Abstract
Background
Increasing physical activity is a cornerstone in the treatment of type 2 diabetes and in general practice it is a challenge to achieve long-term adherence to this life style change. The aim of this study was to investigate in a non-randomised design whether the introduction of motivational interviewing combined with fitness tests in the type 2 diabetes care programme was followed by a change in cardio-respiratory fitness expressed by VO2max, muscle strength of upper and lower extremities, haemoglobin A1c (HbA1c) and HDL-cholesterol.
Methods
Uncontrolled 18-month intervention study with follow-up and effect assessment every 3 months in a primary care unit in Denmark with six general practitioners (GPs). Of 354 eligible patients with type 2 diabetes, 127 (35.9%) were included. Maximum work capacity was tested on a cycle ergometer and converted to VO2max. Muscle strength was measured with an arm curl test and a chair stand test. The results were used in a subsequent motivational interview conducted by one of the GPs. Patients were encouraged to engage in lifestyle exercise and simple home-based self-managed exercise programmes. Data were analysed with mixed models.
Results
At end of study, 102 (80.3%) participants remained in the intervention. Over 18 months, VO2max increased 2.5% (p = 0.032) while increases of 33.2% (p < 0.001) and 34.1% (p < 0.001) were registered for the arm curl test and chair stand test, respectively. HDL-cholesterol increased 8.6% (p < 0.001), but HbA1c remained unchanged (p = 0.57) on a low level (6.8%). Patients without cardiovascular disease or pain from function limitation increased their VO2max by 5.2% (p < 0.0001) and 7.9% (p = 0.0008), respectively.
Conclusions
In this 18-month study, participants who had repeated fitness consultations, including physical testing and motivational interviewing to improve physical activity, improved VO2max, muscle strength, and lipid profile. Our results indicate that physical testing combined with motivational interviewing is feasible in a primary health care setting. Here, a fitness consultation tailored to the individual patient, his/her comorbidities and conditions in the local area can be incorporated into the diabetes programme to improve patients' muscle strength and cardio-respiratory fitness.
Background
Increases in cardio-respiratory fitness, muscle strength and level of physical activity are associated with decreased mortality and protect against age-related disabilities [1-7]. A substantial proportion of patients with type 2 diabetes have low levels of physical fitness and do not engage in the recommended level of physical activity [1,8]. These patients have increased cardiovascular mortality [9] and many comorbidities [10], e. g. hypertension, cardiovascular disease and arthritis, which may preclude some physical activities or require evaluation by a physician before the activities can be undertaken.
Regular exercise in type 2 diabetic individuals may have a significant effect on VO2max and may result in decreased HbA1c [11,12]. Similarly, HDL-cholesterol increases with cardio-respiratory fitness [13,14]. It has also been demonstrated that progressive resistance training increases muscle strength in type 2 diabetic patients [15].
Interventions that include fitness testing and individual exercise prescription are associated with more effect on fitness outcomes than interventions without these elements [16], and in recent years the use of individual behavioural approaches to increase physical activity has been advocated. It seems relevant to use motivational interviewing and include testing of muscle strength, cardio-respiratory fitness as well as exercise prescription in the motivational armamentarium of a diabetes care programme [17-22]. In a study of home-based resistance training in elderly people it was concluded that a positive attitude towards exercise and a sense of control over it were associated with adherence to the exercise regimen [23]. There are many laboratory studies with supervised exercise that demonstrate an effect on cardiovascular fitness and muscle strength, but results from general practice are scarce [11].
The over-all purpose of the present study was to see whether it was feasible for general practitioners (GPs) and their staff to motivate people with type 2 diabetes to increase and maintain their muscle strength and cardio-respiratory fitness by self-managed physical activities during an 18-month intervention period. We assumed that by providing patients with knowledge of their own muscle strength and cardio-respiratory fitness, they would become aware of discrepancies between their current physical fitness and personal goals for future health, and this realisation could induce behavioural changes [16,20]. In those patients who accepted to undergo the intervention, we measured a number of outcomes before, during and after the inclusion of fitness tests and motivational interviewing in the patients' regular diabetes control consultations. The primary outcomes were VO2max (maximal oxygen uptake, ml O2 kg-1 min-1), muscle strength of upper and lower extremities, haemoglobin A1c (HbA1c), and HDL-cholesterol during the 18-month intervention period. Secondary outcomes were waist circumference, body mass index (BMI), systolic and diastolic blood pressure, fasting plasma glucose, total cholesterol, LDL-cholesterol, and triglycerides.
Methods
Study design and participants
The study was designed as an uncontrolled study in a primary health care unit with six GPs providing health services to approx. half of the 20,000 population of a town in Denmark. All health care providers in the unit participated. In Denmark, routine care of patients with type 2 diabetes is usually given by GPs and practice nurses in primary care units in a national structured diabetes programme recommending control every 3 months.
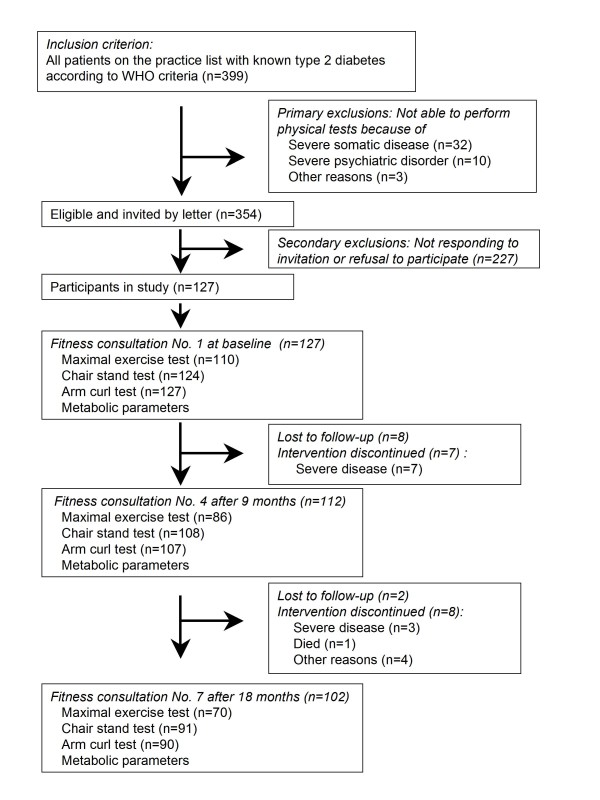
Of 399 patients with known type 2 diabetes, 127 were included (Figure 1). All were Caucasians. During the study, participants were considered lost to follow-up after non-response to one reminder. The reasons for exclusion or loss to follow-up appear from Figure 1. The ethics committee of West Zealand approved the protocol. Patients gave written informed consent.
