Abstract
PURPOSE
Although most researchers agree that platelet-rich plasma (PRP) is a good source of autogenous growth factors, its effect on bone regeneration is still controversial. The purpose of this study was to evaluate whether increasing angiogenic factors in the human PRP to enhance new bone formation through rapid angiogenesis.
MATERIAL AND METHODS
In vitro, the human platelets were activated with application of shear stress, 20 µg/ml collagen, 2 mM CaCl2 and 10U thrombin/1 × 109 platelets. Level of vascular endothelial growth factor (VEGF) and platelet microparticle (PMP) in the activated platelets were checked. In the animal study, human angiogenic factors-enriched PRP was tested in 28 athymic rat's cranial critical bone defects with β-TCP. Angiogenesis and osteogenesis were evaluated by laser Doppler perfusion imaging, histology, dual energy X-ray densinometry, and micro-computed tomography.
RESULTS
In vitro, this human angiogenic factors-enriched PRP resulted in better cellular proliferation and osteogenic differentiation. In vivo, increasing angiogenic potential of the PRP showed significantly higher blood perfusion around the defect and enhanced new bone formation around acellular bone graft material.
CONCLUSION
Angiogenic factor-enriched PRP leads to faster and more extensive new bone formation in the critical size bone defect. The results implicate that rapid angiogenesis in the initial healing period by PRP could be supposed as a way to overcome short term effect of the rapid angiogenesis.
Keywords: Platelet-rich plasma, Angiogenesis, Human, Athymic rat, Cranial defect
INTRODUCTION
Platelets play important role in the initial wound healing; bleeding from the wound leads to rapid activation of platelets that release multiple growth factors and cytokines involved in healing. Since the first demonstration of new bone formation with a combination of autogenous bone graft and PRP, used as a term by Marx,1 those platelet-derived growth factors have been thought to contribute to bone regeneration. However, subsequent studies of its efficacy using different bone graft materials have led to conflicting results.2-4 Results from studies using non-cellular alloplastic graft materials were typically more discouraging, even though there are some positive reports.5-7 However, PRP's potential for delivering multiple factors is particularly appealing, as recent experiments have disclosed that combinations of factors seem to work better than single factors in bone regeneration.8 The preparation and delivery of platelet-derived growth factors (GFs) are likely to be critical, but the biologic significance of different preparations of PRP remain unclear, no standardized method has been developed, and there are only few studies that consider the factors involved in platelet activation and conditions at the PRP delivered site.
Rapid vascularization of bone graft materials is a key step for early and long-term successful osteogenesis, and the degree of angiogenesis is related to the stimuli present in surrounding tissues that allow preexisting vessels to start budding into the freely applied grafts and the graft material itself.9,10 Localized angiogenic factor delivery has proven beneficial for bone regeneration in numerous animal models by promoting neovascularization, bone turnover, osteoblast migration and mineralization.11,12 Alloplastic bone substitutes have no cells in the graft and need more time for vascularization or cell population and matrix production compare to autogenous cancellous bone grafts. Considering that PRP can release factors involved in angiogenesis and the short effective time of platelets and its proteins, an angiogenic effect of PRP might be more important to promote bone healing in alloplastic bone substitutes. This approach is supposed to overcome the short term effect of the PRP reported by previous study.13
In the present study, the effects of increasing angiogenic factors in activated human PRP on the angiogenesis and subsequent osteogenesis were analyzed in vitro and in vivo. Four factors were considered to increase the angiogenic potential of the PRP, including the concentrations of thrombin and calcium for platelet activation, the subsequent release of vascular endothelial growth factor (VEGF), production of platelet microparticles (PMPs), and inclusion of only peripheral blood mononuclear cells (PBMNCs) among white blood cells (WBCs). The first three factors can vary in amount during the process of PRP preparation and influence angiogenesis.14-18 Also, PBMNCs and platelets has been reported to be more potent in improving rat ischemic limb lesions, compared to platelets only or with addition of polymorphonuclear leukocytes (PMNs).19
We hypothesized that enhancement of the angiogenic potential of the human PRP could improve osteogenesis of acellular inorganic graft materials. To test this, β-tricalcium phosphate (β-TCP) was placed, along with PRP, in a critical-size rat calvarial defect model.
