9.7 | 8.3–11.7 | 9.1 | 8.8–9.8 | 10.0 | 9.2–11.1 | 9.1 | 8.3–9.7 | 9.7 | 8.3–18.2 | |||
| Within-group change postintervention | |||||||||||||
| Folate RIA, ng/mL | −0.7 | −3.3 to 0.6 | 3.4 | 0.9 to 6.2 | 3.3 | 3.1 to 9.4 | 13.0 | 7.8 to 28.7 | 52.5 | 5.9 to 102.4 | <0.001 | ||
| Homocysteine, μmol/L | −0.9 | −2.1 to −0.1 | −2.3 | −4.2 to −1.3 | −2.8 | −3.2 to 0.2 | −1.6 | −2.1 to −0.4 | −1.0 | −1.5 to −0.7 | 0.84 | ||
| Between-group change postintervention (active dose vs. placebo) | Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | |||||
| Folate RIA, ng/mL | 4.5 | −1.7, 10.7 | 4.0 | 1.1, 6.9 | 14.7 | 8.4, 21.0 | 98.9 | 87.3, 110.5 | |||||
| Homocysteine, μmol/L | −1.4 | −5.3, 2.5 | −1.9 | −4.3, 0.4 | −0.5 | −1.8, 0.8 | −0.2 | −1.7, 1.2 | |||||
Abbreviations: CI, confidence interval; IQR, interquartile range; RIA, radioimmunoassay.
To convert ng/mL to nmol/L, multiply by 2.27.
25th–75th percentiles.
DISCUSSION
In this dose-response trial of healthy, older persons, we found that serum folate concentration progressively increased with higher doses of supplemental folic acid. Among all participants and in those with the highest homocysteine concentrations at baseline, there was no dose-response relation between folic acid intake and homocysteine level. When analyses were restricted to persons with the lowest plasma folate concentrations at baseline, there was a borderline-significant trend in homocysteine reduction with increasing doses of folic acid.
Our finding of an increase in serum folate concentration with increasing folic acid intake is consistent with results from observational studies (30, 31), as well as randomized clinical trials of fortified foods (32–34) or supplements (35–37). Most relevant to our findings are results from another dose-response study carried out in older adults (26), where 4 weeks of supplementation with placebo and folic acid at doses of 100 μg/day and 400 μg/day produced increases in serum folate concentrations.
Our results complement those of other studies examining the homocysteine-lowering effect of folic acid. These include 2 meta-analyses (2, 38) and 5 dose-response trials (23, 26, 35–37). Results from the 2 dose-response trials that were conducted in healthy, older adults are most relevant to our trial (23, 26). Although our results are in contrast to those of these 2 trials, they are consistent with those of the first Homocysteine Lowering Trialists’ Collaboration meta-analysis (2), which showed no differences in the homocysteine-lowering effect among higher folic acid doses (800, 1,000, or 2,000 μg/day). In this meta-analysis, the investigators concluded that the reduction in homocysteine concentration achieved was comparable (∼25%) for folic acid doses of 500–1,000 μg/day, 1,000–3,000 μg/day, and >3,000 μg/day (2). The second Homocysteine Lowering Trialists’ Collaboration meta-analysis included lower doses of folic acid (<500 μg/day) and found differences in their homocysteine-lowering effects (38). Differences were seen between 200 μg/day and 400 μg/day and between 400 μg/day and 800 μg/day. These results for lower doses of folic acid are in contrast to our findings. Unlike our study, the meta-analyses included persons from a wide age range (17–92 years) with a median preintervention homocysteine concentration of 10.5 μmol/L.
There are 3 potential reasons for the lack of effect of folic acid on homocysteine levels seen in our study. First, our study population was healthier than expected, with relatively high serum folate concentrations and low homocysteine concentrations at baseline. The degree of homocysteine response to folic acid intervention depends on preintervention concentrations of both folate and homocysteine. There is also a nonlinear relation between folate and mean homocysteine levels and a plateau beyond which homocysteine concentrations remain stable (1). Our trial baseline homocysteine concentrations (8.3 μmol/L) were lower than those seen in the dose-response study by van Oort et al. (26) (range of 10.9–12.0 μmol/L by dose group) and in the dose-response study by Rydlewicz et al. (23) (range of 9.5–10.8 μmol/L by dose group). We conducted subgroup analyses in this study to evaluate the effect of the intervention in persons with the lowest preintervention concentrations of folate. We did not have sufficient statistical power for subgroup analyses; thus, sample sizes for these analyses were small. However, we found a near-significant (P = 0.06) dose response among persons in the lowest tertile of serum folate concentration at baseline. Furthermore, the greatest homocysteine reductions in this study were seen among persons with the highest baseline homocysteine concentrations who took 100 μg/day (−2.3 μmol/L) or 400 μg/day (−2.8 μmol/L).
