Abstract
Background
To understand an RNA sequence's mechanism of action, the structure must be known. Furthermore, target RNA structure is an important consideration in the design of small interfering RNAs and antisense DNA oligonucleotides. RNA secondary structure prediction, using thermodynamics, can be used to develop hypotheses about the structure of an RNA sequence.
Results
RNAstructure is a software package for RNA secondary structure prediction and analysis. It uses thermodynamics and utilizes the most recent set of nearest neighbor parameters from the Turner group. It includes methods for secondary structure prediction (using several algorithms), prediction of base pair probabilities, bimolecular structure prediction, and prediction of a structure common to two sequences. This contribution describes new extensions to the package, including a library of C++ classes for incorporation into other programs, a user-friendly graphical user interface written in JAVA, and new Unix-style text interfaces. The original graphical user interface for Microsoft Windows is still maintained.
Conclusion
The extensions to RNAstructure serve to make RNA secondary structure prediction user-friendly. The package is available for download from the Mathews lab homepage at http://rna.urmc.rochester.edu/RNAstructure.html.
Background
The prediction of RNA structure has received increasing attention over the last decade as the number of known functional RNA sequences, called non-coding RNA (ncRNA), has increased [1]. These new ncRNA sequences range in size from microRNAs to Xist [2,3]. They serve numerous roles, from modulating gene expression [4-6] to catalyzing reactions [7,8].
One of the first steps to understanding the mechanism of action of an RNA is to determine its structure [9]. Secondary structure, defined as the set of canonical base pairs (AU, GC, and GU), can be determined using comparative analysis if a large number of sequences are available [10,11]. In comparative analysis, base pairs are determined when they are conserved in multiple sequences and instances of compensating base pair changes occur. Compensating base pair changes demonstrate the conservation of structure in spite of sequence not being conserved, for example a GC base pair in one sequence being replaced by a homologous AU pair in another sequence. Comparative analysis, however, requires both significant user input and a large number of homologous sequences that can be aligned.
As an alternative to comparative analysis, the secondary structure of an RNA can be predicted for a single sequence using thermodynamics [9]. The thermodynamic methods are based on nearest neighbor rules that predict the stability of a structure as quantified by folding free energy change [12-14]. Often, structure prediction is accomplished by finding the lowest free energy structure, which is the single most probable structure in a folding ensemble [15]. Alternatively, structures can be sampled from the Boltzmann ensemble and a centroid, i.e. representative structure, determined [16,17]. Another alternative method for structure prediction is the prediction of a structure with the highest sum of pairing probabilities, called the maximum expected accuracy structure [18,19].
Single sequence secondary structure prediction is reasonably accurate. On average, for sequences of fewer than 700 nucleotides, the accuracy of predicting known base pairs is as high as 73% [14]. The accuracy, however, benchmarked lower when longer sequences were included [20,21]. Additional sources of information can be used to improve accuracy. For example, base pair probabilities can be determined using a partition function and highly probable pairs are more likely to be correctly predicted pairs [22]. Alternatively, using two or more homologous sequences to determine a conserved structure can result in significantly more accurate structure prediction [23-27]. Experimental data, such as enzymatic cleavage [13], chemical mapping [14], oligonucleotide array binding [28], SHAPE [29], and NMR data [30] can all be used to improve structure prediction accuracy.
In addition to structure prediction, the thermodynamic methods can be applied to other problems. For example, antisense oligonucleotide and siRNA design can be improved using thermodynamic predictions of self-structure in the oligonucleotides and target [31-36]. Sequences can be designed to fold to a specific structure [37,38]. Reverse-PCR primers can be designed to avoid self structure in the template that could prevent hybridization [39]. Novel types of ncRNAs can be found in genomes on the basis of folding stability [40-42].
In this contribution, the RNAstructure software package is described. RNAstructure first appeared in the literature in 1998 as a secondary structure prediction package [43]. At that time, it contained a method to predict the lowest free energy structure and a set of low free energy structures [44,45]. It was subsequently expanded to include bimolecular folding and hybridization thermodynamics with OligoWalk [13,31,33]. It was then expanded to include an algorithm for finding lowest free energy structures common to two sequences, Dynalign [23,41,46]; a partition function algorithm [22]; an alternative prediction method that can determine all low free energy structures for a sequence [28,47]; and stochastic sampling of structures [48]. It provides methods for constraining structures with enzymatic data [13], chemical mapping data [14], SHAPE [29], and NMR data [30]. Finally, recent extensions include PARTS [24], which calculates partition functions for secondary structures common to two sequences and can perform stochastic sampling of common structures [48]; MaxExpect, which finds maximum expected accuracy structures [18]; and a method for removal of pseudoknots, leaving behind the lowest free energy pseudoknot-free structure [49]. Several tutorials exist for using RNAstructure [50-52].
RNAstructure has been publicly available with a user-friendly interface for Microsoft Windows. Testing and development occur in-house using Unix/Linux text-based interfaces, but these have generally been available only upon request. The package is coded in C++. Extensive benchmarks of individual components have been published [14,18,22-24,29-31,46,48,53,54].
Here, three major extensions of RNAstructure are reported. First, a new JAVA-based graphical interface (GUI) is available. This interface functions cross-platform and binaries are available for Apple OS-X and Linux. Second, text interfaces are now available for each component of the package. These interfaces use a standard Unix syntax and include online help. Binaries for the text interfaces are available for Microsoft Windows and source code, including Makefiles, are available for download. Finally, a new class library is available for programmers who want to incorporate the RNAstructure functions into C++ programs. Each of these components is available for download and covered by the GNU Public License, version 2.
