Abstract
Objectives
To determine whether serum Cystatin C (CysC) and NTproBNP have prognostic value among patients with long-standing chronic lung disease.
Design
Prospective, observational, non-interventional study.
Setting
CysC and NTproBNP are prognostic markers in several cardiac conditions. In addition, CysC acts as an antiprotease following Cathepsin activation, which has been involved in the pathogenesis of chronic obstructive pulmonary disease.
Participants
Patients with a basal functional status of II-IV (NYHA), admitted for an acute exacerbation of chronic pulmonary diseases and no previous history of symptoms related to pulmonary hypertension or heart failure.
Main outcome measures
NTproBNP and CysC were determined at admission in 107 patients with acute exacerbation of chronic lung disease. During 12-month follow-up, mortality, new hospital admissions and prescription of diuretics were recorded.
Results
During follow-up there were eight patient deaths (7.5%). Mean NTproBNP among the deceased was 1510.20 pg/mL (95% CI 498.44–4628.55) vs 502.70 pg/mL (95% CI 395.44–645.48) among survivors (p = 0.01). Twenty-seven patients (25%) were prescribed loop diuretics. Mean concentration of CysC was 1.45 mg/dL (95% CI 1.21–1.69 mg/dL) vs 1.17 mg/dL (95% IC 1.09–1.25 mg/dL) in those not prescribed (p = 0.004). NTproBNP concentration was 837.14 pg/mL (95% CI 555.57–1274.10 pg/mL) in patients prescribed diuretics vs 473.42 pg/mL (95% CI 357.80–632.70 pg/mL) in those not prescribed (p = 0.03). Kaplan-Meier analysis revealed a significant difference between death and diuretic prescription during follow-up when cut-off value for NTproBNP was 550 pg/mL (p = 0.03 and p = 0.02, respectively). For 1.16mg/dL of CsysC, a significant difference was only observed in diuretic prescription (p = 0.007).
Conclusions
In patients with chronic respiratory diseases NTproBNP has predictive value in terms of mortality whereas CysC does not. However, it is still possible that both can contribute to the early identification of patients at risk of developing clinical ventricular dysfunction.
Introduction
Cardiac biomarkers are tools that should aid the physician in one or more of the following: diagnosis and subsequent risk stratification; risk stratification for secondary prevention; guiding selection of therapy; and, finally, in some cases, serving as a target for therapy. Among them, natriuretic peptides (both BNP and NTproBNP) are the most commonly used for diagnosis and risk stratification in patients with heart failure.1,2 Another emerging biomarker, Cystatin C (CysC), initially used for measuring renal function3 is becoming more frequent in the clinical setting, since its serum levels add important prognostic information when evaluating certain conditions that go beyond the simple renal function estimate. CysC has been found to be a more reliable predictor of cardiovascular risk than either creatinine or the estimated GFR among the elderly,4,5 and also provide significant prognostic information, in terms of morbidity and mortality, among coronary,6 hypertensive,7 acute8 and chronic9 heart failure patients.10,11 CysC adds complementary information to the data provided by NTproBNP alone.11,12
The potential usefulness of natriuretic peptides (not of CysC) has also been tested in several respiratory conditions. Raised NTproBNP concentrations have been found in patients with chronic lung diseases,13 cor pulmonale14,15 and pulmonary embolism.16 B-type natriuretic peptide was a short-term independent predictor of mortality in patients with chronic lung disease13,17 and primary pulmonary hypertension.18
Chronic cor pulmonale is a complication of chronic and severe lung diseases and an independent predictor of death.19 Early diagnosis and treatment of cor pulmonale increases survival rate and improves the patients' quality of life.20 Therefore, identification of patients who are at risk of suffering cor pulmonale could be beneficial for the management and prognosis of the disease. One category of patients potentially at risk are those who develop silent high arterial pulmonary pressures during acute respiratory processes in the absence of a history of heart disease.
We hypothesize that an increase in either NTproBNP or CysC blood concentrations could improve risk stratification among patients with acute exacerbations of CLD prior to onset of symptomatic right heart failure. The aim of the study was to determine the prognostic utility of NTproBNP and CysC in this setting.