The second potential reason for the lack of effect of folic acid in our study is the possibility that the homocysteine-lowering effect of folic acid may have been attenuated by participants’ intake of fortified foods during the fortification transition period. Early fortification of foods may have occurred in the Baltimore area. Although we intended to start this study prior to fortification, industry initiation of fortification was done earlier than the mandate for 1998 and occurred throughout our data collection period. The extent of the effect of fortification on our results is unknown, but it is possible that it influenced our findings. Evidence from clinical trials on cardiovascular disease suggests that fortification has influenced the homocysteine-lowering effect of folic acid (15, 16). Trials conducted in countries with mandatory fortification show narrower differences in homocysteine outcomes for study groups than trials conducted in countries without fortification (2, 38).
The third potential reason for the lack of effect of folic acid in this study is the possibility that supplementation with vitamin B6, B12, or B2 is also necessary for a reduction in homocysteine levels to occur. There are 2 pathways involved in homocysteine metabolism. One pathway is remethylation, and it requires folic acid and vitamin B12 coenzymes. The other pathway is transsulfuration, which requires a vitamin B6 coenzyme. Riboflavin may also be used in the metabolic cycle. Since folic acid fortification began, there have been scientific reports suggesting that other B vitamins have an important role in lowering homocysteine concentration (35, 39). More specifically, associations between vitamin intakes and homocysteine levels have shifted from being primarily dependent on folate status to being more dependent on B-vitamin status (35, 39). Results from these studies suggested that the homocysteine-lowering effect of a regimen including folic acid and B vitamins was highly dependent on the action of vitamin B12. In this study, we did not monitor intake of vitamin B6, B12, or B2.
In summary, although no dose-response relation was seen between folic acid supplementation and homocysteine concentration, our data indicate that healthy, older adults can improve their folate status through supplementation. The benefits, risks, and appropriate dose of folic acid supplementation continue to be investigated for various health outcomes (40–42). These data suggest that a folic acid dose as low as 100 μg/day is effective in raising serum folate concentrations, and increasing folic acid dose leads to greater increases in blood folate concentration, with no evidence of a threshold. In the folic acid fortification era, daily supplementation comparable to usual dietary intake or even high-dose supplements does not lower homocysteine concentrations in healthy, older adults. Older adults with low folate status may benefit from folic acid supplementation.
Acknowledgments
Author affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Cheryl A. M. Anderson, Sun Ha Jee, Jeanne Charleston, Lawrence J. Appel); Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Cheryl A. M. Anderson, Lawrence J. Appel); Division of General Internal Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Lawrence J. Appel); Renaissance Gardens at Charlestown, Catonsville, Maryland (Matthew Narrett); and Department of Epidemiology and Health Promotion, School of Public Health, Yonsei University, Yonsei, South Korea (Sun Ha Jee).
This project was supported by the National Heart, Lung, and Blood Institute (grants 5 UO1 HL 50981 and 1K01HL092595-01), the Johns Hopkins School of Medicine General Clinical Research Center (grant M01-RR00052 from the National Center for Research Resources, National Institutes of Health), and the Seoul R&BD Program, Seoul, South Korea (grant 10526 to S. H. J.).
Conflict of interest: none declared.
Glossary
Abbreviation
- eGFR
estimated glomerular filtration rate
References
- 1.Boushey CJ, Beresford SA, Omenn GS, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274(13):1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 2.Homocysteine Lowering Trialists’ Collaboration. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ. 1998;316(7135):894–898. [PMC free article] [PubMed] [Google Scholar]
- 3.Selhub J, Jacques PF, Wilson PW, et al. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270(22):2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 4.Bostom AG, Silbershatz H, Rosenberg IH, et al. Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Framingham men and women. Arch Intern Med. 1999;159(10):1077–1080. doi: 10.1001/archinte.159.10.1077. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Stampfer MJ, Colditz GA, et al. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993;85(11):875–884. doi: 10.1093/jnci/85.11.875. [DOI] [PubMed] [Google Scholar]
- 6.Cravo M, Fidalgo P, Pereira AD, et al. DNA methylation as an intermediate biomarker in colorectal cancer: modulation by folic acid supplementation. Eur J Cancer Prev. 1994;3(6):473–479. doi: 10.1097/00008469-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Tucker KL, Qiao N, Scott T, et al. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82(3):627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 8.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82(3):636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 9.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369(9557):208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 10.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 11.Morrison HI, Schaubel D, Desmeules M, et al. Serum folate and risk of fatal coronary heart disease. JAMA. 1996;275(24):1893–1896. doi: 10.1001/jama.1996.03530480035037. [DOI] [PubMed] [Google Scholar]
