Abstract
Carbon isotopic signatures (“δ13C”) might reflect consumption of corn- and cane-based sweeteners. The authors hypothesized that the δ13C value of human serum is higher for individuals with high versus low intakes of corn- and cane-based sweeteners (measured as sweetened beverage intake). They conducted a cross-sectional study within the Atherosclerosis Risk in Communities Magnetic Resonance Imaging study (Maryland, 2005–2006). Diet was assessed by food frequency questionnaire, and blinded serum samples were assayed by natural abundance stable isotope mass spectroscopy. Studied were 186 participants (53% male; mean age, 71 years; mean body mass index, 30 kg/m2). Serum δ13C values for individuals with high sweetened beverage intakes were significantly higher than for those with low intakes (−19.15‰ vs. −19.47‰, P < 0.001). Serum δ13C value increased 0.20‰ for every serving/day of sweetened beverages (P < 0.01). The association between sweetened beverages and serum δ13C value remained significant after adjustment for confounding by corn-based product intake (P < 0.001). Serum δ13C values were also associated with waist circumference, body mass index, and waist-to-hip ratio. This study provides the first known evidence that the δ13C value of human serum differs between persons consuming low and high amounts of sweets. Within the proper framework, serum δ13C value could be developed into an objective biomarker promoting more reliable assessment of dietary sweets intake.
Keywords: biological markers, body mass index, diet, isotope labeling, sweetening agents
Dietary assessment is a central feature of many epidemiologic studies. A strong temporal relation has been demonstrated between national trends in increased consumption of corn-based sweeteners (i.e., high fructose corn syrup) and type 2 diabetes (1) and obesity (2). These and other epidemiologic assessments are limited in their ability to evaluate the effects of foods with corn-based sweeteners because of measurement error in self-reported dietary assessment techniques and limited information in food composition databases about the amount of these sweeteners present in foods and beverages (3, 4). A biomarker that objectively reflects intake of sweets would allow for more accurate evaluation of whether corn-based sweeteners affect health, and whether interventions to reduce intake of sweeteners are effective.
The naturally abundant isotope carbon 13 (13C) is a potential biomarker of dietary sweets intake. Both zea (corn) and saccharum (cane) species are known as C4 plants because they use the enzyme phosphoenolpyruvate carboxylase during photosynthesis (this enzyme is not used by the more common C3 plants) (5). Phosphoenolpyruvate carboxylase imparts an enrichment in 13C relative to isotope carbon 12 (12C) in C4 plant tissues (6), leading us to hypothesize that a diet rich in sweetened foods could be detected as a high 13C:12C ratio within human tissues (7). Many studies have quantified the carbon isotope composition (ratio of 13C to 12C, denoted as the δ13C value) of tissues of animals with a known diet (8–14) and upon a change in animal diet (15–24). However, although surveys of the carbon and nitrogen isotope composition of human blood (25–29), serum retinol (30), and other human tissues (31–38) are available, we know of no studies that specifically quantify a correlation between human sweetener consumption and serum δ13C value.
This study tested the hypothesis that the δ13C value of human serum reflects intake of food products containing corn- and cane-based sweeteners (measured as sweetened beverage intake). The secondary aim of the study was to assess associations of serum δ13C value with other corn-related dietary components and anthropometric measures.
MATERIALS AND METHODS
Study population
The study population consisted of Atherosclerosis Risk in Communities (ARIC) Magnetic Resonance Imaging study participants, aged 60–80 years, who lived in Washington County, Maryland. All participants were Caucasian. Each had dietary data and serum collected during their 2005/2006 study visit. Of the total 573 Washington County participants, we excluded those who reported an implausible caloric intake (i.e., <1,000 kcal/day (n = 88) or >4,000 kcal/day (n = 4)), those with diagnosed diabetes who may have had dietary restrictions (n = 89), and those for whom the amount of serum archived was insufficient for analysis (n = 14). Of the remaining 378 individuals, participants were selected for either high or low sweetened beverage consumption according to their responses to food frequency questionnaire (FFQ) items. We sampled all participants who responded that they never drink sweetened beverages (n = 42) and those who responded that they drink 5 or more servings per week (n = 60). We assayed 84 additional samples from a moderate consumption category (1–4 servings/week). Within the moderate consumption category, we sampled all individuals from quintiles 1 and 2 of total sugar intake as well as all individuals from quintiles 4 and 5 of total sugar intake. The final sample size was 186 participants.
Assessment of demographic and anthropometric factors
Methods of collecting data for the Atherosclerosis Risk in Communities study have been previously described (39). Age, gender, height, weight, hip circumference, and waist circumference were measured during the clinic visit by trained personnel using a standardized protocol. Both waist and hip circumferences were measured with a tape measure to the nearest centimeter (rounding down). Hip circumference was taken at the maximal protrusion of the gluteal muscles and waist circumference at the level of the umbilicus. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Assessment of sweetened beverage consumption
Complete dietary intake was measured using the previously validated Willett semiquantitative FFQ (40). Participants received the FFQ in the mail for self-administration prior to the clinical examination. At the study visit, clinic staff reviewed participant responses and queried participants if the questionnaire was incomplete.