Patients and methods
Patients admitted consecutively due to acute exacerbation of chronic pulmonary diseases, and interviewed within 72 hours of admission, were included in the study. Chronic pulmonary diseases considered eligible were: chronic obstructive pulmonary diseases (COPD); chronic bronchitis; emphysema; chronic asthma; pulmonary fibrosis; and pneumoconiosis. Patients considered eligible had a basal functional status of II-IV (NYHA) and no previous history of symptoms related to pulmonary hypertension or right heart failure. Patients diagnosed with heart failure, valvular heart disease, acute or chronic pulmonary embolism, symptomatic ischemic heart disease, renal failure (creatinine >2 mg/mL), liver cirrhosis, hyperthyroidism and Cushing's syndrome were excluded. Patients treated with diuretics (for whatever reason) were also excluded, with the exception of hypertensive patients treated with low dose (12.5 mg or equivalent) hydrochlorotiazide.
The study was carried out in the Internal Medicine Units of the Hospital Clínico Universitario Lozano Blesa, Zaragoza, and Hospital ‘Virgen de la Luz’, Cuenca, Spain. The study protocol was checked and approved by the region's Ethics Committee (Comité Ético de la Comunidad Autónoma de Aragón [CEICA]) under reference code PI-04/16. Signed consent was obtained from each patient. Neither specific interventions nor changes in routine clinical practice were programmed.
Variables
The patients were interviewed and baseline demographic and clinical data prospectively obtained. Data from laboratory and radiology studies were also registered.
Outcomes were assessed at six-month intervals during a follow-up period of at least one year. Data were obtained by revising medical records, outpatient clinic notes, telephone interviews, hospital administrative files and oxygen-delivery company files. For patients impossible to locate, we used the ‘Índice Nacional de Defunciones del Ministerio Español de Sanidad y Consumo’, a nation-wide database of deaths kept by Spain's Ministry of Health and Consumption.
A specific record was made of the following data: date of death; cause of death; date on which signs or symptoms of HF were first recorded; date of initial use of diuretics; date of new admissions to hospital; and in-hospital days.
NTproBNP
Within 72 hours of admission, fasting blood samples were drawn, centrifuged at 3000 rpm for 15 minutes, and plasma aliquots immediately stored at –70°C. All samples were processed sequentially, in one session, in the same clinical laboratory. Plasma NTproBNP was determined by electro-chemo-luminescence immunoassay (Elecsys proBNP 2010, Roche Diagnostics). The system was calibrated whenever a new reactive kit was used. Quality control was performed by processing two control levels (either for high or low values) in each series (Precicontrol Cardiac Elecsys 1 y 2 Ref. 12018209122). This test is not interfered with by hyperbilirubinaemia, haemolysis, hyperlipaemia, biotin or rheumatoid factor to 1500 IU/mL; measurement interval 5–35.000 pg/mL; variation coefficient 0.8–5.8%; functional sensitivity less than 5.9 pmol/L.
Cystatin C
Serum Cystatin C levels were measured by the N latex Cystatin C assay on a BN II System (Dade Behring GmbH, Marburg, Germany). This is a latex-enhanced nephelometric immunoassay using rabbit polyclonal antibodies.21 Within-run and between-run coefficients of variation were ≤1.8% in a concentration range between 0.87–4.65 mg/L.
Statistical analysis
Baseline characteristics of the patients are presented as percentages, for dichotomous variables; or median value, with its interquartile range, for continuous variables. For all variables, a normal distribution was verified using histograms and Kolmogorov–Smirnov test. Non-normally distributed values (NTproBNP) were logarithmically transformed. Sperman rank correlation coefficient between age, NTproBNP, CysC and creatine was calculated. The univariate analysis for continuous variables was performed with the use of Student's T test. Analysis for categorical variables was performed with χ2 test.
Survival curves were generated by means of Kaplan-Meier estimates, and differences in survival were compared with use of the log-rank test.
The ability of serum CysC and NTproBNP concentration to distinguish between patients who will either die or be prescribed diuretics in the year following discharge was evaluated by means of descriptive statistics and receiver-operating characteristic (ROC) curves. The area under the ROC curve (AUC) and 95% confidence intervals were calculated.