- 12.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 13.Schnyder G, Roffi M, Flammer Y, et al. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention. The Swiss Heart Study: a randomized controlled trial. JAMA. 2002;288(8):973–979. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 14.Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, et al. Secondary prevention with folic acid: effects on clinical outcomes. J Am Coll Cardiol. 2003;41(12):2105–2113. doi: 10.1016/s0735-1097(03)00485-6. [DOI] [PubMed] [Google Scholar]
- 15.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 16.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. N Engl J Med. 2006;354(15):1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 17.Bønaa KH, Njølstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Cook NR, Albert CM, et al. Effect of homocysteine-lowering treatment with folic acid and B vitamins on risk of type 2 diabetes in women: a randomized, controlled trial. Diabetes. 2009;58(8):1921–1928. doi: 10.2337/db09-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wald DS, Wald NJ, Morris JK, et al. Folic acid, homocysteine, and cardiovascular disease: judging causality in the face of inconclusive trial evidence. BMJ. 2006;333(7578):1114–1117. doi: 10.1136/bmj.39000.486701.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazzano LA, Reynolds K, Holder KN, et al. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296(22):2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 21.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebbing M, Bleie Ø, Ueland PM, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300(7):795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]
- 23.Rydlewicz A, Simpson JA, Taylor RJ, et al. The effect of folic acid supplementation on plasma homocysteine in an elderly population. QJM. 2002;95(1):27–35. doi: 10.1093/qjmed/95.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Gartler SM, Hornung SK, Motulsky AG. Effect of chronologic age on induction of cystathionine synthase, uroporphyrinogen I synthase, and glucose-6-phosphate dehydrogenase activities in lymphocytes. Proc Natl Acad Sci U S A. 1981;78(3):1916–1919. doi: 10.1073/pnas.78.3.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bostom AG, Lathrop L. Hyperhomocysteinemia in end-stage renal disease: prevalence, etiology, and potential relationship to arteriosclerotic outcomes. Kidney Int. 1997;52(1):10–20. doi: 10.1038/ki.1997.298. [DOI] [PubMed] [Google Scholar]
- 26.van Oort FV, Melse-Boonstra A, Brouwer IA, et al. Folic acid and reduction of plasma homocysteine concentrations in older adults: a dose-response study. Am J Clin Nutr. 2003;77(5):1318–1323. doi: 10.1093/ajcn/77.5.1318. [DOI] [PubMed] [Google Scholar]
- 27.Block G, Woods M, Potosky A, et al. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 28.Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine, National Academy of Sciences. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy of Sciences; 1998. ( http://www.nap.edu). (Accessed August 5, 2010) [PubMed] [Google Scholar]
- 30.Jacques PF, Selhub J, Bostom AG, et al. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340(19):1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 31.Rader JI. Folic acid fortification, folate status and plasma homocysteine. J Nutr. 2002;132(8 suppl) doi: 10.1093/jn/132.8.2466S. 2466S–2470S. [DOI] [PubMed] [Google Scholar]
- 32.Venn BJ, Mann JI, Williams SM, et al. Assessment of three levels of folic acid on serum folate and plasma homocysteine: a randomised placebo-controlled double-blind dietary intervention trial. Eur J Clin Nutr. 2002;56(8):748–754. doi: 10.1038/sj.ejcn.1601388. [DOI] [PubMed] [Google Scholar]
- 33.Neuhouser ML, Beresford SA, Hickok DE, et al. Absorption of dietary and supplemental folate in women with prior pregnancies with neural tube defects and controls. J Am Coll Nutr. 1998;17(6):625–630. doi: 10.1080/07315724.1998.10718812. [DOI] [PubMed] [Google Scholar]
- 34.Johansson M, Witthöft CM, Bruce A, et al. Study of wheat breakfast rolls fortified with folic acid. The effect on folate status in women during a 3-month intervention. Eur J Nutr. 2002;41(6):279–286. doi: 10.1007/s00394-002-0388-9. [DOI] [PubMed] [Google Scholar]
- 35.Tucker KL, Olson B, Bakun P, et al. Breakfast cereal fortified with folic acid, vitamin B-6, and vitamin B-12 increases vitamin concentrations and reduces homocysteine concentrations: a randomized trial. Am J Clin Nutr. 2004;79(5):805–811. doi: 10.1093/ajcn/79.5.805. [DOI] [PubMed] [Google Scholar]
- 36.Brouwer IA, van Dusseldorp M, Duran M, et al. Low-dose folic acid supplementation does not influence plasma methionine concentrations in young non-pregnant women. Br J Nutr. 1999;82(2):85–89. doi: 10.1017/s0007114599001221. [DOI] [PubMed] [Google Scholar]
- 37.Wald DS, Bishop L, Wald NJ, et al. Randomized trial of folic acid supplementation and serum homocysteine levels. Arch Intern Med. 2001;161(5):695–700. doi: 10.1001/archinte.161.5.695. [DOI] [PubMed] [Google Scholar]
- 38.Homocysteine Lowering Trialists’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82(4):806–812. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 39.Miller JW, Green R, Ramos MI, et al. Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2003;78(3):441–447. doi: 10.1093/ajcn/78.3.441. [DOI] [PubMed] [Google Scholar]
- 40.Mason JB, Dickstein A, Jacques PF, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1325–1329. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]
- 41.Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302(19):2119–2126. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- 42.Whitrow MJ, Moore VM, Rumbold AR, et al. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170(12):1496–1493. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]