Figure 1.
Flow of patients through the trial and the number of different physical tests done at fitness consultation No. 1, 4 and 7.
The fitness consultation
The six GPs involved were trained in the principles of aerobic and resistance exercise testing and training in a 3-hour session, and they were introduced to the strategies and techniques of motivational interviewing in another 3-hour session conducted by a researcher with wide experience of motivational interviewing.
During the 18 months of intervention, the patients were seen on two different days every three months in connection with the scheduled visits in the usual diabetes care programme.
On the first day, the practice nurse or the laboratory technician tested maximum work capacity (Wattmax) and muscle strength and measured weight, height, waist circumference, blood pressure and HbA1c. At baseline and after 9 and 18 months, fasting plasma glucose, total cholesterol, HDL-cholesterol, fasting triglycerides, serum creatinine, HbA1c, and urinary albumin/creatinine-ratio were measured as well.
On the second day, the GPs, in conjunction with the usual diabetes control, carried out a fitness consultation. At every fitness consultation the GPs had to judge and note in the patient file the patient's position in relation to the Stages of change model (Pre-contemplation, Contemplation, Preparation, Action, Maintenance and Relapse). The consultation was then held bearing this knowledge in mind. The doctors and patients worked with ambivalent attitudes, using the results of the physical tests, the pro and cons of increased physical activity, resistance, readiness and ability to change. The GPs were recommended not to try to persuade patients to certain behaviour changes but to accept the choices made by the patients.
Results of the physical tests were presented to the patient using age- and sex-stratified nomograms and information about changes since the previous visit [2,24]. Life-style exercise, i.e. increasing physical activity in daily life, actions such as walking up and down stairs instead of using the lift, cycling instead of using the car, gardening etc., were suggested. A realistic goal of physical activity was negotiated, aiming at 2,500 kcal per week corresponding to approximately a half-hour walking and a half-hour cycling a day seven days a week. This is a level where the maximal effect on cardiovascular risk and HbA1c might be expected [4,25,26].
Self-managed resistance exercise was suggested for each of the major muscle groups three times a week gradually progressing to two or three sets with a resistance that could be done between a minimum of 10 times and a maximum of 15 times [24].
Tests of fitness and muscle strength
Before testing, all patients had a 6-minute light warm-up period on the bicycle ergometer with 50% of maximum workload. Tests of muscle strength were done as described in "Senior Fitness Test" [2] by two tests: (a) an arm curl test during which the patient lifted weights from full extension to maximum flexion as many times as possible within 30 seconds. The outcome was the number of flexions; (b) a chair stand test where the patient stood up and sat down from a 40 cm high chair as many times as possible within 30 seconds. The outcome was the number of stand ups. The chair stand test is a measure of lower body strength and has a moderately high correlation to leg press scores (R = 0.78 for men and R = 0.71 for women). The arm curl test is a measure of upper body strength and has a moderately high correlation to combined 1-RM (repetition max) biceps, chest, and upper back (R = 0.84 for men and R = 0.79 for women). The test-retest reliability (95% confidence interval) for the chair stand test is 0.89 (0.79-0.93) and for the arm curl test 0.81 (0.72-0.88) [2].
Cardio-respiratory fitness was tested with an individualized symptom-limited ramp cycle ergometer (Monark®) test where we used a protocol with an individualized initial workload and a 12.5-watt increase per min. aiming at a testing time between six and 10 minutes. The results, i.e. the Wattmax attained, were converted to VO2max (ml O2 kg-1 min-1) by use of conversion formulas [27]. This method of measuring cardio-respiratory fitness has a high correlation, R = 0.97, with measurements of pulmonary ventilation and gas exchange [27].
We did not screen all participants with stress testing before exercise testing, but all were assessed clinically and with a resting ECG with regard to the risk of ischaemic heart disease and, at the discretion of the GP, referred to a cardiologist before testing [28]. Contraindications for the maximal exercise test were blood pressure > 180/110 mmHg, unstable angina pectoris, severe ischaemia on resting ECG, severe heart arrhythmia, aorta stenosis, pacemaker with fixed heart rate, autonomic neuropathy (resting heart rate > 100 bpm, orthostatic vertigo), proliferative retinopathy, and acute disease [24,28]. Patients on insulin treatment with blood glucose < 7 mmol/l were given 20 g glucose 15 minutes before testing. All tests were stopped if the patient felt unwell in any way. The health centre had access to resuscitation equipment including a heart starter.
Biochemical and clinical variables
All blood samples were taken in the morning after an eight-hour overnight fast and a resting period of at least 15 min and no hard physical activity within the foregoing two hours. Samples were analysed at Slagelse Hospital. Fraction of HbA1c was measured by a high performance liquid chromatography method (a Tosoh Automated Glycohaemoglobin Analyzer HLC-723 G. Reference interval: 0.042-0.063). Serum total cholesterol concentration was measured enzymatically with cholesterol esterase-cholesterol oxidase-peroxidase reagent. Serum triglyceride concentrations were determined enzymatically with a lipase-glycerolkinase-glycerol-3-phosphate oxidase-peroxidase reagent. HDL-cholesterol was determined by a homogeneous enzymatic colorimetric method. Plasma glucose was measured by a hexokinase method. In freshly voided morning urine, creatinine was determined by a Jaffé reaction and albumin by an immunoturbidimetric method.