MATERIAL AND METHODS
1. Preparation of platelets for in vitro study
Commercially available human apheresed platelet plasmas were purchased from AllCells (Emeryville, CA, USA). Donors were restricted to males under 30 years-old for quality control. All apheresed platelet plasmas were delivered between 18-24 hours after withdrawal of blood, and the same batch of platelets were used for each experiment. Total 6 samples from different donors were used for the study. To exclude the effects of other factors in in vitro study, platelets were isolated and suspended in HEPES-Tyrode's buffer solution according to a previous report20 at a concentration 1 × 109 /ml. Four different methods of platelet activation described previously were used and modified.1,3,4,21 Group A: platelet-poor plasma and 10% CaCl2·2H2O; Group B: plateletlysate with 0.1% Triton-X (Mallinckrodt, KY, USA); Group C: 142.8 U/ml of thrombin (Sigma, St. Louis, MO, USA) and 14.3 mg/ml CaCl2·2H2O; Group D: 10 U/ml of thrombin and 2 mM CaCl2·2H2O after pre-activation with shear stress and 20 µg/ml collagen. For the application of shear stress, a tabletop vortex machine was used. The rotation rate was 100 rpm for the first 15 seconds and then increased to 3500 rpm for 5 minutes. All samples were centrifuged for 10 minutes at 4000 g and activated PRP supernatants were harvested. All supernatants were immediately stored at -80℃ until further analysis. Because PBMNCs are mixture of heterogeneous cells and mixture of adherent and nonadherent cells, they could not involve in in vitro study.
2. Determination of GF concentrations and PMP formation
GF concentrations of bFGF, PDGF-BB, TGF-β, and VEGF in the supernatants were determined using a Quantikine Assay Kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). Triplicate measurements were performed for all assays (n = 3). The PMPs were collected and the amount was checked depending on the preparation methods by Western blot as previously described.21 Monoclonal antibody SZ22 (Beckman Coulter, Miami, FL, USA) against platelet GPIIbα was used.
3. Cell proliferation and vascular endothelial cell-assisted osteogenesis assay
Human cord blood-derived outgrowth endothelial like cells (hCBOECs) and human bone marrow stromal cells (hBMSCs) (Cambrex, Walkersville, MD, USA) were used. All cells were seeded at a density of 2500 cells per well in 96-well culture plates (BD, Franklin Lakes, NJ, USA) and incubated in the following medium: 90 µl IMDM supplemented with 1% FBS, 100 U/ml of penicillin, and 100 µg/ml of streptomycin and 10 µl of activated PRP supernatant from each condition (A-D) for 48 hours. The final cell numbers were assessed with MTS method (CellTiter96, Promega, Madison, WI, USA) according to the manufacturer's instruction.
For analysis of in vitro osteogenic differentiation assisted by vascular endothelial cells, 1 × 104 /cm2 hBMSCs were co-cultured directly with 0.5 × 104 /cm2 hCBOECs under EBM medium supplemented with various activated platelet supernatants (10% v/v), 1% FBS and 0.04% (v/v) hydrocortisone. Activity of Alkaline phosphatase (ALP) at 5-th day after co-culture was evaluated with EnzoLyte FDP ALP Assay Kits (AnaSpec, San Jose, CA, USA) and also, concentration of secreted osteocalcin (OCN) at 10-th day was measured with Mid-Tact Human OCN ELISA Kits (Biomedical Technologies, Stoughton, MA, USA) as markers of early and late osteoblast differentiation respectively.
4. Animal Experiment
In this study, the PRP containing high amount of VEGF and PMP and PBMNCs without PBPMNs was called angiogenic factor-enriched PRP. PBMNCs and platelets were separately isolated from the whole blood by density gradient centrifugation (Histopaque-1077, Sigma, St. Louis, MO, USA) according to the manufacturer's instruction. PBMNCs and PRP were collected by additional centrifugation (200 g and 1400 g, respectively) and PRP was activated with the method described above. In control groups using PRP, the group treated with plateletpoor plasma and 10% CaCl2·2H2O was excluded because no significant difference was found between it and the group followed Marx's protocol in in vitro study.