All statistical procedures were carried out with Statistical Package for the Social Sciences (SPSS), version 11.5.
Results
One hundred and seven patients with acute chronic lung disease exacerbation were enrolled during the inclusion period (1 November 2004–1 May 2006) and followed for one year (until May 2007). Eligibility was determined by admission for a recent recrudescence of their respiratory symptoms, usually an increase in the degree of dyspnoea and hypoxemia or the worsening of an existing one.
The general characteristics of the sample are shown in Table 1; 82.2% were men, and 17.8% women. The most frequent causes of chronic lung disease were smoking related diseases (COPD) (69%), chronic asthma (20.5%) and the rest (10.5%) had either pulmonary fibrosis or pneumoconiosis. Acute exacerbations were mostly due to non-pneumonic respiratory tract infection in over 95% of cases.
Table 1.
General characteristics of patients at baseline
| Median value | Interquartile range | |
|---|---|---|
| Age (years) | 77 | 71–80 |
| PaO2 (mmHg) | 57.15 | 52.92–66.15 |
| PaCO2 (mmHg) | 37.65 | 33.92–43.52 |
| HCO3 (mmol/L) | 26.35 | 24.10–29.07 |
| Haemoglobin (g/dL) | 14 | 12.6–15.2 |
| Leukocyte count (103/mm3) | 11.10 | 7.9–13.7 |
| Albumin (g/dL) | 3.6 | 3.15–3.9 |
| Total protein (g/dL) | 6.5 | 5.9–7.1 |
| Glucose (mg/dL) | 124 | 100–155 |
| Sodium (mEq/L) | 139 | 137–141 |
| Potasium (mEq/L) | 4.3 | 4.02–4.7 |
| Blood urea (mg/dL) | 47 | 38–58 |
| Creatinine (mg/dL) | 0.9 | 0.7–1.0 |
| NTproBNP (pg/mL) | 573.76 | 199.32–1348.6 |
| Cystatin (mg/dL) | 1.11 | 0.95–1.43 |
PaO2 = arterial oxygen pressure; PaCO2 = arterial carbon dioxide pressure; NTproBNP = N-terminal fragment of pro BNP
The correlation coefficient between CysC and creatinine concentrations was 0.59 (p < 0.001). Between CysC and age it was 0.31 (p = 0.001). Between CysC and urea it was 0.68 (p < 0.001) and between CysC and NTproBNP it was 0.24 (p = 0.012). The correlation between NTproBNP, creatinine and urea was not statistically significant. Age showed a significant correlation with urea (r = 0.23, p = 0.017), with creatinine (r = 0.26, P = 0.006) and with NTproBNP (r = 0.34, p < 0.001). The correlation coefficient between creatine and urea was 0.59 (p < 0.001).
During the 12-month follow-up period, eight patient deaths occurred (7.5%). Mean NTproBNP concentration among the deceased was 1510.20 pg/mL (95 CI% 498.44–4628.55) vs 502.70 pg/mL (95 CI% 395.44–645.48) among the survivors. This difference was statistically significant (p = 0.01).
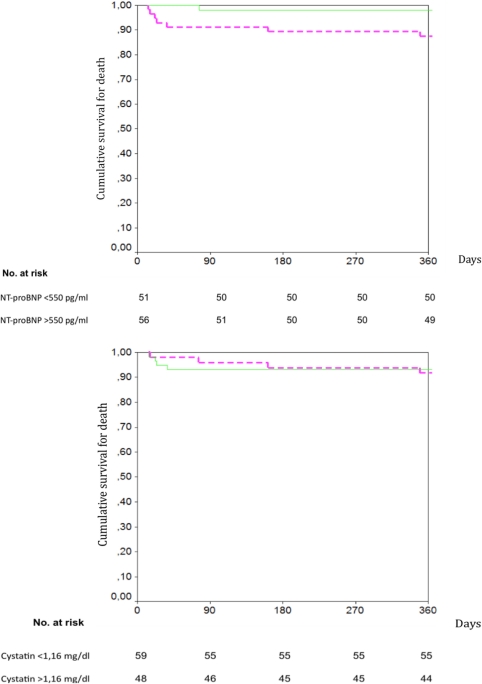
Survival Kaplan-Maier curves showed significant differences for a cut-point value of NTproBNP of 550 pg/mL (p = 0.03) (Figure 1a). Cut-off point was obtained by ROC analysis for the prediction of one-year mortality for different concentrations of NTproBNP. The selected cut-off value had a sensitivity of 87.5%, a specificity of 50.5% and AUC of 0.74 (95% CI 0.56–0.92, p = 0.02).