Body weight and height were measured without shoes and outer garments on the same scales throughout the study. BMI was calculated as (weight in kg)/(height in metres)2. Waist circumference was measured to the nearest cm in the mid-horizontal plane between lowest rib and iliac crest. Blood pressure was measured after 10 min. rest in the seated position as the lowest of three values using a mercury sphygmomanometer.
Pain with function limitation was defined as pain from joints and/or muscles in arms, shoulders, legs and/or back which reduced the performance at the physical tests at the 9- and/or 18-month follow-up as indicated by the patient. Cardiovascular disease (CVD) was defined as history of myocardial infarction and/or verified stenosis of coronary arteries and/or stroke and/or arteriosclerosis of the lower extremities verified by distal pressure measurement recorded at baseline and/or after 9 and/or 18 months.
Statistical analysis
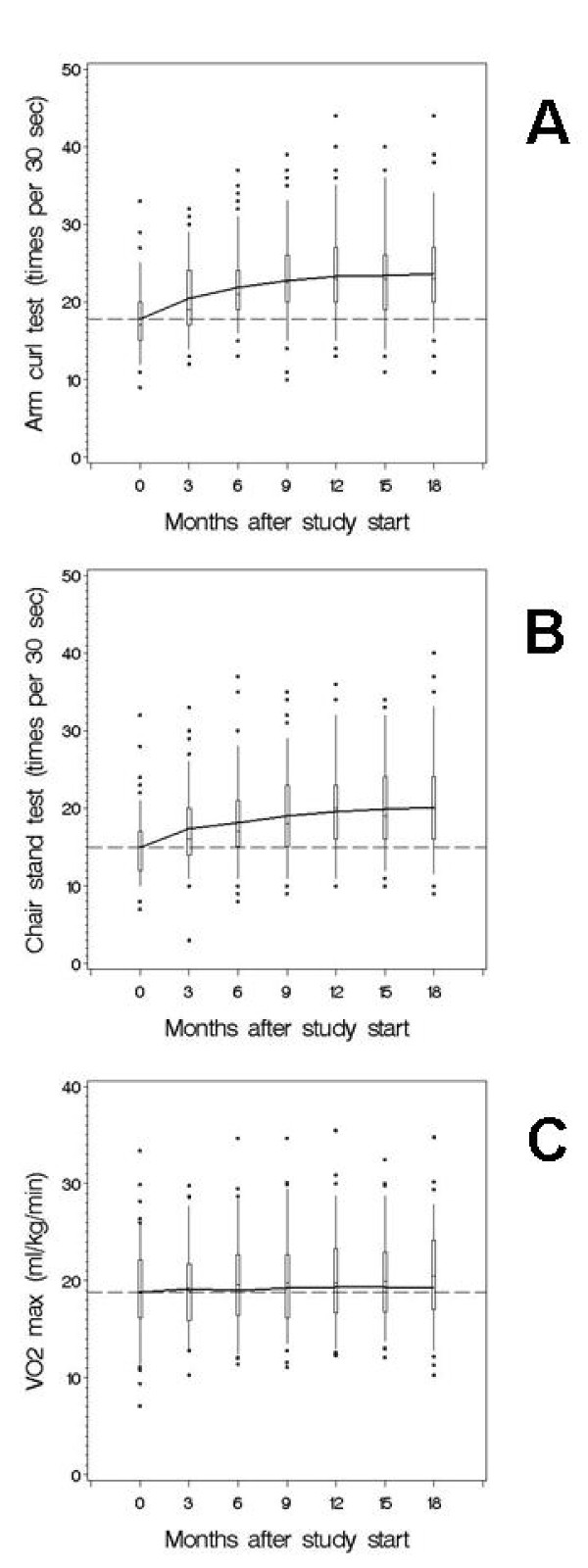
Differences between study subjects and excluded patients were investigated by χ2 tests or Kruskal-Wallis tests. The development of each outcome variable was described in a mixed model with a separate fixed effect for each examination, and a random patient intercept [29]. The concatenation of fixed effects was interpreted as the average development of the outcome, and was superimposed on the cross-sectional distributions of the outcome (shown as box-plots) in Figure 2. Whether the outcome remains the same over time was tested by a Wald test for the null hypothesis that all parameters of the fixed effects were the same. A heuristic measure of increase was Δ: the difference between the modelled baseline outcome and the modelled outcome at the seventh examination. A power calculation shows that the study has a power of 80% to detect a difference in change in VO2max from 0 (no change) to 1.3 ml O2 kg-1 min-1 during 18 months when n = 127.
Figure 2.
Changes over 18 months in arm curl test (A), chair stand test (B), and VO2max (C).
Subgroup analyses were done by adding the stratification variable to the mixed model both as a main fixed effect and interacting with the fixed effects of the separate examinations. This allowed for completely different developments in the subgroups. The test for the significance of the difference between the developments was the Wald test for the null hypothesis that all parameters of fixed effects containing the subgroup variable were zero. Here Δ was the difference between the subgroup-specific modelled baseline and seventh examination outcome. Significance of the comparison tests was determined controlling for the false discovery rate at 5% [30]. Data were analysed with SAS PROC MIXED.