Implementation
C++ class library
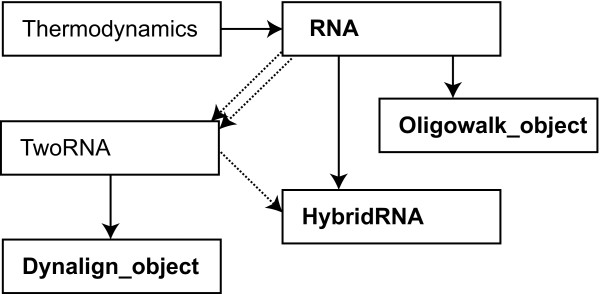
The C++ class library encapsulates the I/O functions of RNAstructure and also the secondary structure prediction and analysis methods. Four main classes are provided for accessing these functions: RNA, for single sequence structure predictions; Dynalign_object, for Dynalign calculations [23]; HybridRNA, for bimolecular structure prediction [33]; and Oligowalk_object, for OligoWalk [31,33] and OligoScreen [55] calculations. Two other notable classes are utilized by inheritance by the four main classes. Thermodynamics is a class that handles reading and storage of the nearest neighbor parameters. TwoRNA is a class that contains two RNA classes and is inherited by Dynalign_object and instantiated by HybridRNA. A class inheritance diagram is provided as Figure 1.
Figure 1.
Class inheritance diagram. The four main classes that provide functionality, named in boldface, are RNA, Oligowalk_object, HybridRNA, and Dynalign_object. Solid lines show inheritance and the dotted lines indicate that a class contains an instance of another. TwoRNA contains two instances of RNA and HybridRNA contains an instance of TwoRNA.
The classes are designed to be easily included in C++ projects. The classes are compiled to Linux/Unix shared libraries, Windows dynamic link libraries, or Macintosh dynamic shared libraries. An included Makefile provides facility for this. Furthermore, the header (*.h) files are commented using Doxygen-formatted comments that facilitate the creation of a manual http://www.stack.nl/~dimitri/doxygen/. Programmers can either compile their own manuals into html or latex using Doxygen or they can refer to precompiled html manuals included in the source code and posted on the RNAstructure website.
Text interfaces
The new text interfaces are designed to provide the features of RNAstructure for use on the command line and in scripts. Most programs available in the graphical user interfaces are provided with text interfaces. Each of these programs provides a brief description of the parameters when invoked without parameters. Additionally, invoking most programs with "-h," "-H," or "--help" will return a more detailed description of the parameters.
The text interfaces are built with ANSI-standard C++. Makefiles are included for compiling the programs in a Unix/Linux/OS X environment. Binaries for Microsoft Windows are also available for download.
The RNAstructure class library is used to implement most of the functions in the text interfaces. Therefore, the text interfaces can be used as tutorials by programmers implementing the RNAstructure algorithms in their own programs.
JAVA Graphical User Interface
The new JAVA GUI is a cross-platform re-implementation of the RNAstructure Windows GUI. This is designed to be user-friendly to make these algorithms accessible to a large audience. Executables are available for Apple OS-X and both 32-bit and 64-bit Linux. A Makefile is provided to facilitate local compilation on Unix/Linux environments.
The JAVA GUI utilizes the new RNAstructure C++ class library to implement the algorithms. The connection between the JAVA front end and the C++ back end is made using SWIG http://www.swig.org/. SWIG wraps the C++ classes, making them accessible to JAVA. The SWIG-wrapped code is made available as part of the RNAstructure code download, so that the JAVA interface can be built on local machines that do not have SWIG installed.
Availability of algorithms
Table 1 shows the availability of the different single-sequence structure prediction and analysis algorithms in RNAstructure. It shows the names of the text interface programs, the corresponding function name and class library, and the menu name in the JAVA and Windows GUIs. Similarly, Table 2 shows the corresponding information for the multi-sequence methods (either for common secondary structure prediction or prediction of nucleic acid hybridization).
Table 1.
Single Sequence Methods.
| Feature: | Text Interface Program: | JAVA/Windows GUI Menu Item: | Class Library and Function Name: |
|---|---|---|---|
| Free energy minimization structure prediction [14] | Fold | Fold RNA Single Strand |
RNA::FoldSingleStrand |
| Maximum expected accuracy structure prediction [18] | MaxExpect | Predict Maximum Expected Accuracy Structure |
RNA:MaximizeExpectedAccuracy |
| Partition function [22] | partition | Partition Function RNA |
RNA::PartitionFunction |
| Efn2 (energy calculator) [13] | efn2 | Efn2 RNA | RNA:: CalculateFreeEnergy |
| Free energy minimization and generation of all suboptimal structures [28,47] | AllSub | Generate All Suboptimal RNA Structures |
RNA::GenerateAllSuboptimalStructures |
| Stochastic sampling of structures [17] | stochastic | Stochastic RNA Sampling |
RNA::Stochastic |
| Remove Pseudoknots [49] | RemovePseudoknots | Break Pseudoknots |
RNA::BreakPeudoknots |
| Prediction of structures with pairs above specified pairing probability threshold [22] | ProbablePair | Output Probable Structure |
RNA::PredictProbablePairs |
| Drawing secondary structure diagrams | draw | Draw | RNA::DetermineDrawingCoordinates |
| NAPSS [30] | NAPSS | - | - |
Table 2.