Because sweetened beverages contribute the largest share of corn-based sweeteners (i.e., high fructose corn syrup) intake (2, 41), we used sweetened beverage consumption, characterized by FFQ, as a proxy for corn-based sweeteners intake. The following 4 FFQ items about sweetened beverages were used: 1) “Coke, Pepsi, or other soda with sugar”; 2) “caffeine-free Coke, Pepsi, or other cola with sugar”; 3) “other carbonated beverages with sugar (e.g., 7-Up)”; and 4) “Hawaiian Punch, lemonade, or other noncarbonated fruit drinks.” The frequency and amount of each type of sweetened beverage were determined. For frequency, there were 9 possible responses: never, <1/month, 1–3/month, 2–4/week, 5–6/week, 1/day, 2–3/day, 4–5/day, ≥6/day. Amounts were given as one glass or one bottle/can. Total servings per day were derived from responses to all 4 items summed for each participant. We categorized participants by low (≤1/week), moderate (2–4/week), and high (≥5/week) consumption. Although not specifically validated for the Atherosclerosis Risk in Communities Magnetic Resonance Imaging study cohort, similar self-reports of beverage intake from FFQs have been validated in other populations, with high correlation between FFQ data and data from daily diaries (42, 43).
Assessment of other dietary factors
Total intake of calories, carbohydrate, protein, animal protein, fat, animal fat, and different types of sugars (i.e., glucose, fructose) is presented in Table 1 and was calculated from FFQ data using the Harvard Nutrient Database (40). Macronutrient intakes (i.e., carbohydrate, fat, protein) are presented as a percentage of total caloric intake. Total meat consumption was derived from combined reports of intake of bacon, meat sandwiches, hamburger, hot dogs, processed meats, liver, and meat as a main or mixed dish. Red meat intake included hamburger, sandwiches, hot dogs, or main dishes containing beef, pork or lamb, and liver. Seafood consumption was considered canned tuna, breaded fish cakes/sticks, dark meat fish, or other fish, as well as shrimp, lobster, scallops, or clams as a main dish. Corn consumption from foods other than corn-based sweeteners consisted of whole corn (44) and foods such as corn chips and tortillas.
Table 1.
Participant Characteristicsa at the 2005–2006 ARIC-MRI Study Visit by Sweetened Beverage Consumption (Servings/Week), Washington County, Maryland
| Servings/Week | ||||
| Total | ≤1 | 2–4 | ≥5 | |
| No. of participants | 186 | 87 | 39 | 60 |
| Age, years | 71 (5) | 70 (5) | 72 (5) | 72 (5) |
| Male gender, no. (%) | 99 (53) | 30 (34) | 28 (71) | 41 (68) |
| Weight, kg | 82.4 (17) | 81 (18) | 86 (17) | 82 (15) |
| Body mass index, kg/m2 | 30 (6) | 30 (6) | 30 (5) | 29 (5) |
| Waist, cm | 104 (15) | 104 (15) | 106 (15) | 103 (13) |
| Waist-to-hip ratio | 0.96 (0.07) | 0.95 (0.06) | 0.97 (0.08) | 0.97 (0.07) |
| Total calories, kcal | 1,783 (594) | 1,454 (385) | 2,032 (558) | 2,099 (622) |
| Protein, % kcal | 16.5 (3) | 18.1 (3) | 16.1 (3) | 14.6 (2) |
| Carbohydrates, % kcal | 50.5 (8) | 48.6 (9) | 50.9 (7) | 53.0 (6) |
| Total fat, % kcal | 33.9 (6) | 34.4 (7) | 34.6 (6) | 32.9 (5) |
| Animal fat, % kcal | 16.4 (5) | 16.8 (5) | 16.2 (5) | 16.0 (5) |
Abbreviation: ARIC-MRI, Atherosclerosis Risk in Communities Magnetic Resonance Imaging.
All values are expressed as mean (standard deviation), unless indicated otherwise.
Laboratory methods
Blood was drawn after an 8-hour fast into silica-coated serum tubes free of any carbon-containing additives. Serum was stored at −70°C after collection and was thawed after approximately 2 years for measurement in 2007–2008. To measure δ13C value, serum samples were quantitatively combusted to carbon monoxide in a EuroVector elemental analyzer (EA3000; EuroVector, Milan, Italy) configured with a continuous-flow stable isotope ratio mass spectrometer (Isoprime; Micromass UK Ltd., Manchester, United Kingdom). The reporting standard is Vienna Pee Dee Belemnite, characterized by the International Atomic Energy Agency in Vienna, Austria. The value of 13C/12C in Vienna Pee Dee Belemnite is independently fixed; a high sample 13C/12C value corresponds to a high sample δ13C value (in units of per mill, designated ‰). Organic standards were introduced every 15 samples, and blanks were introduced every 12 samples.
