Abstract
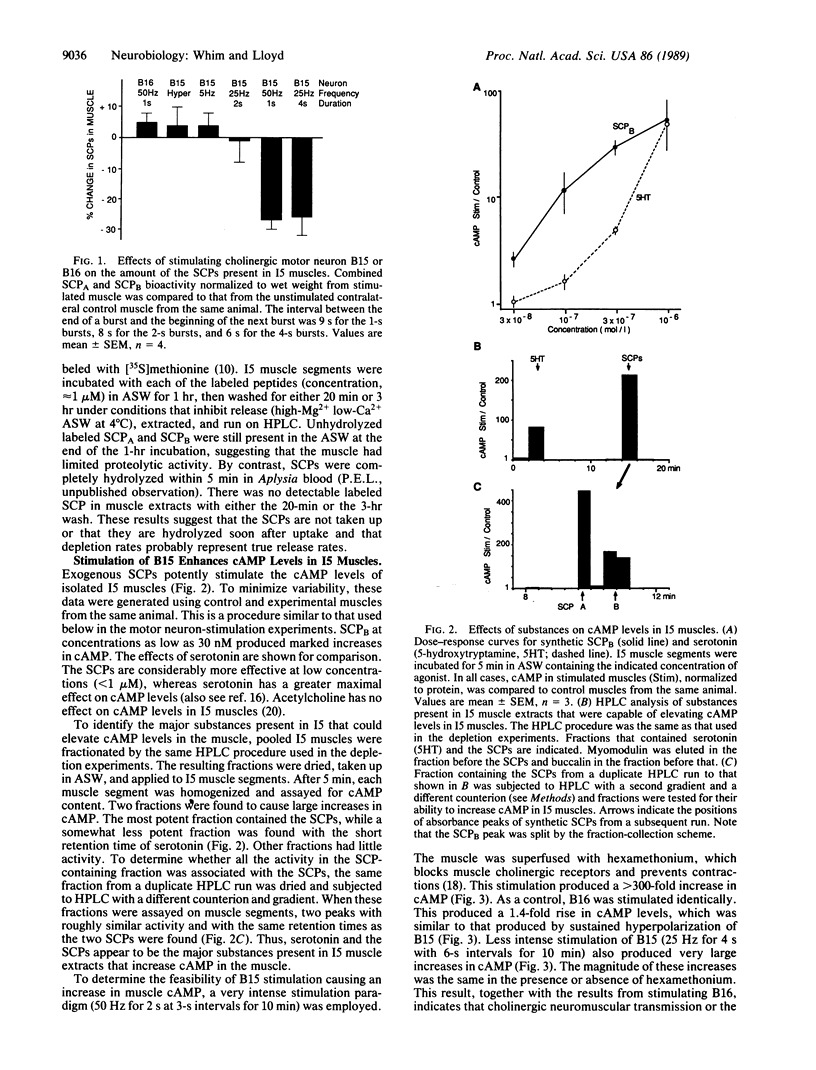
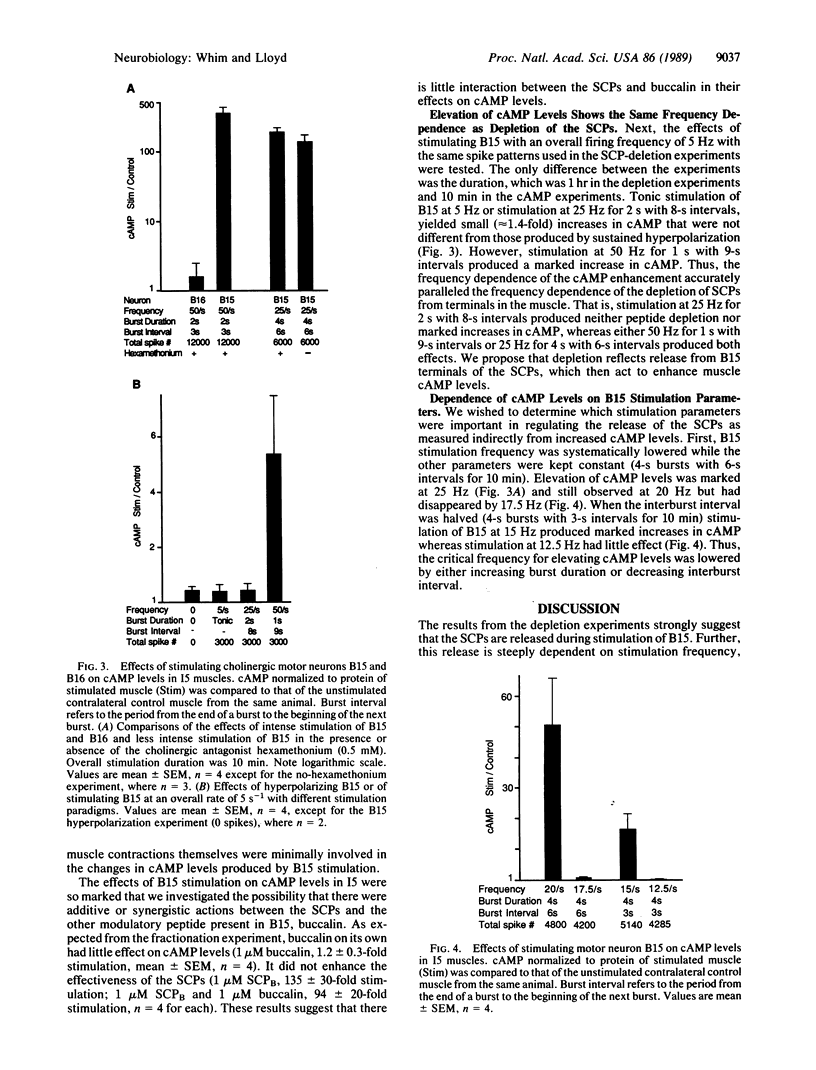
We have investigated the release of two peptide cotransmitters from the terminals of a cholinergic motor neuron in Aplysia. Identified motor neuron B15 synthesizes the two small cardioactive peptides (SCP) A and B in addition to acetylcholine. A symmetrical pair of B15 neurons innervate symmetrical buccal muscles, termed I5, which are involved in generating biting movements. The amplitude of I5 contractions is enhanced by the SCPs. Intracellular stimulation of one B15 produces depletion of the SCPs from the stimulated muscle as compared to the unstimulated control muscle. Significant depletion requires either high-frequency stimulation or prolonged bursts at lower frequencies. A second cholinergic motor neuron, B16, also innervates I5 but does not synthesize the SCPs. Stimulation of B16 produced no depletion of the SCPs. Exogenous SCPs potently increase cAMP levels in the muscle. If depletion is a reflection of release, it should be possible to demonstrate an effect of B15 stimulation on muscle cAMP levels. Indeed, stimulation of B15 did elevate cAMP levels in I5. Stimulation of B16 had no effect on cAMP levels. Increases in cAMP were observed only when B15 was stimulated in a manner that would produce significantly facilitated acetylcholine release. This facilitation could be produced by increased stimulation frequency, longer burst durations, or shorter interburst intervals. However, B15 is capable of producing cholinergically mediated contractions with stimulation parameters that would not cause release of the SCPs. Thus, B15 appears to function as a purely cholinergic motor neuron when firing slowly, and as a cholinergic/peptidergic neuron when firing rapidly.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams T. W., Castellucci V. F., Camardo J. S., Kandel E. R., Lloyd P. E. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T., Iverfeldt K., Fisone G., Serfözö P. Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol Toxicol. 1988;28:285–310. doi: 10.1146/annurev.pa.28.040188.001441. [DOI] [PubMed] [Google Scholar]
- Bishop C. A., Wine J. J., Nagy F., O'Shea M. R. Physiological consequences of a peptide cotransmitter in a crayfish nerve-muscle preparation. J Neurosci. 1987 Jun;7(6):1769–1779. doi: 10.1523/JNEUROSCI.07-06-01769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. Cotransmission. Annu Rev Pharmacol Toxicol. 1987;27:51–70. doi: 10.1146/annurev.pa.27.040187.000411. [DOI] [PubMed] [Google Scholar]
- Cohen J. L., Weiss K. R., Kupfermann I. Motor control of buccal muscles in Aplysia. J Neurophysiol. 1978 Jan;41(1):157–180. doi: 10.1152/jn.1978.41.1.157. [DOI] [PubMed] [Google Scholar]