Multiple Sequence Methods.
| Feature: | Text Interface Program: | JAVA/Windows GUI Menu Item: | Class Library and Function Name: |
|---|---|---|---|
| Dynalign [23,41,46] | dynalign | Dynalign RNA | Dynalign_object::Dynalign |
| OligoWalk [31-33] | OligoWalk | OligoWalk | Oligowalk_object::Oligowalk |
| OligoScreen [55] | oligoscreen | OligoScreen | Oligowalk_object::OligoScreen |
| Bimolecular structure prediction with intramolecular pairs [33] | bifold | Fold RNA Bimolecular |
HybridRNA::FoldBimolecular |
| PARTS [24,48] | PARTS | - | - |
| Bimolecular partition function (no intramolecular pairs) [31] | bipartition | Partition Function RNA Bimolecular |
HybridRNA::PartitionFunctionBimolecular |
| Bimolecular structure prediction without intramolecular pairs | DuplexFold | - | HybridRNA::FoldDuplex |
Thermodynamic parameters
The algorithms implemented in RNAstructure use nearest neighbor parameters to predict the stability of secondary structures. These include both free energy change parameters at 37°C and enthalpy change parameters to allow prediction of conformation stability at an arbitrary temperature. For RNA, these parameters are those most recent parameters from the Turner group [12,14,54]. For DNA, the parameters are derived from the experimental literature [56-92]. Most algorithms for RNA structure prediction can be invoked for DNA structure prediction using the class, text interfaces, or GUIs. DNA-RNA hybridization parameters are also used by OligoWalk [93].
Unit testing
The RNAstructure package now includes a facility for unit testing. A Makefile automates testing of the text interfaces and comparison with calculation standards. The same tests can be used to test the GUIs, but this requires manual selection of the input. Unit testing is helpful when changes are made to the algorithms to ensure that structure prediction is unchanged. Unit testing is also important when RNAstructure is installed in environments that have not been previously tested, to ensure that the installation worked correctly.
Results
To demonstrate the utility of RNAstructure, an example of secondary structure prediction is provided, showing the input and output. The method utilized is free energy minimization and the example sequence is the 5S rRNA from Pneumocystis carinii [94].
Figure 2 shows a screenshot of the "Fold RNA Single Strand" input form from the JAVA GUI. This program predicts the lowest free energy structure for an input sequence and a set of low free energy structures called suboptimal structures. The name of the input sequence is already selected in this screenshot by having clicked the "Sequence File" button. The default output (CT) file and the default parameters for determining suboptimal structures were determined by the program. Save files can be written to accelerate the prediction of an alternative set of suboptimal structures with a subsequent calculation. This view of the GUI shows the checkbox unchecked and so no save file will be written. The three remaining parameters control the prediction of suboptimal structures. "Max % Energy Difference" and "Max Number of Structures" place limits on the number of suboptimal structures predicted, with a maximum of the percentage difference in free energy change above the lowest free energy structure or an absolute limit on the number of structures, respectively. Suboptimal structures will be generated until the percent energy difference above the lowest free energy structure is reached, 10% in this case, or 20 structures have been generated. "Window Size" determines how different the suboptimal structures must be from each other in the set [44]. Zero places no restriction and larger integers become increasingly more stringent in the number of different pairs required between structures.
Figure 2.
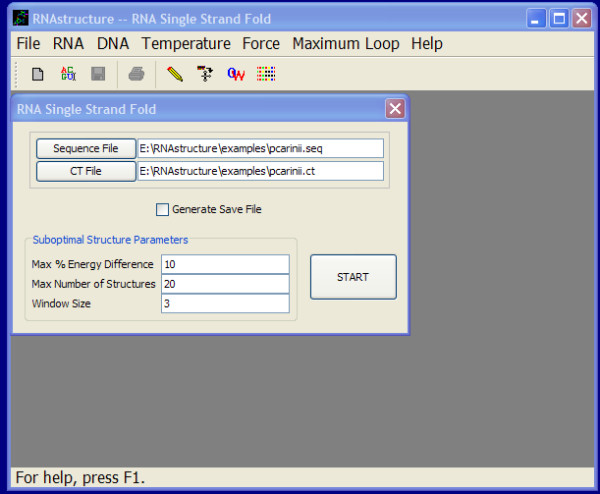
A screenshot of the JAVA GUI for predicting the lowest free energy structure for sequence "pcarinii.seq".
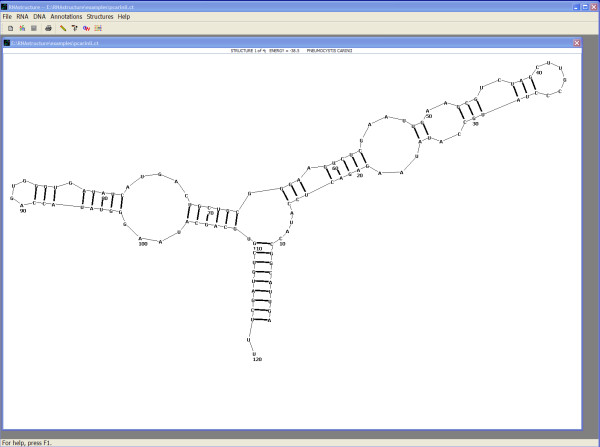
After clicking "Start," the calculation proceeds and the structure is then drawn as shown in Figure 3. The lowest free energy structure is shown by default and the user can display other structures by selecting the "Draw" menu item or by typing control-up-arrow or control-down-arrow.