Each sample was analyzed in triplicate, and the mean value was used in statistical analysis. Total variability across the 3 measurements never exceeded 0.1‰. An analytical uncertainty of <±0.1‰ is associated with each sample measurement, resulting in an intraassay coefficient of variation of 0.1‰. All samples were analyzed, with blinding to sample source, in the Jahren laboratory at the Krieger School of Arts and Sciences, The Johns Hopkins University. Two internal laboratory standards referenced to Vienna Pee Dee Belemnite were used, for a 2-point calibration that encompassed the range of δ13C values present in our samples (−22.9‰ to −15.6‰).
Statistical methods
Data were checked for outliers, and distributions of δ13C values and other variables were assessed for normality. A t test was conducted of the difference in serum δ13C value between those consuming high versus low amounts of sweetened beverages. Multiple linear regression was used to examine the association between serum δ13C value and sweetened beverage intake, adjusting for potential confounders. Partial Spearman correlations and multiple linear regression were used to characterize dietary macronutrients (i.e., protein and fat intake) associated with δ13C value as well as its relation with anthropometric variables (i.e., BMI and waist circumference). All analyses were carried out using Stata version 9.2 software (Stata Corporation, College Station, Texas). All 2-sided tests of significance were set at a type 1 error (alpha) level of 0.05.
RESULTS
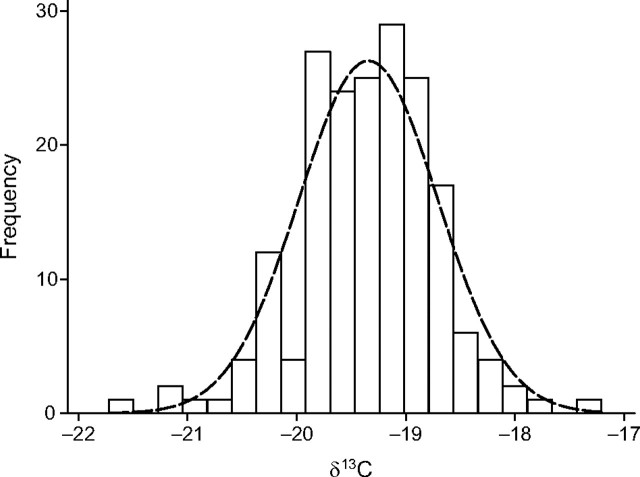
Table 1 shows participant characteristics by low (≤1/week), moderate (2–4/week), and high (≥5/week) sweetened beverage consumption. Consumers of low amounts of sweetened beverages were predominantly female (66%), whereas consumers of medium and high amounts were predominantly male. No significant association between sweetened beverage consumption and anthropometric measures was found. The δ13C distribution for all participants is shown in Figure 1. Mean δ13C value was −19.34‰ (range, −17.21 to −21.72).
Figure 1.
Distribution of serum ratio of isotope carbon 13 to isotope carbon 12 (δ13C) values among 186 participants in the Atherosclerosis Risk in Communities Magnetic Resonance Imaging (ARIC-MRI) study superimposed upon a normal distribution. Mean serum δ13C value was −19.34‰ (range, −17.21 to −21.72). Values were considered “low δ13C” if they measured on the upper end of the range; values were considered “high δ13C” if they were on the low end of the range. Participants were sampled from the Washington County, Maryland, site at the 2005–2006 ARIC-MRI study visit.
Association between serum δ13C value and sweetened beverage consumption
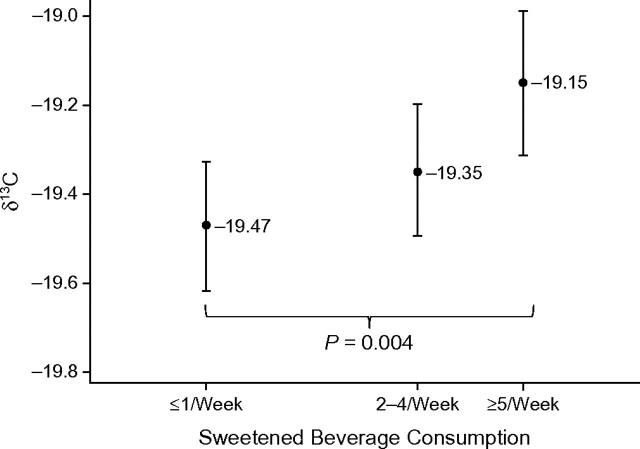
Figure 2 shows mean δ13C values stratified by the 3 levels of sweetened beverage consumption. The a priori aim of the study was to determine whether dichotomized sweetened beverage consumption was associated with serum δ13C value by testing the mean difference for low (≤1/week) versus high (≥5/week) levels of consumption. The mean serum δ13C value was lower for consumers of low (−19.47‰) compared with high (−19.15‰) amounts of sweetened beverages. This difference of 0.32‰ was statistically significant (P < 0.01). Combining those with moderate (2–4/week) intake with those with high intake decreased this difference (−0.24‰), but it remained significant (P < 0.01).
Figure 2.
Mean (95% confidence interval) serum ratio of isotope carbon 13 to isotope carbon 12 (δ13C) values stratified by 3 levels of sweetened beverage consumption in the Atherosclerosis Risk in Communities Magnetic Resonance Imaging study, Washington County, Maryland, 2005–2006. Mean serum δ13C value was significantly lower for those consuming sweetened beverages infrequently (≤1/week) compared with those consuming such beverages at a high frequency (≥5/week, P = 0.004 by t test of difference). Mean serum δ13C value of those reporting moderately frequent intake (2–4/week) was in between that for the infrequent and extremely frequent consumers.