Results
Of 354 eligible patients, 127 (35.9%) participated in the study. There was no statistically significant difference between the 227 non-responders and the 127 participants with regard to age (67.5/67.0 years, p = 0.77), gender (women, 42.3/42.5%, p = 0.96) and HbA1c (6.8/6.9%, p = 0.30) before the start of intervention (Figure 1). At baseline, participants were characterised by a low HbA1c and a high comorbidity (Table 1). Thirteen patients had atypical courses due to severe disease (myocardial infarction, stroke, accidents, and cancer) and one patient started participation in a placebo trial after inclusion. Test results from these patients were excluded from analysis from the date of the event.
Table 1.
Baseline characteristics of the patients
| Characteristic | |
|---|---|
| Sex (men/women) | 73 (57%)/54 (43%) |
| Age (years) | 67.0 (59.8-73.8) |
| Diabetes duration (years) | 3 (2-8) |
| School education (basic/further) | 39 (31%)/88 (69%) |
| Marital status (single/cohabiting) | 29 (23%)/98 (77%) |
| VO2max (ml kg-1 min-1) | 19.0 (17.0-22.5) |
| Arm curl test (times per 30 sec) | 17 (14-19) |
| Chair stand test (times per 30 sec) | 14 (12-17) |
| Haemoglobin A1c (%) | 6.8 (6.4-7.5) |
| HDL-cholesterol (mmol/l) | 1.3 (1.1-1.5) |
| Total cholesterol (mmol/l) | 5.0 (4.2-5.6) |
| LDL-cholesterol (mmol/l) | 2.7 (2.2-3.3) |
| Fasting triglycerides (mmol/l) | 1.5 (1.1-2.3) |
| Waist circumference (cm, men) | 105 (101-115) |
| Waist circumference (cm, women) | 99 (92-107) |
| Body mass index (kg/m2) | 29.6 (26.3-33.3) |
| Systolic pressure (mmHg) | 140 (130-150) |
| Diastolic pressure (mmHg) | 80 (75-85) |
| Fasting plasma glucose (mmol/l) | 7.7 (6.8-9.1) |
| Antihypertensive medication (N/Y) | 36 (28%)/91 (72%) |
| Antidiabetic treatment (diet/oral agents/insulin) | 37 (29%)/70 (55%)/20 (16%) |
| Statin treatment (N/Y) | 49 (39%)/78 (61%) |
| Smoking (N/Y) | 99 (78%)/28 (22%) |
| Albumin/creatinine ratio (≤ 3.5/> 3.5) | 97 (76%)/30 (24%) |
| Cardiovascular diseasea (N/Y) | 61 (48%)/66 (52%) |
| Pain with function limitationb (N/Y) | 53 (42%)/74 (58%) |
b Recorded after 9 and/or 18 months
Primary outcome measures
Over the 18 months, clear average increases of approximately one third were obtained for both arm curl test and chair stand test, while VO2max increased moderately (Table 2). The average increase in muscle strength abated over the 18 months but never declined (Figure 2). HDL-cholesterol also increased, but HbA1c remained unchanged on a low level.
Table 2.
Developments in primary outcome measures during the study according to baseline variables
| VO2max (ml/kg/min) |
Arm curl test (times per 30 sec) |
Chair stand test (times per 30 sec) |
Haemoglobin A1c (%) |
HDL-cholesterol (mmol/l) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Δ | SE | % | p a | Δ | SE | % | p a | Δ | SE | % | p a | Δ | SE | % | p a | Δ | SE | % | p a | ||
| Total | 127 | 0.46 | 0.20 | 2.5 | 0.032 | 5.9 | 0.30 | 33.2 | < 0.0001 | 5.1 | 0.31 | 34.1 | < 0.0001 | 0.020 | 0.066 | 0.3 | 0.57 | 0.11 | 0.02 | 8.6 | < 0.0001 | |
| Sex | Male | 73 | 0.58 | 0.26 | 2.9 | * | 6.3 | 0.40 | 34.2 | 5.6 | 0.40 | 36.4 | -0.069 | 0.088 | -1.0 | 0.09 | 0.03 | 7.0 | ||||
| Female | 54 | 0.34 | 0.31 | 2.0 | 5.3 | 0.46 | 31.8 | 4.4 | 0.47 | 31.0 | 0.129 | 0.098 | 1.8 | 0.14 | 0.03 | 10.2 | ||||||
| Age | ≤ 67 years | 63 | 0.58 | 0.26 | 2.7 | * | 7.0 | 0.42 | 37.9 | * | 6.0 | 0.41 | 37.9 | * | 0.042 | 0.092 | 0.6 | 0.10 | 0.03 | 8.1 | ||
| > 67 years | 64 | 0.32 | 0.31 | 2.0 | 4.7 | 0.43 | 27.8 | 4.1 | 0.45 | 29.2 | -0.002 | 0.096 | -0.0 | 0.13 | 0.03 | 9.3 | ||||||
| Diabetes duration | ≤ 1 year | 31 | 0.16 | 0.37 | 0.8 | 7.0 | 0.60 | 38.4 | 6.8 | 0.58 | 45.2 | * | -0.066 | 0.130 | -1.0 | 0.14 | 0.04 | 10.0 | ||||
| > 1 year | 96 | 0.58 | 0.23 | 3.2 | 5.5 | 0.35 | 31.2 | 4.5 | 0.36 | 30.0 | 0.049 | 0.077 | 0.7 | 0.11 | 0.03 | 8.1 | ||||||
| School education | Further | 88 | 0.56 | 0.23 | 2.9 | 6.1 | 0.36 | 33.3 | 4.9 | 0.36 | 31.4 | 0.079 | 0.078 | 1.1 | 0.08 | 0.03 | 6.2 | |||||
| Basic | 39 | 0.21 | 0.38 | 1.2 | 5.3 | 0.57 | 32.6 | 5.7 | 0.58 | 42.0 | -0.128 | 0.123 | -1.8 | 0.19 | 0.04 | 14.8 | ||||||