Figure 3.
A screenshot of the JAVA GUI displaying the predicted lowest free energy structure for the sequence input as shown in Figure 2.
The same calculation could have been performed on the command line using the program "Fold." The command line for the same default method is "Fold pcarinii.seq pcarinii.ct." A set of postscript images of the predicted structure can then be rendered using "draw pcarinii.ct pcarinii.ps."
Behind the scenes, the structure prediction is performed using the RNA class. For this example, the class was instantiated using a constructor that reads sequences: RNA::RNA("pcarinii.seq", 2, true). The integer 2 indicates that the file is a sequence file and the bool true indicates that the sequence is RNA (as opposed to DNA). Structure prediction is then accomplished using RNA::FoldSingleStrand(10, 20, 3), where the parameters that control suboptimal prediction are the Max % Energy Difference, the Max number of structures, and the Window Size, respectively, as appeared in the GUI (Figure 2). The pairing can then be queried using RNA::GetPair or the drawing coordinates can be determined using RNA::DetermineDrawCoordinates.
Conclusions
RNAstructure is a software package for RNA secondary structure prediction and analysis. It is designed to make algorithms accessible for a variety of user needs. User-friendly GUIs are available for Windows, using native Windows code, and for Linux/Unix and Macintosh OS-X using JAVA. Text interfaces are provided for performing calculations on the command line or for scripting. Finally, a C++ class library is available to implement the algorithms into new programs. The package can be downloaded at http://rna.urmc.rochester.edu/RNAstructure.html.
Availability and requirements
• Project name: RNAstructure, version 5.0 and later
• Project home page: http://rna.urmc.rochester.edu/RNAstructure.html
• Operating system(s):
Text interfaces: Compilation is platform independent.
Executables are provided for Microsoft Windows and for 32- and 64-bit Linux.
Class library: Compilation is platform independent.
Windows GUI: Windows XP or later.
JAVA GUI: Compilation is platform independent, but requires Sun JDK 1.6 or higher. Executables are provided for Macintosh OS-X (version 10.5 or later) and Linux.
• Programming language:
Text interfaces, Class library, Windows GUI: C++
JAVA GUI: JAVA
• Other requirements:
Windows GUI: Compilation requires Microsoft Foundation Classes (MFC) as found in Microsoft Visual Studio 2005 or later and the Intel C++ compiler.
JAVA GUI: Requires Sun JAVA JDK version 1.6 or later. Recompilation of SWIG interface requires SWIG version 1.3.39 or later.
• License: GNU GPL
• Any restrictions to use by non-academics: None.
Abbreviations
GUI: graphical user interface; ncRNA: non-coding RNA; siRNA: Small interfering RNA
Authors' contributions
JSR wrote the JAVA interface and the new text interfaces. DHM wrote the C++ class library. Both authors contributed to the debugging of the code. DHM drafted the manuscript and both authors contributed to revisions.
Contributor Information
Jessica S Reuter, Email: Jessica_Reuter@urmc.rochester.edu.
David H Mathews, Email: David_Mathews@urmc.rochester.edu.
Acknowledgements
This work was supported by National Institutes of Health grant R01GM076485 to D.H.M. The authors thank those who contributed to the code base.
References
- Eddy SR. Non-coding RNA genes and the modern RNA world. Nature Reviews. 2001;2:919–929. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- Mello CC, Conte D Jr. Revealing the world of RNA interference. Nature. 2004;431(7006):338–342. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- Chow J, Heard E. X inactivation and the complexities of silencing a sex chromosome. Curr Opin Cell Biol. 2009;21(3):359–366. doi: 10.1016/j.ceb.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Wu L, Belasco JG. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell. 2008;29(1):1–7. doi: 10.1016/j.molcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Storz G, Gottesman S. In: The RNA World. third. Gesteland RF, Cech TR, Atkins JF, editor. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2006. Versatile Roles of Small RNA Regulators in Bacteria; pp. 567–594. [Google Scholar]
- Tucker BJ, Breaker RR. Riboswitches as versatile gene control elements. Curr Opin Struct Biol. 2005;15:342–348. doi: 10.1016/j.sbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Rodnina MV, Beringer M, Wintermeyer W. How ribosomes make peptide bonds. Trends Biochem Sci. 2007;32(1):20–26. doi: 10.1016/j.tibs.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Doudna J, Cech T. The chemical repertoire of natural ribozymes. Nature. 2002;418:222–228. doi: 10.1038/418222a. [DOI] [PubMed] [Google Scholar]
- Mathews DH, Turner DH. Prediction of RNA secondary structure by free energy minimization. Curr Opin Struct Biol. 2006;16(3):270–278. doi: 10.1016/j.sbi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gutell RR, Lee JC, Cannone JJ. The accuracy of ribosomal RNA comparative structure models. Curr Opin Struct Biol. 2002;12:301–310. doi: 10.1016/S0959-440X(02)00339-1. [DOI] [PubMed] [Google Scholar]