Association between δ13C value and other dietary factors
Given the potential for association of δ13C with other food sources of corn and cane, we report in Table 2 the Spearman correlations between serum δ13C value and various dietary variables adjusted for gender. As expected, whole-corn consumption was associated with higher serum δ13C value, although the correlation was very weak (r = 0.15, P = 0.04). Significant correlations were found between serum δ13C value and animal sources of protein (r = 0.28, P < 0.001) and fat (r = 0.37, P < 0.001). Furthermore, these correlations were similar even among individuals consuming low amounts of sweetened beverages (r = 0.28 (P < 0.01) for protein and r = 0.31 (P < 0.01) for fat). Total meat consumption (r = 0.22, P = 0.003) was less correlated with serum δ13C value than red meat consumption (r = 0.33, P < 0.001). No correlations were found with seafood (P = 0.76) (data not shown). These patterns were expected given the widespread use of corn as feed for animals butchered for red meat, while not part of the diet of fish, for example. Total carbohydrate consumption was negatively associated with serum δ13C value initially (r = −0.21), but this finding was due to its strong negative correlation with animal fat (r = −0.57) such that adjustment for animal fat intake completely eliminated the correlation (r = −0.02, P = 0.79). Total sugar, glucose, sucrose, and fructose intake was not correlated with serum δ13C value after adjusting for gender.
Table 2.
Partial Spearman Correlations Between Serum δ13C Value and Dietary Factors Adjusted for Gender, Atherosclerosis Risk in Communities Magnetic Resonance Imaging Study, 2005–2006 Visit
| Gender Adjusted | P Value | |
| Sweetened beverages, servings/day | 0.18 | 0.01 |
| Whole corn, servings/day | 0.15 | 0.04 |
| Total calories, kcal | 0.08 | 0.26 |
| Total protein, % calories | 0.11 | 0.12 |
| Animal protein, % calories | 0.28 | <0.001 |
| Total fat, % calories | 0.10 | 0.19 |
| Animal fat, % calories | 0.37 | <0.001 |
| Carbohydrate, % calories | −0.21 | 0.004 |
| Glycemic index | 0.06 | 0.40 |
| Glycemic load | −0.003 | 0.97 |
| Total sugar, g | 0.11 | 0.13 |
| Glucose, g | 0.06 | 0.39 |
| Sucrose, g | 0.06 | 0.41 |
| Fructose, g | 0.06 | 0.42 |
Abbreviation: δ13C, ratio of isotope carbon 13 to isotope carbon 12.
We also tested whether the linear association between sweetened beverage consumption and δ13C was independent of corn and animal protein/fat intake (Table 3). Adjustment for dietary factors was conducted by adding factors consecutively. Because of the strong correlation between animal fat and animal protein (r = 0.81), only animal fat was included to avoid overadjustment. The fully adjusted model (Table 3, model 4) shows that mean serum δ13C values continued to be associated with increased sweetened beverage consumption, even after accounting for animal fat consumption (β = 0.18‰, P < 0.01). Table 4 displays the coefficients of the full model, where animal fat remained significantly associated with serum δ13C value, with an increase of 0.05‰ (P < 0.001) observed with each additional percentage of calories of animal fat consumed. The variance in serum δ13C value explained by the full model reached 29%.
Table 3.
Beta Coefficient (95% Confidence Interval) of Association Between Serum δ13C Values and Sweetened Beverage Consumption, Atherosclerosis Risk in Communities Magnetic Resonance Imaging Study, 2005–2006 Visit
| Model | β Coefficient for Sweetened Beverage Consumption | 95% CI | P Value | Adjusted r-Squared Value |
| 1: Univariate | 0.21 | 0.09, 0.32 | <0.001 | 0.06 |
| 2: + Corn consumption (≥1/week) | 0.20 | 0.08, 0.31 | 0.001 | 0.08 |
| 3: + Male gender | 0.13 | 0.02, 0.25 | 0.02 | 0.13 |
| 4: + Animal fat (% total calories/day) | 0.18 | 0.08, 0.29 | 0.001 | 0.29 |
Abbreviations: CI, confidence interval; δ13C, ratio of isotope carbon 13 to isotope carbon 12.
Table 4.
Results of Linear Regression With Serum δ13C Value as the Dependent Variable and Covariates From a Model Including Corn Consumption, Male Gender, and Animal Fat, Atherosclerosis Risk in Communities Magnetic Resonance Imaging Study, 2005–2006 Visit
| β Coefficient | 95% CI | P Value | |
| Sweetened beverages, servings/day | 0.18 | 0.08, 0.29 | 0.001 |
| Corn consumption (≥1/week) | 0.18 | 0.03, 0.34 | 0.02 |
| Male gender | 0.24 | 0.10, 0.42 | 0.004 |
| Animal fat (% total calories/day) | 0.05 | 0.03, 0.06 | <0.001 |
Abbreviations: CI, confidence interval; δ13C, ratio of isotope carbon 13 to isotope carbon 12.