Figure 1.
Cumulative survival according to NTproBNP (top panel) and CysC (bottom panel) concentrations at admission. Solid line denotes a baseline concentration of NTproBNP above 550 pg/mL. Dotted line denotes a baseline concentration of NTproBNP below 550 pg/mL (top panel). Solid line denotes a baseline concentration of CysC above 1.16 mg/dL. Dotted line denotes a baseline concentration of CysC below 1.16 mg/dL (bottom panel)
Mean concentration of CysC at admission among dead patients was 1.49 mg/dL (95% CI media 1.00–1.98 mg/dL) and 1.22 mg/ dL (95% CI media 1.13–1.31 mg/dL) among survivors. This difference was statistically non-significant (p = 0.1). Survival Kaplan-Maier curves showed no significant difference for a CysC concentration cut-off of 1.16 mg/dL (p = 0.78) (Figure 1b). After a follow-up period of one year, there were no significant differences in urea and creatinine concentrations between the group of survivors and the group of deceased patients.
During the 12-month follow-up period, 29 patients (27%) were readmitted due to acute exacerbation of their respiratory illness. Mean concentration of NTproBNP among patients readmitted was 632.70 pg/mL (95% CI media 391.50–1022.49 pg/mL) vs 518.01 pg/mL (95% CI media 391.50–685.39 pg/mL) among non-hospitalized (p = 0.46).
Mean concentration of CysC among patients needing hospitalization, during follow-up, was 1.28 mg/dL (95% CI media 1.13–1.43 mg/dL) vs 1.23 mg/dL (95% CI media 1.12–1.33 mg/dL) among non-hospitalized ones (p = 0.59).
During the 12-month follow-up period, 27 patients (25%) were prescribed with loop diuretics, due to appearance of leg or pedal oedema. In univariate analysis, admission NTproBNP concentration was predictive of indication of diuretics during follow-up. Admission NTproBNP concentration was 837.14 pg/mL (95% CI media 555.57–1274.10 pg/mL) among those patients who were prescribed vs 473.42 pg/mL (IC 95% media 357.80–632.70 pg/mL) among those not prescribed during follow-up (p = 0.03). Serum concentration of CysC was also predictive of ‘de novo’ prescription of diuretics during the 12-month follow-up period. Patients who received diuretics during follow-up had a mean concentration of CysC of 1.45 mg/dL (95% CI media 1.21–1.69 mg/dL) vs 1.17 mg/dL (95% IC media 1.09–1.25 mg/dL) in those who were not prescribed diuretics (p = 0.004).
Admission creatinine concentrations were not predictive of ‘de novo’ indication of diuretics during the same period. Mean creatinine concentration of patients prescribed diuretics was 0.98 mg/dL (95% CI media 0.85–1.11 mg/dL) vs 0.91 mg/dL (95% CI media 0.85–0.97 mg/dL) among those not prescribed (p = 0.251).
Nine (15%) of 59 and 19 (37.5%) of 48 patients with a concentration of CysC at admission below or above 1.16 mg/dL, respectively, were prescribed with diuretics during the one-year follow-up period (p = 0.008). Predictive value of CysC concentration for diuretics prescription at one year was studied by means of ROC analysis so as to ascertain sensitivity and specificity. AUC was 0.633 (95% CI 0.514–0.753; p = 0.04). The best cut-off point was 1.16 mg/dL (sensitivity 66.6% and specificity 62.5%).