- Pace NR, Thomas BC, Woese CR. In: The RNA World. 2. Gesteland RF, Cech TR, Atkins JF, editor. Cold Spring Harbor Laboratory Press; 1999. Probing RNA structure, function, and history by comparative analysis; pp. 113–141. [Google Scholar]
- Xia T, SantaLucia J Jr, Burkard ME, Kierzek R, Schroeder SJ, Jiao X, Cox C, Turner DH. Thermodynamic parameters for an expanded nearest-neighbor model for formation of RNA duplexes with Watson-Crick pairs. Biochemistry. 1998;37:14719–14735. doi: 10.1021/bi9809425. [DOI] [PubMed] [Google Scholar]
- Mathews DH, Sabina J, Zuker M, Turner DH. Expanded sequence dependence of thermodynamic parameters provides improved prediction of RNA secondary structure. J Mol Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- Mathews DH, Disney MD, Childs JL, Schroeder SJ, Zuker M, Turner DH. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc Natl Acad Sci USA. 2004;101:7287–7292. doi: 10.1073/pnas.0401799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M, Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981;9:133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Chan CY, Lawrence CE. RNA secondary structure prediction by centroids in a Boltzmann weighted ensemble. RNA. 2005;11(8):1157–1166. doi: 10.1261/rna.2500605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Lawrence CE. A statistical sampling algorithm for RNA secondary structure prediction. Nucleic Acids Res. 2003;31(24):7280–7301. doi: 10.1093/nar/gkg938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZJ, Gloor JW, Mathews DH. Improved RNA secondary structure prediction by maximizing expected pair accuracy. RNA. 2009;15:1805–1813. doi: 10.1261/rna.1643609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Kiryu H, Sato K, Mituyama T, Asai K. Prediction of RNA secondary structure using generalized centroid estimators. Bioinformatics. 2009;25(4):465–473. doi: 10.1093/bioinformatics/btn601. [DOI] [PubMed] [Google Scholar]
- Dowell RD, Eddy SR. Evaluation of several lightweight stochastic context-free grammars for RNA secondary structure prediction. BMC Bioinformatics. 2004;5(1):71. doi: 10.1186/1471-2105-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi KJ, Cannone JJ, Cobaugh CW, Gutell RR. Evaluation of the suitability of free-energy minimization using nearest-neighbor energy parameters for RNA secondary structure prediction. BMC Bioinformatics. 2004;5(1):105. doi: 10.1186/1471-2105-5-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DH. Using an RNA secondary structure partition function to determine confidence in base pairs predicted by free energy minimization. RNA. 2004;10:1178–1190. doi: 10.1261/rna.7650904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DH, Turner DH. Dynalign: An algorithm for finding the secondary structure common to two RNA sequences. J Mol Biol. 2002;317:191–203. doi: 10.1006/jmbi.2001.5351. [DOI] [PubMed] [Google Scholar]
- Harmanci AO, Sharma G, Mathews DH. PARTS: Probabilistic Alignment for RNA joinT Secondary structure prediction. Nucleic Acids Res. 2008;36:2406–2417. doi: 10.1093/nar/gkn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havgaard JH, Lyngso RB, Stormo GD, Gorodkin J. Pairwise local structural alignment of RNA sequences with sequence similarity less than 40% Bioinformatics. 2005;21(9):1815–1824. doi: 10.1093/bioinformatics/bti279. [DOI] [PubMed] [Google Scholar]
- Hofacker IL, Fekete M, Stadler PF. Secondary structure prediction for aligned RNA sequences. J Mol Biol. 2002;319:1059–1066. doi: 10.1016/S0022-2836(02)00308-X. [DOI] [PubMed] [Google Scholar]
- Lück R, Steger G, Riesner D. Thermodynamic prediction of conserved secondary structure: Application to the RRE element of HIV, the tRNA-like element of CMV and the mRNA of prion protein. J Mol Biol. 1996;258:813–826. doi: 10.1006/jmbi.1996.0289. [DOI] [PubMed] [Google Scholar]
- Duan S, Mathews DH, Turner DH. Interpreting oligonucleotide microarray data to determine RNA secondary structure: application to the 3' end of Bombyx mori R2 RNA. Biochemistry. 2006;45(32):9819–9832. doi: 10.1021/bi052618x. [DOI] [PubMed] [Google Scholar]
- Deigan KE, Li TW, Mathews DH, Weeks KM. Accurate SHAPE-directed RNA structure determination. Proc Natl Acad Sci USA. 2009;106(1):97–102. doi: 10.1073/pnas.0806929106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JM, Kennedy SD, Mathews DH, Turner DH. NMR-assisted prediction of RNA secondary structure: identification of a probable pseudoknot in the coding region of an R2 retrotransposon. J Am Chem Soc. 2008;130(31):10233–10239. doi: 10.1021/ja8026696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZJ, Mathews DH. Efficient siRNA selection using hybridization thermodynamics. Nucleic Acids Res. 2007;36:640–647. doi: 10.1093/nar/gkm920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZJ, Mathews DH. Fundamental differences in the equilibrium considerations for siRNA and antisense oligodeoxynucleotide design. Nucleic Acids Res. 2008;36:3738–3745. doi: 10.1093/nar/gkn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DH, Burkard ME, Freier SM, Wyatt JR, Turner DH. Predicting oligonucleotide affinity to nucleic acid targets. RNA. 1999;5:1458–1469. doi: 10.1017/S1355838299991148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafer H, Ameres SL, Obernosterer G, Gebeshuber CA, Schroeder R, Martinez J, Hofacker IL. The impact of target site accessibility on the design of effective siRNAs. Nat Biotechnol. 2008;26(5):578–583. doi: 10.1038/nbt1404. [DOI] [PubMed] [Google Scholar]