Association between δ13C and anthropometric measures
Univariate associations between serum δ13C value and the anthropometric variables of weight, BMI, waist, and waist-to-hip ratio were examined (data not shown). The associations were in the hypothesized direction in that higher serum δ13C values corresponded to higher waist circumference, BMI, and waist-to-hip ratio. All anthropometric variables were positively associated with serum δ13C value in linear regression after adjustment for gender and intake of corn, sweetened beverages, and animal fat. On the other hand, the FFQ-derived measurements of sweetened beverage, total sugar (BMI, P = 0.60), or animal fat (BMI, P = 0.10) intake were not associated cross-sectionally with these anthropometric measures when the same regression models were used.
DISCUSSION
In this proof-of-principle approach, we confirmed our hypothesis that δ13C values are higher in individuals with higher intakes of sweetened beverages. The significant association between serum δ13C value and self-reported consumption of sweetened beverages was independent of gender, corn consumption, and animal fat intake. Furthermore, serum δ13C value was associated with adiposity, particularly waist circumference, suggesting that serum δ13C measurements could be useful in studying aspects of the modern diet that may contribute to diabetes and obesity. Although this technique is novel and promising, several issues have not been resolved, and further studies are needed to decipher its specificity to intake of sweets and its kinetic properties.
To our knowledge, this is the first evidence that the δ13C value of human serum is different for individuals who drink different amounts of sweetened beverages. Recent studies have investigated aspects of dietary intake on the δ13C value of various biologic samples including hair protein (37, 38), plasma protein (37), plasma glucose (29), serum retinol (30), and red blood cells (26). Our results are in line with those from recent studies showing a correlation between the δ13C value of serum retinol and specific nutritional components (i.e., provitamin A increase) (30) and long-term animal protein intake and hair δ13C value (37). It was also recently proposed that urinary sucrose and fructose excretion be used as biomarkers for sugar consumption (45), and urinary sugar excretion (46) has been shown to reflect immediate prior intake even in people without diabetes. The kinetics of δ13C in serum are not known, so we are unsure of the amount of time it would take for a change in diet to be reflected in a change in δ13C.
A recent cross-over feeding study measured plasma glucose δ13C values in 5 individuals fed differing amounts of C4 sugars (5%, 16%, or 32%) over 3 periods lasting 1 week each (29). The investigators found no differences in fasting plasma glucose δ13C values at the end of each period and concluded that fasting levels cannot be a marker of habitual intake. Nevertheless, important points must be raised in accessing this evidence, including the small number of participants (n = 5), which might not have enabled precise differences to be tested, the short period of feeding (7 days), and the different biologic media (plasma glucose).
To our knowledge, the distribution of δ13C in components of serum has not been specifically studied, although it is likely to reflect predominantly carbon in amino acids and in fatty acids. These presumably are in equilibrium with large body stores, not simply reflecting immediate dietary intake. The long half-life of serum components (e.g., albumin) suggests that serum δ13C represents a time-integrated reflection of carbon intake. This is distinct from urinary sugar excretion, which presumably reflects blood glucose over antecedent hours. Experiments expressly designed to measure turnover time of δ13C in specific human tissues are needed to fully determine the integrated intake period reflected by serum δ13C value (24).
Our data suggest that δ13C, when confirmed by further research, could be extremely useful in epidemiologic and public health settings. Dietary assessment is intrinsically cumbersome to administer and inaccurate, being dependent on self-report alone. The FFQ is subject to measurement error and may be systematically associated with factors such as BMI (e.g., overweight people underreporting their sweets intake), introducing error and biasing associations between subgroups. Even in the case of “nondifferential” misclassification, the resulting error introduces bias and underestimates diet-disease associations.
The measurement of serum δ13C value, as an objective laboratory measurement, could supplement or replace self-report of consumption of sweetened foods and beverages in epidemiologic studies. Presumably, clinical use as a laboratory assessment of individuals will require refinements to improve accuracy.
δ13C as a biomarker could address long-standing questions such as whether nutritively sweetened beverage consumption is in fact a cause of excessive weight gain. The relation between nutritively sweetened beverage consumption, obesity, and diabetes remains unclear despite a number of studies that have demonstrated an association between sweetened beverages and development of type 2 diabetes (47–51). Likewise, it is unclear whether changing the availability of sweetened beverages affects total caloric consumption, and how a change in sweets intake impacts glycemic control in diabetes.
The limitations of our work include our inability to capture and adjust for consumption of other forms of sugar (i.e., beet sugar) and potential confounding from other dietary sources of corn. Because beets do not share the conspicuously high 13C:12C value of corn and cane plants (i.e., beet is a “C3” plant), serum δ13C value does not capture the consumption of sugar from beets, which accounts for a nontrivial amount of the raw sugar used in the United States (48). It is unlikely that the source of raw sugar differed much between individuals in this study, all of whom resided in Washington County, Maryland, but the extent to which beet versus cane sugar contributes to caloric content globally would need to be studied. Less prominent sources of high fructose corn syrup such as certain types of breads, cereals, grains, and artificially sweetened dairy products could also conceivably affect serum δ13C; however, FFQs also cannot determine high fructose corn syrup intake from these sources. Another limitation of our work is the limitation of the comparator: if the FFQ is not in fact an accurate “gold standard,” then it is possible that the δ13C measurement is actually closer to “reality” than the FFQ. Lastly, because of the cross-sectional design of the study, we were unable to determine causal associations. Future longitudinal studies are needed to determine whether serum δ13C value reliably reflects long-term intake and whether the small, but significant difference in δ13C value detected here reflects an important biologic difference in metabolic risk.