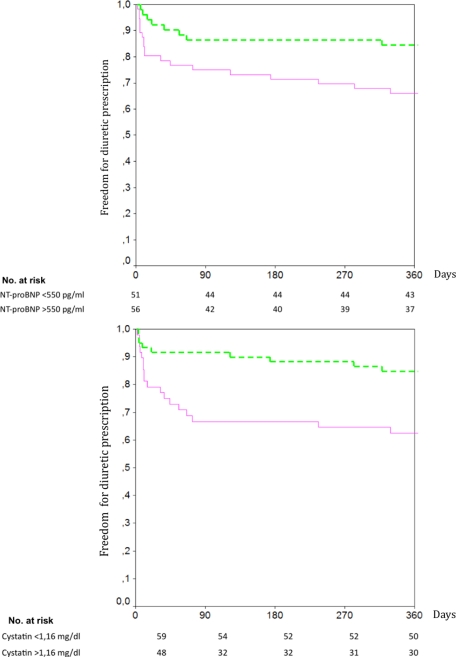
Kaplan-Meier analysis of diuretics prescription during follow-up, taking a cut-off value for CysC of 1.16 mg/dL, showed a significant difference between survivors and deceased (p = 0.007). A cut-off point of 550 pg/mL for NTproBNP, also showed significant differences in prescription of diuretics during follow-up (p = 0.02) (Figure 2a and 2b). Diuretics were less frequently prescribed among patients in whom concentrations of either of the two biomarkers were below the selected cut-off point.
Figure 2.
Cumulative interval free from diuretics according to NTproBNP (top panel) and CysC (bottom panel) concentrations at admission. Solid line denotes a baseline concentration of NTproBNP above 550 pg/mL. Dotted line denotes a baseline concentration of NTproBNP below 550 pg/mL (top panel). Solid line denotes a baseline concentration of CysC above 1.16 mg/dL. Dotted line denotes a baseline concentration of CysC below 1.16 mg/dL (bottom panel)
Deaths and readmissions were assessed through revision of hospital records. For cases (25 patients) without follow-up records, a telephone interview was conducted. It was answered by 13 patients or relatives. For death occurrence we also reviewed the ‘Índice Nacional de Defunciones del Ministerio Español de Sanidad y Consumo’. No cases of death were overlooked; although it is possible we might have excluded some of the readmissions among the 12 patients we were unable to locate. For ‘de novo’ diuretic prescription we used data from discharge reports or outpatient clinic notes or telephone interviews.
Discussion
In our study, we included patients previously diagnosed with chronic lung disease (chronic bronchitis, emphysema and chronic asthma accounted for more than 90%) – the majority, with a prior smoking habit. COPD involves a chronic inflammation that leads to fixed narrowing of small airways and alveolar wall destruction (emphysema). This is produced by the release of multiple inflammatory mediators, including serine proteases, cathepsins and matrix metalloproteinases.22,23
In our cohort of patients, NTproBNP was significantly higher in the deceased than in the survivors, while CysC failed to predict mortality at 12 months after discharge for acute exacerbation. However, both NTproBNP and CysC were significantly higher among patients who were more likely to be prescribed diuretics during follow-up.
Since 20–30% of COPD24 patients suffer from heart failure as co-morbidity, it is possible that an underlying, clinically silent, heart failure may possibly explain these results. However, clinical signs of heart failure were excluded by means of a thorough physical examination performed by two experienced physicians. All patients with rales, jugular distension, oedema or liver enlargement were ruled out prior to inclusion. Consequently, although some patients with asymptomatic ventricular dysfunction may have been included, overt heart failure was certainly excluded.
There are two possible explanations for the ‘de novo’ prescription of diuretics. Firstly, CysC may merely be a good measurement of renal function, thereby predicting a higher degree of renal function decline that leads to fluid retention, oedema and prescription of diuretics. However, this explanation seems unlikely in our series, since significant renal dysfunction was an exclusion criterion and the creatinine concentration of most of the patients was within a normal range and below 2 mg/dL in all cases. In addition, neither creatinine nor urea correlated with diuretic prescription.