- Cropper E. C., Lloyd P. E., Reed W., Tenenbaum R., Kupfermann I., Weiss K. R. Multiple neuropeptides in cholinergic motor neurons of Aplysia: evidence for modulation intrinsic to the motor circuit. Proc Natl Acad Sci U S A. 1987 May;84(10):3486–3490. doi: 10.1073/pnas.84.10.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper E. C., Miller M. W., Tenenbaum R., Kolks M. A., Kupfermann I., Weiss K. R. Structure and action of buccalin: a modulatory neuropeptide localized to an identified small cardioactive peptide-containing cholinergic motor neuron of Aplysia californica. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6177–6181. doi: 10.1073/pnas.85.16.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper E. C., Tenenbaum R., Kolks M. A., Kupfermann I., Weiss K. R. Myomodulin: a bioactive neuropeptide present in an identified cholinergic buccal motor neuron of Aplysia. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5483–5486. doi: 10.1073/pnas.84.15.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Goldstein M. Chemical anatomy of the brain. Science. 1984 Sep 21;225(4668):1326–1334. doi: 10.1126/science.6147896. [DOI] [PubMed] [Google Scholar]
- Kreiner T., Sossin W., Scheller R. H. Localization of Aplysia neurosecretory peptides to multiple populations of dense core vesicles. J Cell Biol. 1986 Mar;102(3):769–782. doi: 10.1083/jcb.102.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I., Weiss K. R. Activity of an identified serotonergic neuron in free moving Aplysia correlates with behavioral arousal. Brain Res. 1982 Jun 10;241(2):334–337. doi: 10.1016/0006-8993(82)91072-1. [DOI] [PubMed] [Google Scholar]
- Lloyd P. E. Fast axonal transport of modulatory neuropeptides from central ganglia to components of the feeding system in Aplysia. J Neurosci. 1988 Sep;8(9):3507–3514. doi: 10.1523/JNEUROSCI.08-09-03507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd P. E., Frankfurt M., Stevens P., Kupfermann I., Weiss K. R. Biochemical and immunocytological localization of the neuropeptides FMRFamide, SCPA, SCPB, to neurons involved in the regulation of feeding in Aplysia. J Neurosci. 1987 Apr;7(4):1123–1132. doi: 10.1523/JNEUROSCI.07-04-01123.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd P. E., Kupfermann I., Weiss K. R. Evidence for parallel actions of a molluscan neuropeptide and serotonin in mediating arousal in Aplysia. Proc Natl Acad Sci U S A. 1984 May;81(9):2934–2937. doi: 10.1073/pnas.81.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd P. E., Kupfermann I., Weiss K. R. Sequence of small cardioactive peptide A: a second member of a class of neuropeptides in Aplysia. Peptides. 1987 Jan-Feb;8(1):179–184. doi: 10.1016/0196-9781(87)90184-7. [DOI] [PubMed] [Google Scholar]
- Lloyd P. E., Mahon A. C., Kupfermann I., Cohen J. L., Scheller R. H., Weiss K. R. Biochemical and immunocytological localization of molluscan small cardioactive peptides in the nervous system of Aplysia californica. J Neurosci. 1985 Jul;5(7):1851–1861. doi: 10.1523/JNEUROSCI.05-07-01851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd P. E., Schacher S., Kupfermann I., Weiss K. R. Release of neuropeptides during intracellular stimulation of single identified Aplysia neurons in culture. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9794–9798. doi: 10.1073/pnas.83.24.9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon A. C., Lloyd P. E., Weiss K. R., Kupfermann I., Scheller R. H. The small cardioactive peptides A and B of Aplysia are derived from a common precursor molecule. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3925–3929. doi: 10.1073/pnas.82.11.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvy J. F., Blumberg S. Inactivation and metabolism of neuropeptides. Annu Rev Neurosci. 1986;9:415–434. doi: 10.1146/annurev.ne.09.030186.002215. [DOI] [PubMed] [Google Scholar]
- Morris H. R., Panico M., Karplus A., Lloyd P. E., Riniker B. Elucidation by FAB-MS of the structure of a new cardioactive peptide from Aplysia. Nature. 1982 Dec 16;300(5893):643–645. doi: 10.1038/300643a0. [DOI] [PubMed] [Google Scholar]
- O'Shea M., Schaffer M. Neuropeptide function: the invertebrate contribution. Annu Rev Neurosci. 1985;8:171–198. doi: 10.1146/annurev.ne.08.030185.001131. [DOI] [PubMed] [Google Scholar]
- Pearson W. L., Lloyd P. E. Immunocytological localization of pedal peptide in the central nervous system and periphery of Aplysia. J Neurosci. 1989 Jan;9(1):318–325. doi: 10.1523/JNEUROSCI.09-01-00318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W., Weiss K. R., Lloyd P. E., Kupfermann I., Chen M., Bailey C. H. Association of neuroactive peptides with the protein secretory pathway in identified neurons of Aplysia californica: immunolocalization of SCPA and SCPB to the contents of dense-core vesicles and the trans face of the Golgi apparatus. J Comp Neurol. 1988 Jun 15;272(3):358–369. doi: 10.1002/cne.902720306. [DOI] [PubMed] [Google Scholar]
- Rosen S. C., Kupfermann I., Goldstein R. S., Weiss K. R. Lesion of a serotonergic modulatory neuron in Aplysia produces a specific defect in feeding behavior. Brain Res. 1983 Jan 31;260(1):151–155. doi: 10.1016/0006-8993(83)90778-3. [DOI] [PubMed] [Google Scholar]
- Rosen S. C., Weiss K. R., Goldstein R. S., Kupfermann I. The role of a modulatory neuron in feeding and satiation in Aplysia: effects of lesioning of the serotonergic metacerebral cells. J Neurosci. 1989 May;9(5):1562–1578. doi: 10.1523/JNEUROSCI.09-05-01562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H. Axonal transport: components, mechanisms, and specificity. Annu Rev Neurosci. 1979;2:467–504. doi: 10.1146/annurev.ne.02.030179.002343. [DOI] [PubMed] [Google Scholar]
- Weiss K. R., Cohen J. L., Kupfermann I. Modulatory control of buccal musculature by a serotonergic neuron (metacerebral cell) in Aplysia. J Neurophysiol. 1978 Jan;41(1):181–203. doi: 10.1152/jn.1978.41.1.181. [DOI] [PubMed] [Google Scholar]
- Weiss K. R., Mandelbaum D. E., Schonberg M., Kupfermann I. Modulation of buccal muscle contractility by serotonergic metacerebral cells in Aplysia: evidence for a role of cyclic adenosine monophosphate. J Neurophysiol. 1979 May;42(3):791–803. doi: 10.1152/jn.1979.42.3.791. [DOI] [PubMed] [Google Scholar]