- Shao Y, Chan CY, Maliyekkel A, Lawrence CE, Roninson IB, Ding Y. Effect of target secondary structure on RNAi efficiency. RNA. 2007;13(10):1631–1640. doi: 10.1261/rna.546207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D, Lee R, Williams P, Chan CY, Ambros V, Ding Y. Potent effect of target structure on microRNA function. Nat Struct Mol Biol. 2007;14(4):287–294. doi: 10.1038/nsmb1226. [DOI] [PubMed] [Google Scholar]
- Aguirre-Hernandez R, Hoos HH, Condon A. Computational RNA secondary structure design: empirical complexity and improved methods. BMC Bioinformatics. 2007;8:34. doi: 10.1186/1471-2105-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm C, Hofacker IL, Maurer-Stroh S, Stadler PF, Zehl M. Design of multistable RNA molecules. RNA. 2001;7:254–265. doi: 10.1017/S1355838201000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JM, Dang KK, Gorelick RJ, Leonard CW, Bess JW Jr, Swanstrom R, Burch CL, Weeks KM. Architecture and secondary structure of an entire HIV-1 RNA genome. Nature. 2009;460(7256):711–716. doi: 10.1038/nature08237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washietl S, Hofacker IL, Stadler PF. Fast and reliable prediction of noncoding RNAs. Proc Natl Acad Sci USA. 2005;102(7):2454–2459. doi: 10.1073/pnas.0409169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzilov AV, Keegan JM, Mathews DH. Detection of non-coding RNAs on the basis of predicted secondary structure formation free energy change. BMC Bioinformatics. 2006;7(1):173. doi: 10.1186/1471-2105-7-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torarinsson E, Sawera M, Havgaard JH, Fredholm M, Gorodkin J. Thousands of corresponding human and mouse genomic regions unalignable in primary sequence contain common RNA structure. Genome Res. 2006;16(7):885–889. doi: 10.1101/gr.5226606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DH, Andre TC, Kim J, Turner DH, Zuker M. In: Molecular Modeling of Nucleic Acids. Leontis NB, SantaLucia J Jr, editor. American Chemical Society; 1998. An updated recursive algorithm for RNA secondary structure prediction with improved thermodynamic parameters; pp. 246–257. [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989;244:48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- Steger G, Hofmann H, Fortsch J, Gross HJ, Randles JW, Sanger HL, Riesner D. Conformational transitions in viroids and virusoids: Comparison of results from energy minimization algorithm and from experimental data. J Biomol Struct Dyn. 1984;2(3):543–571. doi: 10.1080/07391102.1984.10507591. [DOI] [PubMed] [Google Scholar]
- Harmanci AO, Sharma G, Mathews DH. Efficient pairwise RNA structure prediction using probabilistic alignment constraints in Dynalign. BMC Bioinformatics. 2007;8:130. doi: 10.1186/1471-2105-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuchty S, Fontana W, Hofacker IL, Schuster P. Complete suboptimal folding of RNA and the stability of secondary structures. Biopolymers. 1999;49:145–165. doi: 10.1002/(SICI)1097-0282(199902)49:2<145::AID-BIP4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Harmanci AO, Sharma G, Mathews DH. Stochastic sampling of the RNA structural alignment space. Nucleic Acids Res. 2009;37:4063–4075. doi: 10.1093/nar/gkp276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit S, Rother K, Heringa J, Knight R. From knotted to nested RNA structures: a variety of computational methods for pseudoknot removal. RNA. 2008;14(3):410–416. doi: 10.1261/rna.881308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DH. In: Current Protocols in Bioinformatics. Baxevanis AD, Davison DB, Page RDM, Petsko GA, Stein LD, Stormo GD, editor. New York: John Wiley and Sons, Inc; 2006. RNA secondary structure analysis using RNAstructure; pp. 12.16.11–12.16.14. [Google Scholar]
- Mathews DH. In: Current Protocols in Bioinformatics. Baxevanis AD, Davison DB, Page RDM, Petsko GA, Stein LD, Stormo GD, editor. New York: John Wiley and Sons, Inc; 2005. Predicting the secondary structure common to two sequences with Dynalign; pp. 12.14.11–12.14.11. [Google Scholar]
- Mathews DH, Turner DH, Zuker M. In: Current Protocols in Nucleic Acid Chemistry. Beaucage SL, Bergstrum DE, Glick GD, Jones RA, editor. Vol. 11. New York: John Wiley and Sons; 2000. RNA secondary structure prediction; pp. 2.1–2.10. [Google Scholar]