| Marital status | Single | 29 | -0.37 | 0.49 | -2.3 | 3.9 | 0.68 | 23.0 | 3.4 | 0.71 | 24.2 | * | 0.168 | 0.153 | 2.3 | 0.14 | 0.05 | 10.7 | ||||
| Co-habiting | 98 | 0.62 | 0.22 | 3.2 | 6.4 | 0.34 | 35.4 | 5.5 | 0.34 | 36.4 | -0.013 | 0.073 | -0.2 | 0.11 | 0.02 | 8.2 | ||||||
| BMI | < 29.62 kg/m2 | 64 | 0.48 | 0.28 | 2.5 | 5.9 | 0.43 | 31.9 | 5.6 | 0.43 | 36.7 | 0.009 | 0.094 | 0.1 | 0.14 | 0.03 | 10.2 | * | ||||
| ≥ 29.62 kg/m2 | 63 | 0.44 | 0.29 | 2.4 | 5.9 | 0.43 | 34.7 | 4.5 | 0.44 | 31.2 | 0.030 | 0.093 | 0.4 | 0.09 | 0.03 | 6.9 | ||||||
| Waist circum-ference | < ♂/♀ median | 60 | 0.84 | 0.27 | 4.3 | 5.9 | 0.44 | 32.0 | 5.6 | 0.43 | 36.6 | -0.001 | 0.096 | -0.0 | 0.10 | 0.03 | 7.4 | |||||
| ≥ ♂/♀ median | 67 | 0.04 | 0.29 | 0.2 | 5.9 | 0.43 | 34.3 | 4.6 | 0.44 | 31.4 | 0.039 | 0.092 | 0.5 | 0.12 | 0.03 | 9.8 | ||||||
| Systolic pressure | ≤ 130 mmHg | 50 | 0.48 | 0.32 | 2.6 | 5.6 | 0.50 | 31.2 | 3.9 | 0.50 | 25.9 | 0.137 | 0.109 | 1.9 | 0.11 | 0.04 | 8.5 | |||||
| > 130 mmHg | 75 | 0.51 | 0.26 | 2.7 | 6.3 | 0.39 | 35.5 | 6.0 | 0.40 | 40.0 | -0.050 | 0.086 | -0.7 | 0.12 | 0.03 | 8.8 | ||||||
| Diastolic pressure | ≤ 80 mmHg | 89 | 0.27 | 0.25 | 1.5 | 5.9 | 0.37 | 32.9 | 5.0 | 0.38 | 33.4 | 0.063 | 0.081 | 0.9 | 0.12 | 0.03 | 9.0 | |||||
| > 80 mmHg | 36 | 0.93 | 0.35 | 4.9 | 6.2 | 0.54 | 36.3 | 5.6 | 0.55 | 36.9 | -0.070 | 0.121 | -1.0 | 0.10 | 0.04 | 8.2 | ||||||
| Antihypertensive medi-cation |
No | 36 | 1.07 | 0.35 | 5.4 | 7.3 | 0.57 | 40.6 | 6.1 | 0.58 | 40.1 | 0.128 | 0.127 | 1.9 | 0.16 | 0.04 | 12.1 | |||||
| Yes | 91 | 0.17 | 0.24 | 0.9 | 5.3 | 0.36 | 30.2 | 4.7 | 0.36 | 31.7 | -0.019 | 0.077 | -0.3 | 0.10 | 0.03 | 7.3 | ||||||
| Albumin/ creatinine ratio |
≤ 3.5 | 97 | 0.58 | 0.22 | 3.1 | 6.7 | 0.34 | 38.1 | * | 5.8 | 0.35 | 39.1 | * | 0.033 | 0.075 | 0.5 | * | 0.12 | 0.02 | 8.7 | ||
| > 3.5 | 30 | -0.05 | 0.45 | -0.3 | 3.4 | 0.60 | 18.5 | 2.9 | 0.61 | 19.5 | -0.036 | 0.134 | -0.5 | 0.10 | 0.04 | 8.2 | ||||||
| Fasting plasma glucose |
≤ 7 mmol/l | 38 | 0.21 | 0.36 | 1.1 | 6.6 | 0.54 | 37.0 | 6.1 | 0.55 | 42.3 | 0.064 | 0.119 | 1.0 | * | 0.11 | 0.04 | 7.9 | ||||
| > 7 mmol/l | 89 | 0.58 | 0.24 | 3.2 | 5.6 | 0.37 | 31.5 | 4.6 | 0.37 | 30.5 | -0.005 | 0.079 | -0.1 | 0.11 | 0.03 | 8.9 | ||||||
| Diabetes treatment |
Diet alone | 37 | 0.42 | 0.36 | 2.2 | 7.0 | 0.57 | 38.8 | 6.7 | 0.55 | 44.8 | * | 0.128 | 0.126 | 2.0 | 0.16 | 0.04 | 11.3 | ||||
| Oral agents | 70 | 0.73 | 0.27 | 3.9 | 5.9 | 0.40 | 33.7 | 5.0 | 0.41 | 32.9 | -0.016 | 0.089 | -0.2 | 0.11 | 0.03 | 8.5 | ||||||
| Insulin | 20 | -0.31 | 0.54 | -1.7 | 3.9 | 0.75 | 21.8 | 2.4 | 0.78 | 16.9 | -0.041 | 0.159 | -0.6 | 0.06 | 0.05 | 4.5 | ||||||
| Statin treatmentb | None | 38 | 1.36 | 0.36 | 7.5 | 6.3 | 0.56 | 36.1 | 5.3 | 0.57 | 37.8 | 0.192 | 0.120 | 2.7 | 0.18 | 0.04 | 13.3 | |||||
| Un-changed | 78 | 0.16 | 0.26 | 0.9 | 5.9 | 0.39 | 33.0 | 5.0 | 0.40 | 33.0 | -0.061 | 0.086 | -0.9 | 0.08 | 0.03 | 5.8 | ||||||
| Initiated | 11 | -0.33 | 0.62 | -1.7 | 4.8 | 0.92 | 26.5 | 5.0 | 0.91 | 31.2 | -0.043 | 0.204 | -0.6 | 0.13 | 0.07 | 9.7 | ||||||
| Smoking | No | 99 | 0.45 | 0.21 | 2.3 | 5.9 | 0.33 | 33.2 | 5.2 | 0.34 | 33.9 | 0.115 | 0.072 | 1.6 | * | 0.09 | 0.02 | 6.7 | ||||
| Yes | 28 | 0.55 | 0.54 | 3.1 | 5.6 | 0.72 | 32.6 | 4.9 | 0.77 | 35.0 | -0.454 | 0.157 | -6.3 | 0.24 | 0.05 | 18.5 | ||||||
| Pain with function limitation |
No | 53 | 1.53 | 0.32 | 7.9 | * | 6.0 | 0.49 | 32.4 | 5.7 | 0.48 | 37.7 | -0.006 | 0.108 | -0.1 | 0.17 | 0.03 | 12.2 | ||||
| Yes | 74 | -0.15 | 0.25 | -0.8 | 5.9 | 0.39 | 34.1 | 4.7 | 0.40 | 32.0 | 0.036 | 0.084 | 0.5 | 0.08 | 0.03 | 6.5 | ||||||
| Cardiovascular disease |
No | 66 | 1.03 | 0.23 | 5.2 | * | 6.0 | 0.39 | 33.3 | 5.5 | 0.38 | 35.2 | 0.012 | 0.084 | 0.2 | 0.12 | 0.02 | 8.9 | ||||
| Yes | 45 | -0.77 | 0.37 | -4.3 | 5.7 | 0.54 | 32.6 | 4.1 | 0.55 | 29.4 | -0.082 | 0.118 | -1.1 | 0.05 | 0.03 | 3.8 | ||||||