The magnitude of the difference we detected (Figure 2) is small compared with the δ13C variability in serum across a large anonymous population (25), suggesting that other factors influence the overall carbon isotope composition of human blood. Dietary carbon comes not only from ingested carbohydrates such as corn, sugar cane, and other plants but also from fat and protein in the meat of animals that themselves consume corn or cane-based foods (44). Because livestock or chicken are fed corn, for example, the high δ13C value is incorporated into their tissues, and presumably into the humans who consume this meat. Our finding that δ13C was associated with animal fat and animal protein, particularly from beef, is consistent with the animals having been at least partially corn-fed. Because the serum δ13C value of our participants was affected by corn and animal fat intake, serum δ13C value cannot be considered specific to dietary sweets. Nevertheless, the highly significant association of δ13C with sweetened beverage intake, after adjustment for these known confounding factors, showed that differences in corn or animal fat intake did not eliminate the sweetened beverage intake signal. Controlled trials are needed to fully tease apart the contributions of each food item, but several studies suggest that consumption of sweetened beverages and high amounts of animal fat are each part of an unhealthy dietary pattern associated with obesity and type 2 diabetes (45, 49).
We utilized whole plasma samples in this study, but it is possible to target specific components in plasma, such as amino acid, lipid, or carbohydrate molecules. Measuring δ13C in one component of plasma may be a way to better distinguish the carbon source from animal (e.g., via protein δ13C) (37) versus plant (e.g., via glucose δ13C) sources (29). Evaluating the individual kinetics of different plasma components, each of which presumably reflects the contribution of a different, larger, whole body pool, will require more research.
Extensive archives of blood serum exist from large national and international epidemiologic studies. These archives can be used, as in this work, to further evaluate the relation of diet and disease. Less than 1 μL of serum is required for δ13C analysis, and we have shown that serum δ13C value is not affected by the archiving process or by preservation or declotting additives (25). Thus, the δ13C value in serum could easily be determined with existing serum archives or incorporated into the planning of epidemiologic studies.
In summary, these data provide the first known evidence that serum δ13C value is associated, to a highly statistically significant degree, with dietary exposure to sweetened beverage intake. Further research is needed to better characterize the contribution of individual nutritional components that affect serum δ13C value, such as animal fat. Research is also needed to elucidate the time course over which changes in diet are reflected in serum δ13C value. With further confirmation, serum δ13C value could become an objective, sensitive measure of sweets intake, promoting more reliable studies of diet on obesity, type 2 diabetes, and cardiovascular disease.
Acknowledgments
Author affiliations: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Edwina H. Yeung, Wen Hong Linda Kao, Josef Coresh, Cheryl A. M. Anderson); Welch Center for Prevention, Epidemiology, and Clinical Research, Baltimore, Maryland (Edwina H. Yeung, Wen Hong Linda Kao, Josef Coresh, Cheryl A. M. Anderson); Department of General Internal Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland (Wen Hong Linda Kao, Josef Coresh, Cheryl A. M. Anderson); Department of Endocrinology and Metabolism, Johns Hopkins School of Medicine, Baltimore, Maryland (Christopher D. Saudek, Melissa Islas); School of Earth and Ocean Science and Technology, University of Hawaii, Honolulu, Hawaii (A. Hope Jahren, Rebecca Kraft).
The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute (NHLBI), Bethesda, Maryland. This work was supported by NHLBI contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. This work was supported by grants DK-62707 to E. H. Y. and DK-67207 to W. H. K. from the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland. This work was also supported by an American Diabetes Association Innovation Award (Principal Investigator: C. D. S.), an Innovation Grant Award from the Johns Hopkins University Center for a Livable Future (Principal Investigator: C. A. M. A.), and a grant from the Seaver Institute (Pasadena, California) (Principal Investigator: A. H. J.).