- Lu ZJ, Mathews DH. OligoWalk: An online siRNA design tool utilizing hybridization thermodynamics. Nucleic Acids Res. 2008;36:W104–W108. doi: 10.1093/nar/gkn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZJ, Turner DH, Mathews DH. A set of nearest neighbor parameters for predicting the enthalpy change of RNA secondary structure formation. Nucleic Acids Res. 2006;34:4912–4924. doi: 10.1093/nar/gkl472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveeva OV, Mathews DH, Tsodikov AD, Shabalina SA, Gesteland RF, Atkins JF, Freier SM. Thermodynamic criteria for high hit rate antisense oligonucleotide design. Nucleic Acids Res. 2003;31(17):4989–4994. doi: 10.1093/nar/gkg710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allawi HT, SantaLucia J Jr. Thermodynamics and NMR of internal G.T mismatches in DNA. Biochemistry. 1997;36(34):10581–10594. doi: 10.1021/bi962590c. [DOI] [PubMed] [Google Scholar]
- Allawi HT, SantaLucia J Jr. NMR solution structure of a DNA dodecamer containing single G.T mismatches. Nucleic Acids Res. 1998;26(21):4925–4934. doi: 10.1093/nar/26.21.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allawi HT, SantaLucia J Jr. Nearest-neighbor thermodynamics of internal A.C mismatches in DNA: sequence dependence and pH effects. Biochemistry. 1998;37(26):9435–9444. doi: 10.1021/bi9803729. [DOI] [PubMed] [Google Scholar]
- Allawi HT, SantaLucia J Jr. Thermodynamics of internal C.T mismatches in DNA. Nucleic Acids Res. 1998;26(11):2694–2701. doi: 10.1093/nar/26.11.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allawi HT, SantaLucia J Jr. Nearest neighbor thermodynamic parameters for internal G.A mismatches in DNA. Biochemistry. 1998;37(8):2170–2179. doi: 10.1021/bi9724873. [DOI] [PubMed] [Google Scholar]
- Peyret N, Seneviratne PA, Allawi HT, SantaLucia J Jr. Nearest-neighbor thermodynamics and NMR of DNA sequences with internal A.A, C.C, G.G, and T.T mismatches. Biochemistry. 1999;38:3468–3477. doi: 10.1021/bi9825091. [DOI] [PubMed] [Google Scholar]
- Plum GE, Grollman AP, Johnson F, Breslauer KJ. Influence of the oxidatively damaged adduct 8-oxodeoxyguanosine on the conformation, energetics, and thermodynamic stability of a DNA duplex. Biochemistry. 1995;34(49):16148–16160. doi: 10.1021/bi00049a030. [DOI] [PubMed] [Google Scholar]
- Bolewska K, Zielenkiewicz A, Wierzchowski KL. Deoxydodecanucleotide heteroduplex d(TTTTATAATAAA). d(TTTATTATAAAA) containing the promoter Pribnow sequence TATAAT. I. Double-helix stability by UV spectrophotometry and calorimetry. Nucleic Acids Res. 1984;12(7):3245–3256. doi: 10.1093/nar/12.7.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslauer KJ, Frank R, Blocker H, Marky LA. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci USA. 1986;83(11):3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard GA, Thomson J, Watson WP, Brown T. High-resolution structure of a mutagenic lesion in DNA. Proc Natl Acad Sci USA. 1990;87(24):9573–9576. doi: 10.1073/pnas.87.24.9573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibanyenda N, De Bruin SH, Haasnoot CAG, Marel GA Van Der, Van Boom JH, Hilbers CW. The effect of single base-pair mismatches on the duplex stability of d(T-A-T-T-A-A-T-A-T-C-A-A-G-T-T-G) . d(C-A-A-C-T-T-G-A-T-A-T-T-A-A-T-A) Eur J Biochem. 1984;139:19–27. doi: 10.1111/j.1432-1033.1984.tb07970.x. [DOI] [PubMed] [Google Scholar]
- Plum GE, Grollman AP, Johnson F, Breslauer KJ. Influence of an exocyclic guanine adduct on the thermal stability, conformation, and melting thermodynamics of a DNA duplex. Biochemistry. 1992;31(48):12096–12102. doi: 10.1021/bi00163a019. [DOI] [PubMed] [Google Scholar]
- Arnold FH, Wolk S, Cruz P, Tinoco I Jr. Structure, dynamics, and thermodynamics of mismatched DNA oligonucleotide duplexes d(CCCAGGG)2 and d(CCCTGGG)2. Biochemistry. 1987;26(13):4068–4075. doi: 10.1021/bi00387a049. [DOI] [PubMed] [Google Scholar]
- Ratmeyer L, Vinayak R, Zhong YY, Zon G, Wilson WD. Sequence specific thermodynamic and structural properties for DNA.RNA duplexes. Biochemistry. 1994;33(17):5298–5304. doi: 10.1021/bi00183a037. [DOI] [PubMed] [Google Scholar]
- Williams AP, Longfellow CE, Freier SM, Kierzek R, Turner DH. Laser temperature-jump, spectroscopic, and thermodynamic study of salt effects on duplex formation by dGCATGC. Biochemistry. 1989;28(10):4283–4291. doi: 10.1021/bi00436a025. [DOI] [PubMed] [Google Scholar]
- Sugimoto N, Honda K, Sasaki M. Application of the thermodynamic parameters of DNA stability prediction to double-helix formation of deoxyribooligonucleotides. Necleosides & Nucleotides. 1994;13:1311–1317. doi: 10.1080/15257779408012153. [DOI] [Google Scholar]
- Wu P, Nakano S, Sugimoto N. Temperature dependence of thermodynamic properties for DNA/DNA and RNA/DNA duplex formation. Eur J Biochem. 2002;269(12):2821–2830. doi: 10.1046/j.1432-1033.2002.02970.x. [DOI] [PubMed] [Google Scholar]
- Hall KB, McLaughlin LW. Thermodynamic and structural properties of pentamer DNA.DNA, RNA.RNA, and DNA.RNA duplexes of identical sequence. Biochemistry. 1991;30(44):10606–10613. doi: 10.1021/bi00108a002. [DOI] [PubMed] [Google Scholar]