n is number of patients. Δ is a measure of absolute increase defined as the difference between the (subgroup-specific) modelled baseline and the 18-month examination outcome. The corresponding increase, relative to the modelled baseline examination outcome, is indicated under %. a Differences between subgroups are assessed by a Wald test for the time-subgroup interaction. To account for multiple testing, the tests that remained significant after controlling for the false discovery rate at 5% are indicated with a *. b Indicates whether patients had unchanged statin treatment during the study or stated statin treatment during the study.
Subgroup analyses
The development of VO2max varied with age, sex, CVD and pain with function limitation (Table 2). In a full multivariate model including all the baseline variables listed in Table 2 as predictors and VO2max as outcome, only CVD (p = 0.001) and pain with function limitation (p = 0.023) were statistically significant. Patients without cardiovascular disease or pain from function limitation increased their VO2max by 5.2% (p < 0.0001) and 7.9% (p = 0.0008), respectively. All subgroups increased their muscle strength, but high age and microalbuminuria were associated with relatively small improvements in muscle strength tests.
Secondary outcome measures
Waist circumference, BMI and fasting plasma glucose did not change, and there was a slight increase in systolic and diastolic pressure. The lipid profile improved (Table 3).
Table 3.
Changes in secondary outcome measures during the study period
| Secondary outcome measure | Δ | SE | p |
|---|---|---|---|
| Waist circumference (cm) | 0.28 | 0.54 | 0.93 |
| BMI (kg/m2) | 0.13 | 0.09 | 0.13 |
| Systolic blood pressure (mmHg) | 2.50 | 1.83 | 0.037 |
| Diastolic blood pressure (mmHg) | 1.38 | 0.92 | 0.0009 |
| Fasting plasma glucose (mmol/l) | 0.12 | 0.19 | 0.79 |
| Total cholesterol (mmol/l) | -0.25 | 0.08 | 0.006 |
| LDL-cholesterol (mmol/l) | -0.31 | 0.07 | < 0.0001 |
| Triglycerides (mmol/l) | -0.09 | 0.14 | 0.66 |
Δ is defined as the difference between the modelled baseline and the 18-month examination outcome. SE, standard error. n = 127. For baseline values see Table 1
The course of the intervention
Ten of the 127 participants were referred to initial supervision by a physiotherapist, and two attended local fitness centres, but most chose lifestyle exercise or self-managed home-based exercise programmes or both. The programmes included aerobic training using an exercise bike and resistance training with weights or use of own body weight [2]. The intervention was safe and well tolerated with a dropout rate of 19.6% despite a high degree of comorbidity (Table 1). One maximal exercise test was stopped because the patient felt unwell, but there were no other complications associated with the test procedures except for slight tenderness of joints and muscles. Reasons for not performing all three tests at the final session were musculoskeletal disease (14/102), blood pressure >180/110 (7/102), heart disease (9/102), and acute illness (1/102). The extra time attributed to the expansion of the usual diabetes control to include a fitness consultation was estimated to be 10 min.
Discussion
In this 18-month uncontrolled intervention study, repeated fitness consultations including fitness testing and motivational interviewing resulted in the participants having increased muscle strength and VO2max, and an improved lipid profile, while HbA1c remained unchanged on a low level. Among the secondary outcome measures, waist circumference, BMI and fasting plasma glucose were unchanged, blood pressures increased slightly, whereas total cholesterol and LDL-cholesterol decreased.
Comparison with relevant literature
In the studies by David et al. [31,32], six months of supervised progressive high-intensity resistance training three times a week in older (mean age 67.6 years) type 2 diabetic patients with few comorbidities resulted in a 41.7% increase in upper body muscle strength and a 28.0% increase in lower body muscle strength. Additional home-based resistance training for 6 months was effective in maintaining the gymnasium-based improvements in muscle strength. These results are comparable with the increase and maintenance of muscle strength attained in the present study considering its less intensive intervention (Table 2 and Figure 2).