The authors thank William M. Hagopian for laboratory assistance. They also thank the staff of the ARIC study for their important contributions.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- FFQ
food frequency questionnaire
- δ13C
ratio of isotope carbon 13 (13C) to isotope carbon 12 (12C)
References
- 1.Gross LS, Li L, Ford ES, et al. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79(5):774–779. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 3.Elliott SS, Keim NL, Stern JS, et al. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 4.Winkler JT. The fundamental flaw in obesity research. Obes Rev. 2005;6(3):199–202. doi: 10.1111/j.1467-789X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 5.Whelan T, Sackett WM. Enzymatic fractionation of carbon isotopes by phosphoenolpyruvate carboxylase from C4 plants. Plant Physiol. 1973;51(6):1051–1054. doi: 10.1104/pp.51.6.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farquhar GD. On the nature of carbon isotope discrimination in C4 species. Aust J Plant Physiol. 1983;10:205–226. [Google Scholar]
- 7.Jahren AH, Saudek C, Yeung E, et al. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr. 2006;84(6):1380–1384. doi: 10.1093/ajcn/84.6.1380. [DOI] [PubMed] [Google Scholar]
- 8.Bol R, Pflieger C. Stable isotope (13C,15N, and 34S) analysis of the hair of modern humans and their domestic animals. Rapid Commun Mass Spectrom. 2002;16(23):2195–2200. doi: 10.1002/rcm.706. [DOI] [PubMed] [Google Scholar]
- 9.DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978;42:495–506. [Google Scholar]
- 10.DeNiro MJ, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta. 1981;45:341–351. [Google Scholar]
- 11.Hobson KA, Stirling I. Low variation in blood δ13C among Hudson Bay polar bears: implications for metabolism and tracing terrestrial foraging. Mar Mamm Sci. 1997;13(3):359–367. [Google Scholar]
- 12.Minson DJ, Ludlow MM, Troughton JH. Differences in natural carbon isotope ratios of milk and hair from cattle grazing tropical and temperate pastures. Nature. 1975;256(5518):602. doi: 10.1038/256602a0. [DOI] [PubMed] [Google Scholar]
- 13.Podlesak DW, McWilliams SR. Metabolic routing of dietary nutrients in birds: effects of diet quality and macronutrient composition revealed using stable isotopes. Physiol Biochem Zool. 2006;79(3):534–549. doi: 10.1086/502813. [DOI] [PubMed] [Google Scholar]
- 14.Sponheimer M, Lee-Thorpe JA, DeRuiter DJ, et al. Diets of southern African Bovidae: stable isotope evidence. J Mammal. 2003;84(2):471–479. [Google Scholar]
- 15.Arneson LS, MacAvoy SE, Bassett E. Metabolic protein replacement drives tissue turnover in adult mice. Can J Zool. 2006;84(7):983–993. [Google Scholar]
- 16.Ayliffe LK, Cerling TE, Robinson T, et al. Turnover of carbon isotopes in tail hair and breath CO2 of horses fed an isotopically varied diet. Oecologia. 2004;139(1):11–22. doi: 10.1007/s00442-003-1479-x. [DOI] [PubMed] [Google Scholar]
- 17.Cerling TE, Ayliffe LK, Dearing MD, et al. Determining biological tissue turnover using stable isotopes: the reaction progress variable. Oecologia. 2007;151(2):175–189. doi: 10.1007/s00442-006-0571-4. [DOI] [PubMed] [Google Scholar]
- 18.Hobson KA, Clark RG. Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor. 1992;94:181–188. [Google Scholar]
- 19.Jim S, Jones V, Ambrose SH, et al. Quantifying dietary macronutrient sources of carbon for bone collagen biosynthesis using natural abundance stable carbon isotope analysis. Br J Nutr. 2006;95(6):1055–1062. doi: 10.1079/bjn20051685. [DOI] [PubMed] [Google Scholar]
- 20.Klaassen M, Thums M, Hume ID. Effects of diet change on carbon and nitrogen stable-isotope ratios in blood cells and plasma of the long-nosed bandicoot (Perameles nasuta) Aust J Zool. 2004;52(6):635–647. [Google Scholar]
- 21.MacAvoy SE, Arneson LS, Bassett E. Correlation of metabolism with tissue carbon and nitrogen turnover rate in small mammals. Oecologia. 2006;150(2):190–201. doi: 10.1007/s00442-006-0522-0. [DOI] [PubMed] [Google Scholar]
- 22.Sponheimer M, Robinson T, Ayliffe LK, et al. An experimental study of carbon-isotope fractionation between diet, hair, and feces of mammalian herbivores. Can J Zool. 2003;81(5):871–876. [Google Scholar]
- 23.Sponheimer M, Robinson TF, Cerling TE, et al. Turnover of stable carbon isotopes in the muscle, liver, and breath CO2 of alpacas (Lama pacos) Rapid Commun Mass Spectrom. 2006;20(9):1395–1399. doi: 10.1002/rcm.2454. [DOI] [PubMed] [Google Scholar]
- 24.Tieszen LL, Boutton TW, Tesdahl KG, et al. Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia. 1983;57:32–37. doi: 10.1007/BF00379558. [DOI] [PubMed] [Google Scholar]