Secondly, above certain concentrations, both NTproBNP and CysC may indicate a certain degree of asymptomatic ventricular dysfunction (either left side, right side or both sides). Patients with a concentration of either NTproBNP or CysC above a cut-off point of 550 pg/mL or 1.16 mg/dL, respectively, were more frequently prescribed diuretics during the following six months. We have recently shown, in a cohort of 192 patients with CLD followed for six months, that NTproBNP provided significant prognostic information. A concentration of NTproBNP above 500 pg/mL increased the risk of death 11-fold, and above a concentration as low as 350 pg/mL, prescription of diuretics was three-fold that of patients below this level.17 We could hypothesize that ‘de novo’ prescriptions of diuretics could be a surrogate marker of ventricular dysfunction, clinically manifested by the appearance of oedema. If this interpretation were correct, NTproBNP and CysC might act as early predictors of subclinical ventricular dysfunction.
It seems plausible that CysC is a specific marker of cardiovascular remodelling rather than the systemic inflammation or the inflammation of any specific organ. In a recent study of a group of 52 non-diabetic patients with advanced chronic kidney disease, Font et al.25 analysed the correlation between renal function, CysC and markers of inflammation (C-reactive protein [CRP], Interleukin-6 [IL-6], fibrinogen) or oxidative stress (oxidized low-density lipoprotein [LDLox] antibodies, paroxonase 1 [PON1]). CysC correlated with either creatinine or eGFR, but not with inflammatory or oxidative stress markers. By regulating protease activities, protease inhibitors such as CysC, play a pivotal role in tissue remodelling.26 In fact, CysC serum levels have been shown to be independently associated with left ventricular mass, concentricity and wall thickness measured by magnetic resonance imaging (MRI).27
Limitations of the study: COPD patients included in our study had been previously diagnosed following standard GOLD criteria. However, a respiratory function test was not prospectively performed, thereby limiting the interpretation of our results. Heart failure was ruled out according to clinical judgement. It would have been possible to include subclinical cases of heart failure, but even if this had been done, the information yielded by the two biomarkers would still have been significant. The cohort of patients included in the follow-up is small in number, thus limiting the strength of our conclusions. However, duration of follow-up and methodology used for assessing outcomes seem to be sufficiently accurate for us to draw our preliminary conclusions.
In conclusion, our study suggests, that in patients with acute exacerbation of chronic lung disease, measurement of serum levels of NTproBNP, but not CysC, may identify a group of patients with high risk of death. However, both can contribute to the early identification of patients at risk of developing clinical ventricular dysfunction.
Further studies designed to elucidate the role of NTproBNP and CysC in chronic respiratory diseases are needed.
DECLARATIONS
Competing interests
None declared
Funding
This work has been partly funded by the Junta de Comunidades de Castilla la Mancha, proyecto 06061-00. Roche Diagnostics, Spain granted the kits for analytical determination of NTproBNP
Ethical approval
Comité Ético de la Comunidad Autónoma de Aragón (CEICA)
Guarantor
JIP-C
Contributorship
JIPC, MSM, JANR designed the study; MSM, JLMR and JANR screened patients and did the follow-up; JIPC, FJRR and JANR did the clinical assessment; MSM, FJRR and JLMR collected the data; FJRR and JANR performed the statistical analysis; all authors approved the final version of the paper
Acknowledgements
The authors wish to thank Michael B Smith for revising the English version of the manuscript
Reviewer
Ricardo José
References
- 1.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161–7 [DOI] [PubMed] [Google Scholar]
- 2.Januzzi JL Jr, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 2005;95:948–54 [DOI] [PubMed] [Google Scholar]
- 3.McMurray MD, Trivax J, McCullough PA Serum Cystatin C, renal filtration function, and left ventricular remodelling. Circ Heart Fail 2009;2:86–9 [DOI] [PubMed] [Google Scholar]