A meta-analysis has reported an 11.8% increase of VO2max in structured aerobic exercise studies with the following average characteristics: 3.4 sessions per week, 49 min. per session for 20 weeks with exercise intensities of 50-75% of VO2max [11]. The study populations in the meta-analysis were selected so they had a minimum of cardiovascular or orthopaedic limitations and were on average 12 years younger than the present study population. During our intervention, there was a slight (2.4%) but significant increase in VO2max for the whole group. There was a more substantial increase of 7,9% in the subgroup (n = 53) without function-limiting pain and 5,2% in the subgroup (n = 61) without CVD (Table 2). In light of these post hoc explanatory subgroup analyses, our study indicated that physical tests and motivational interviewing had an impact comparable with supervised exercise sessions on muscle strength and VO2max.
There is a steep inverse relationship between cardio-respiratory fitness and mortality in men with documented diabetes [5]. This could mean that an improvement in fitness, like the one we observed, is of clinical significance. In an observational prospective study of men, an increase of 7.0 ml/kg/min in VO2max over 4.9 years was associated with an estimated reduction of 30% in mortality risk during the following 5.1 years [7]. HbA1c did not change in participants during the present study, which perhaps is explained by the low baseline level of HbA1c [33]. The increase in HDL-cholesterol may be a result of increased muscle strength and increased VO2max [34]. The intervention had no impact on BMI and waist circumference. Nevertheless the intervention may have had a clinically significant effect on health as the inverse gradient between fitness and mortality in men with documented diabetes mentioned above is independent of BMI [4,5]. The improvement in glycaemic control following endurance and strength training may also be observed with unchanged BMI [35].
Strengths and limitations of the study
The study was done in the setting of a primary health care unit using primary care practitioners to carry out the intervention. The whole town was aware of the project, which made it difficult and demanding to do a randomised controlled trial, e.g. using the idea of waiting list controls. Unlike most other studies, patients with cardiovascular or musculoskeletal disease were not excluded [11]. The idea of physical testing was completely new to the patients and this, in combination with the relatively high median age, was probably the major reason for the low participation rate.
The lack of a control group is a major limitation of the study which leaves the possibility that the improvements in outcomes could be due to the general development in the natural history of type 2 diabetes and, for the physical tests in particular, to some degree of habituation. However, it is unlikely that the observed improvements in cardio-respiratory fitness and muscle strength can be explained entirely by these effects. Firstly, control groups in previous randomised studies show a decrease in VO2max of 1% over a period of 20 weeks and small non-significant increases of 1.5% and 5% in upper and lower body strength [11,31]. Secondly, the expected age-related decline in muscle strength and VO2max over 18 months can be estimated to be 2.5% and 2.2% respectively in the population of the present study [2,36]. Thirdly, if habituation explained the improvement, the same development should be expected in the different subgroups, and this is not the case (Table 2).
The arm curl test and the chair stand test are validated methods for measuring muscle strength in upper and lower extremities. These tests have a high test-retest reliability, are simple to use in everyday practice, and can be done by nearly all patients [2] (Figure 1). Until now, experience with these tests has been limited to a population over 60 years of age. In this study, it was assumed that they could also be used with younger people to measure changes in muscle strength. The bicycle ergometer test is known to give an accurate estimate of VO2max [24,27], but about one third of the type 2 diabetic participants were unable to do the test because of contraindications and comorbidity (Figure 1). However, all the participants were able to perform at least one of the three tests so all got a result that could be used in the motivational interview.
Conclusion
Clinical implementation of increased physical activity in the treatment of type 2 diabetes is still far from being standard practice. Our results indicate that physical testing combined with motivational interviewing can be done in a primary health care setting. Here, a fitness consultation tailored to the individual patient, his/her comorbidities and conditions in the local area can be incorporated into the diabetes programme to improve patients' muscle strength and cardio-respiratory fitness. The extra workload caused by fitness consultations in primary care could for instance be carried by practice nurses or physiotherapists who have received training in motivational interviewing and physical testing. Randomised trials are needed to confirm our findings and to optimise recommendations for the content and the frequency of the fitness consultations [37].
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HL made substantial contributions to conception and design of the study, planned and conducted it, collected, analysed and interpreted data and drafted the manuscript,. VS did the statistical analysis and interpreted data and has been involved in drafting the manuscript and revising it critically for important intellectual content.,. NO made substantial contributions to conception, design, analysis and interpretation. He has revised the manuscript critically for important intellectual content.
All authors read and approved the final manuscript.
Funding
The study was supported by The Danish Board of Health and the Region of West-Zealand.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Henning Lohmann, Email: Lohmann@dadlnet.dk.
Volkert Siersma, Email: siersma@sund.ku.dk.
Niels F Olivarius, Email: olivarius@sund.ku.dk.
Acknowledgements
We acknowledge the contributions of the participating patients. We thank our colleagues P. E. Elkjær-Andersen, J. Essam, J. Knabe, S. Sand, and A. Stærke for doing the fitness consultations and L. Nielsen, C. Krumphardt, J. Lynge Olsen and W. Karlslund for technical and logistical support. T. Thorsen gave instruction to the GPs in motivational interviewing. We thank the two reviewers for their valuable comments and suggestions.