- 25.Kraft RA, Jahren AH, Saudek CD. Clinical-scale investigation of stable isotopes in human blood: δ13C and δ15N from 406 patients at the Johns Hopkins Medical Institutions. Rapid Commun Mass Spectrom. 2008;22(22):3683–3692. doi: 10.1002/rcm.3780. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson MJ, Yai Y, O'Brien DM. Age-related variation in red blood cell stable isotope ratios (δ13C and δ15N) from two Yupik villages in southwest Alaska: a pilot study. Int J Circumpolar Health. 2007;66(1):31–41. doi: 10.3402/ijch.v66i1.18222. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien DM, Kristal AR, Jeannet MA, et al. Red blood cell δ15N: a novel biomarker of dietary eicosapentaenoic acid and docosahexaenoic acid intake. Am J Clin Nutr. 2009;89(3):913–919. doi: 10.3945/ajcn.2008.27054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metges CC, Petzke KJ. Measurement of 15N/14N isotopic composition in individual plasma free amino acids of human adults at natural abundance by gas chromatography-combustion isotope ratio mass spectrometry. Anal Biochem. 1997;247(1):158–164. doi: 10.1006/abio.1997.2037. [DOI] [PubMed] [Google Scholar]
- 29.Cook CM, Alvig AL, Liu YQ, et al. The natural 13C abundance of plasma glucose is a useful biomarker of recent dietary caloric sweetener intake. J Nutr. 2010;140(2):333–337. doi: 10.3945/jn.109.114777. [DOI] [PubMed] [Google Scholar]
- 30.Howe JA, Valentine AR, Hull AK, et al. 13C natural abundance in serum retinol acts as a biomarker for increases in dietary provitamin A. Exp Biol Med (Maywood) 2009;234(2):140–147. doi: 10.3181/0806-RM-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petzke KJ, Boeing H, Klaus S, et al. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr. 2005;135(6):1515–1520. doi: 10.1093/jn/135.6.1515. [DOI] [PubMed] [Google Scholar]
- 32.Petzke KJ, Boeing H, Metges CC. Choice of dietary protein of vegetarians and omnivores is reflected in their hair protein 13C and 15N abundance. Rapid Commun Mass Spectrom. 2005;19(11):1392–1400. doi: 10.1002/rcm.1925. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Schoeller DA, Winkler FJ, et al. Geographical variations in the carbon isotope composition of the diet and hair in contemporary man. Biomed Mass Spectrom. 1982;9(9):390–394. doi: 10.1002/bms.1200090906. [DOI] [PubMed] [Google Scholar]
- 34.Minagawa M. Reconstruction of human diet from δ13C and δ15N in contemporary Japanese hair: a stochastic method for estimating multi-source contribution by double isotopic tracers. Appl Geochem. 1992;7:145–158. [Google Scholar]
- 35.Nardoto GB, Silva S, Kendall C, et al. Geographical patterns of human diet derived from stable-isotope analysis of fingernails. Am J Phys Anthropol. 2006;131(1):137–146. doi: 10.1002/ajpa.20409. [DOI] [PubMed] [Google Scholar]
- 36.Macko SA, Engel MH, Andrusevich V, et al. Documenting the diet in ancient human populations through stable isotope analysis of hair. Philos Trans R Soc Lond B Biol Sci. 1999;354(1379):65–75. doi: 10.1098/rstb.1999.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petzke KJ, Lemke S. Hair protein and amino acid 13C and 15N abundances take more than 4 weeks to clearly prove influences of animal protein intake in young women with a habitual daily protein consumption of more than 1 g per kg body weight. Rapid Commun Mass Spectrom. 2009;23(16):2411–2420. doi: 10.1002/rcm.4025. [DOI] [PubMed] [Google Scholar]
- 38.Huelsemann F, Flenker U, Koehler K, et al. Effect of a controlled dietary change on carbon and nitrogen stable isotope ratios of human hair. Rapid Commun Mass Spectrom. 2009;23(16):2448–2454. doi: 10.1002/rcm.4039. [DOI] [PubMed] [Google Scholar]
- 39.The Atherosclerosis Risk in Communities (ARIC) study design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 40.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 41.Forshee RA, Storey ML, Allison DB, et al. A critical examination of the evidence relating high fructose corn syrup and weight gain. Crit Rev Food Sci Nutr. 2007;47(6):561–582. doi: 10.1080/10408390600846457. [DOI] [PubMed] [Google Scholar]
- 42.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 43.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 44.Jahren AH, Kraft RA. Carbon and nitrogen stable isotopes in fast food: signatures of corn and confinement. Proc Natl Acad Sci U S A. 2008;105(46):17855–17860. doi: 10.1073/pnas.0809870105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tasevska N, Runswick SA, McTaggart A, et al. Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1287–1294. doi: 10.1158/1055-9965.EPI-04-0827. [DOI] [PubMed] [Google Scholar]
- 46.Tasevska N, Runswick SA, Welch AA, et al. Urinary sugars biomarker relates better to extrinsic than to intrinsic sugars intake in a metabolic study with volunteers consuming their normal diet. Eur J Clin Nutr. 2009;63(5):653–659. doi: 10.1038/ejcn.2008.21. [DOI] [PubMed] [Google Scholar]
- 47.McNaughton SA, Mishra GD, Brunner EJ. Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the Whitehall II Study. Diabetes Care. 2008;31(7):1343–1348. doi: 10.2337/dc07-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer JR, Boggs DA, Krishnan S, et al. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168(14):1487–1492. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 50.Haley S, Ali M. Sugar Backgrounder. Washington, DC: USDA Economic Research Service; 2007. (Report no. SSS-249-01) [Google Scholar]
- 51.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82(3):675–684. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]