- Sugimoto N, Nakano S, Yoneyama M, Honda K. Improved thermodynamic parameters and helix initiation factor to predict stability of DNA duplexes. Nucleic Acids Res. 1996;24(22):4501–4505. doi: 10.1093/nar/24.22.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommarito S, Peyret N, SantaLucia J Jr. Thermodynamic parameters for DNA sequences with dangling ends. Nucleic Acids Res. 2000;28(9):1929–1934. doi: 10.1093/nar/28.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior M, Jones RA, Breslauer KJ. Influence of dangling thymidine residues on the stability and structure of two DNA duplexes. Biochemistry. 1988;27(10):3879–3885. doi: 10.1021/bi00410a053. [DOI] [PubMed] [Google Scholar]
- Aboul-ela F, Koh D, Tinoco I Jr, Martin FH. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A, C, G, T) Nucleic Acids Res. 1985;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccelli PV, Hilario J, Gallo FJ, Young AP, Benight AS. DNA and RNA oligomer sequences from the 3' noncoding region of the chicken glutamine synthetase gene from intramolecular hairpins. Biochemistry. 1996;35(48):15364–15372. doi: 10.1021/bi9615203. [DOI] [PubMed] [Google Scholar]
- Rentzeperis D, Alessi K, Marky LA. Thermodynamics of DNA hairpins: contribution of loop size to hairpin stability and ethidium binding. Nucleic Acids Res. 1993;21(11):2683–2689. doi: 10.1093/nar/21.11.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone PM, Paner TM, Hilario J, Lane MJ, Faldasz BD, Benight AS. Melting studies of short DNA hairpins: influence of loop sequence and adjoining base pair identity on hairpin thermodynamic stability. Biopolymers. 1999;50(4):425–442. doi: 10.1002/(SICI)1097-0282(19991005)50:4<425::AID-BIP8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Hilbers CW, Haasnoot CA, de Bruin SH, Joordens JJ, Marel GA van der, van Boom JH. Hairpin formation in synthetic oligonucleotides. Biochimie. 1985;67(7-8):685–695. doi: 10.1016/S0300-9084(85)80156-5. [DOI] [PubMed] [Google Scholar]
- Antao VP, Tinoco I Jr. Thermodynamic parameters for loop formation in RNA and DNA hairpin tetraloops. Nucleic Acids Res. 1992;20:819–824. doi: 10.1093/nar/20.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antao VP, Lai SY, Tinoco I Jr. A thermodynamic study of unusually stable RNA and DNA hairpins. Nucleic Acids Res. 1991;19:5901–5905. doi: 10.1093/nar/19.21.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody EM, Bevilacqua PC. Thermodynamic coupling of the loop and stem in unusually stable DNA hairpins closed by CG base pairs. J Am Chem Soc. 2003;125(8):2032–2033. doi: 10.1021/ja029831q. [DOI] [PubMed] [Google Scholar]
- Nakano M, Moody EM, Liang J, Bevilacqua PC. Selection for thermodynamically stable DNA tetraloops using temperature gradient gel electrophoresis reveals four motifs: d(cGNNAg), d(cGNABg), d(cCNNGg), and d(gCNNGc) Biochemistry. 2002;41(48):14281–14292. doi: 10.1021/bi026479k. [DOI] [PubMed] [Google Scholar]
- LeBlanc DA, Morden KM. Thermodynamic characterization of deoxyribooligonucleotide duplexes containing bulges. Biochemistry. 1991;30(16):4042–4047. doi: 10.1021/bi00230a031. [DOI] [PubMed] [Google Scholar]
- Morden KM, Chu YG, Martin FH, Tinoco I Jr. Unpaired cytosine in the deoxynucleotide duplex dCA3CA3G.dCT6G is outside of the helix. Biochemistry. 1983;22:5557–5563. doi: 10.1021/bi00293a016. [DOI] [Google Scholar]
- Li Y, Zon G, Wilson WD. Thermodynamics of DNA duplexes with adjacent G.A mismatches. Biochemistry. 1991;30(30):7566–7572. doi: 10.1021/bi00244a028. [DOI] [PubMed] [Google Scholar]
- Li Y, Agrawal S. Oligonucleotides containing G.A pairs: effect of flanking sequences on structure and stability. Biochemistry. 1995;34(31):10056–10062. doi: 10.1021/bi00031a030. [DOI] [PubMed] [Google Scholar]
- Kadrmas JL, Ravin AJ, Leontis NB. Relative stabilities of DNA three-way, four-way and five-way junctions (multi-helix junction loops): unpaired nucleotides can be stabilizing or destabilizing. Nucleic Acids Res. 1995;23:2212–2222. doi: 10.1093/nar/23.12.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontis NB, Kwok W, Newman JS. Stability and structure of three-way DNA junctions containing unpaired nucleotides. Nucleic Acids Res. 1991;19:759–766. doi: 10.1093/nar/19.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbury JE, Sturtevant JM, Leontis NB. The thermodynamics of formation of a three-strand, DNA three-way junction complex. Biochemistry. 1994;33(22):6828–6833. doi: 10.1021/bi00188a011. [DOI] [PubMed] [Google Scholar]
- Sugimoto N, Nakano S, Katoh M, Matsumura A, Nakamuta H, Ohmichi T, Yoneyama M, Sasaki M. Thermodynamic parameters to predict stability of RNA/DNA hybrid duplexes. Biochemistry. 1995;34:11211–11216. doi: 10.1021/bi00035a029. [DOI] [PubMed] [Google Scholar]
- Szymanski M, Barciszewska MZ, Barciszewski J, Erdmann VA. 5S ribosomal RNA database Y2K. Nucleic Acids Res. 2000;28:166–167. doi: 10.1093/nar/28.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]