References
- Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132:605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- Rikli RE, Jones CJ. Senior Fitness Test Manual. Human Kinetics, Champaign IL; 2001. [Google Scholar]
- Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports. 2003;13:3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. 2005;28:799–805. doi: 10.2337/diacare.28.4.799. [DOI] [PubMed] [Google Scholar]
- Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27:83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl HW III, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. Jama. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl HW III, Barlow CE, Paffenbarger RS Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. Jama. 1995;273:1093–1098. doi: 10.1001/jama.273.14.1093. [DOI] [PubMed] [Google Scholar]
- Plotnikoff RC, Taylor LM, Wilson PM, Courneya KS, Sigal RJ, Birkett N, Raine K, Svenson LW. Factors associated with physical activity in Canadian adults with diabetes. Med Sci Sports Exerc. 2006;38:1526–1534. doi: 10.1249/01.mss.0000228937.86539.95. [DOI] [PubMed] [Google Scholar]
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care. 1998;21:1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- Struijs JN, Baan CA, Schellevis FG, Westert GP, van den Bos GA. Comorbidity in patients with diabetes mellitus: impact on medical health care utilization. BMC Health Serv Res. 2006;6:84. doi: 10.1186/1472-6963-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia. 2003;46:1071–1081. doi: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- Boule NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Rao DC. et al. Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care. 2005;28:108–114. doi: 10.2337/diacare.28.1.108. [DOI] [PubMed] [Google Scholar]
- Boule NG, Bouchard C, Tremblay A. Physical fitness and the metabolic syndrome in adults from the Quebec Family Study. Can J Appl Physiol. 2005;30:140–156. doi: 10.1139/h05-111. [DOI] [PubMed] [Google Scholar]
- Verity LS, Ismail AH. Effects of exercise on cardiovascular disease risk in women with NIDDM. Diabetes Res Clin Pract. 1989;6:27–35. doi: 10.1016/0168-8227(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Ibanez J, Izquierdo M, Arguelles I, Forga L, Larrion JL, Garcia-Unciti M, Idoate F, Gorostiaga EM. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care. 2005;28:662–667. doi: 10.2337/diacare.28.3.662. [DOI] [PubMed] [Google Scholar]
- Nielsen PJ, Hafdahl AR, Conn VS, Lemaster JW, Brown SA. Meta-analysis of the effect of exercise interventions on fitness outcomes among adults with type 1 and type 2 diabetes. Diabetes Res Clin Pract. 2006;74:111–120. doi: 10.1016/j.diabres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55:305–312. [PMC free article] [PubMed] [Google Scholar]
- Kirk AF, Mutrie N, MacIntyre PD, Fisher MB. Promoting and maintaining physical activity in people with type 2 diabetes. Am J Prev Med. 2004;27:289–296. doi: 10.1016/j.amepre.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Rollnick S, Mason P, Butler C. Health Behavior Change: A guide for practitioners. London: Churchill Livingstone; 1999. [Google Scholar]
- Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns. 2004;53:147–155. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Di Loreto C, Fanelli C, Lucidi P, Murdolo G, De CA, Parlanti N, Santeusanio F, Brunetti P, De FP. Validation of a counseling strategy to promote the adoption and the maintenance of physical activity by type 2 diabetic subjects. Diabetes Care. 2003;26:404–408. doi: 10.2337/diacare.26.2.404. [DOI] [PubMed] [Google Scholar]
- Kirk AF, Barnett J, Mutrie N. Physical activity consultation for people with Type 2 diabetes. Evidence and guidelines. Diabet Med. 2007;24:809–816. doi: 10.1111/j.1464-5491.2007.02190.x. [DOI] [PubMed] [Google Scholar]
- Jette AM, Rooks D, Lachman M, Lin TH, Levenson C, Heislein D, Giorgetti MM, Harris BA. Home-based resistance training: predictors of participation and adherence. Gerontologist. 1998;38:412–421. doi: 10.1093/geront/38.4.412. [DOI] [PubMed] [Google Scholar]
- American college of sports medicine. ACSM´s Guidelines for Exercise Testing and Presciption. Lippincott Williams & Wilkins, Philadelphia; 2000. [Google Scholar]
- Hu FB, Stampfer MJ, Solomon C, Liu S, Colditz GA, Speizer FE, Willett WC, Manson JE. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134:96–105. doi: 10.7326/0003-4819-134-2-200101160-00009. [DOI] [PubMed] [Google Scholar]
- Di Loreto C. Make Your Diabetic Patients Walk. Diabetes Care. 2005;28:1295–1302. doi: 10.2337/diacare.28.6.1295. [DOI] [PubMed] [Google Scholar]
- Storer TW, Davis JA, Caiozzo VJ. Accurate prediction of VO2max in cycle ergometry. Med Sci Sports Exerc. 1990;22:704–712. doi: 10.1249/00005768-199010000-00024. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer series in statistics. Springer, New York; 2000. [Google Scholar]
- Benjamini Y, H Y. "Controlling for the false discovery rate: a practical and powerful approach to multiple testing". Journal of the Royal Statistical Society. 1995;57(1):289–300. [Google Scholar]
- Dunstan DW, Daly RM, Owen N, Jolley D, De Court, Shaw J, Zimmet P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25:1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- Dunstan DW, Daly RM, Owen N, Jolley D, Vulikh E, Shaw J, Zimmet P. Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care. 2005;28:3–9. doi: 10.2337/diacare.28.1.3. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud'homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P. et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- Balducci S, Leonetti F, Di MU, Fallucca F. Is a long-term aerobic plus resistance training program feasible for and effective on metabolic profiles in type 2 diabetic patients? Diabetes Care. 2004;27:841–842. doi: 10.2337/diacare.27.3.841. [DOI] [PubMed] [Google Scholar]
- Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, Wagner O, Georg P, Prager R, Kostner K. et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil. 2005;86:1527–1533. doi: 10.1016/j.apmr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Paterson DH, Cunningham DA, Koval JJ, St Croix CM. Aerobic fitness in a population of independently living men and women aged 55-86 years. Med Sci Sports Exerc. 1999;31:1813–1820. doi: 10.1097/00005768-199912000-00018. [DOI] [PubMed] [Google Scholar]
- Praet SF, van Loon LJ. Exercise: the brittle cornerstone of type 2 diabetes treatment. Diabetologia. 2008;51:398–401. doi: 10.1007/s00125-007-0910-y. [DOI] [PMC free article] [PubMed] [Google Scholar]