- 4.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005;352:2049–60 [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Katz R, Fried LF, et al. ; Cardiovascular Health Study. Cystatin C and aging success. Arch Intern Med 2008;168:147–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García Acuña JM, González-Babarro E, Grigorian Shamagian L, et al. La cistatina C aporta más información que otros parámetros de función renal en la estratificación del riesgo de los pacientes con síndrome coronario agudo. Rev Esp Cardiol 2009;62:510–1919406065 [Google Scholar]
- 7.Watanabe S, Okura T, Liu J, et al. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens Res 2003;26:895–9 [DOI] [PubMed] [Google Scholar]
- 8.Lassus J, Harjola VP, Sund R, et al. Prognostic value of Cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J 2007;28:1841–7 [DOI] [PubMed] [Google Scholar]
- 9.Arimoto T, Takeishi Y, Niizeki T, et al. Cystatin C, a novel measure of renal function, is an independent predictor of cardiac events in patients with heart failure. J Card Fail 2005;11:595–601 [DOI] [PubMed] [Google Scholar]
- 10.Moran A, Katz R, Smith NL, et al. Cystatin C concentration as a predictor of systolic and diastolic heart failure. J Cardiac Fail 2008;14:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alehagen U, Dahlstro U, Lindahl TL Cystatin C and NT-proBNP, a powerful combination of biomarkers for predicting cardiovascular mortality in elderly patients with heart failure: results from a 10 year study in primary care. Eur J Heart Failure 2009;11:354–60 [DOI] [PubMed] [Google Scholar]
- 12.Tang WH, Van Lente F, Shrestha K, et al. Impact of myocardial function on Cystatin C measurements in chronic systolic heart failure. J Cardiac Fail 2008;14:394–9 [DOI] [PubMed] [Google Scholar]
- 13.Ishii J, Nomura M, Ito M, et al. Plasma concentration of brain natriuretic peptide as a biochemical marker for the evaluation of right ventricular overload and mortality in chronic respiratory disease. Clin Chim Acta 2000;301:19–30 [DOI] [PubMed] [Google Scholar]
- 14.Bando M, Ishii Y, Sugiyama Y, Kitamura S Elevated plasma brain natriuretic peptide levels in chronic respiratory failure with cor pulmonale. Respir Med 1999;93:507–14 [DOI] [PubMed] [Google Scholar]
- 15.Morrison LK, Harrison A, Krishnaswamy P, Kazanegra R, Clopton P, Maisel A Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol 2002;39:202–9 [DOI] [PubMed] [Google Scholar]
- 16.Klok FA, Mos IC, Huisman MV Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008;178:425–30 [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Marteles M, Molina A, Bermejo E, Ruiz-Laiglesia F, Nieto JA, Pérez-Calvo JI Valor pronóstico del NTproBNP en pacientes con Enfermedad Pulmonar Crónica reagudizada. Med Clin (Barc) 2010;135:441–6 [DOI] [PubMed] [Google Scholar]
- 18.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation 2000;102:865–70 [DOI] [PubMed] [Google Scholar]
- 19.Traver GA, Cline NG, Burrows B Predictors of mortality in chronic obstructive pulmonary disease. Am Rev Resp Dis 1979;119:895–902 [DOI] [PubMed] [Google Scholar]
- 20.Long term oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema Report of the Medical Research Council Working Party. Lancet 1981;1:681–6 [PubMed] [Google Scholar]
- 21.Erlandsen EJ, Randers E, Kristensen JH Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest 1999;59:1–8 [DOI] [PubMed] [Google Scholar]
- 22.Barnes PJ, Shapiro SD, Pauwels RA Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003;22:672–88 [DOI] [PubMed] [Google Scholar]
- 23.Thurmond RL, Sun S, Karlsson L, Edwards JP Cathepsin S inhibitors as novel immunomodulators. Curr Opin Investig Drugs 2005;6:473–82 [PubMed] [Google Scholar]
- 24.Fabbri LM, Luppi F, Beghé B, Rabe KF Complex chronic comorbidities of COPD. Eur Respir J 2008;31:204–12 [DOI] [PubMed] [Google Scholar]
- 25.Font R, Prats M, Gutiérrez C, et al. ¿Existe relación entre los niveles de cistatina C y el estado inflamatorio, el estrés oxidativo y otros factores de riesgo cardiovascular en pacientes no diabéticos con enfermedad renal crónica? Nefrología 2009;29:228–35 [DOI] [PubMed] [Google Scholar]
- 26.Sukhova GK, Shi G-P, Simon DI, Chapman HA, Libby P Expression of elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest 1998;102:576–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel PC, Ayers CR, Murphy SA Association of Cystatin C with left ventricular structure and function: the Dallas Heart Study. Circ Heart Fail 2009;2:98–104 [DOI] [PubMed] [Google Scholar]