Harvard University and National Institute of Health (NIH) animal care guidelines were followed in all procedures. Twenty-eight NIH athymic rats, weighing approximately 200 g, were used and critical size (8 mm) cranial defects were made. Anaesthesia and pain control followed recommended routines for the species. The animals were anaesthetized using isoflurane inhalation anaesthesia (E-Z Anesthesia, Euthanex Corp., Palmer, PA, USA). Buprenorphine HCl, 0.02 - 0.03 mg/kg, was administered pre-surgically. In each animal, one calvarial through-and-through osteotomy was trephined into the central portion of the cranium using a dental handpiece and trephine bur. 0.05 g of synthetic β-TCP (Synthograft®, Bicon, Boston, MA, USA) mixed with 50 µl of the angiogenic factor-enriched PRP (activated PRP with the modified method + 2 × 106 PBMNCs) was implanted into the defect. In control groups, β-TCP or β-TCP with 50 µl of conventional PRPs were grafted.
1) Analysis of blood vessel formation
After 2 weeks, periosteal blood flow over the cranial defect was measured using a laser Doppler perfusion imaging (LDPI) system (PeriScan, Perimed AB, Stockholm, Sweden). After skin incision and meticulous supraperiosteal dissection under inhalation anaesthesia, consecutive perfusion measurements were obtained by scanning (the size of ROI; 10 × 10 mm2) just above the defects. Blood flow in the tail of the same animal was used as internal control. After measuring periosteal blood flow, animals were sacrificed with 100% carbon dioxide and cervical dislocation. Cranial bones including the original defect were harvested immediately after euthanization and processed for immunohistochemistry. The tissue sections were immunostained for von Willebrant factor (vWF) using a commercial staining kit (Chemicon, Temecula, CA, USA) and imaged by means of a Nikon Eclipse E800 light microscope and a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA). Blood vessels, indicated by vWF staining, were counted manually at 100x magnification in randomly selected areas of the defect, and normalized to tissue area with the use of NIH Image J Software.
2) Analysis of New Bone Formation
At 8 weeks, the animals were euthanized, and the implants were retrieved and fixed in 10% zinc-buffered formalin. Following fixation, implants were scanned to measure bone mineral content (BMC, in grams) and bone mineral density (BMD, in g/cm2) in the region of interest (ROI) sized 8 × 8 mm2 using dual energy x-ray absorptiometry (DEXA, ODR2000+, Hologic Inc., Waltham, MA, USA), three dimensionally with micro-computed tomography (µCT40, ScanoMedical, Bassersdorf, Switzerland) and decalcified H&E staining.
5. Statistical analysis
Wilcoxon Rank Sum test was applied to evaluate the control groups compared to experimental group. The level of significance was set at 5%.
RESULTS
1.VEGF release and PMP production
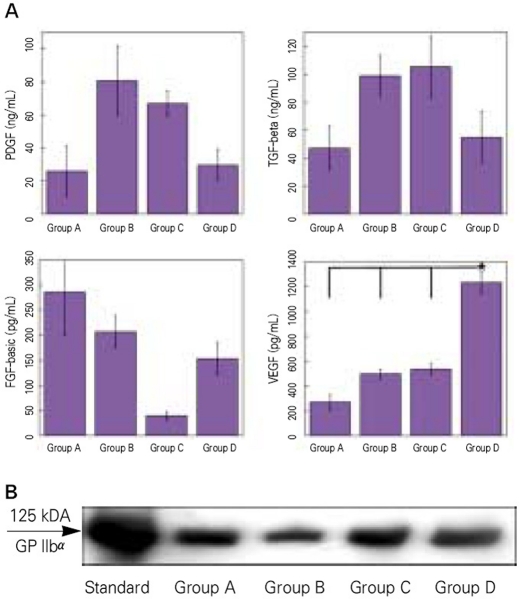
Activation with 0.1% Triton-X (Group B) evoked relatively high release of all growth factors except for VEGF. Activation with shear force, collagen, and low amount of calcium and thrombin (Group D) showed a significant increase in the release of VEGF (mean concentration 1264 pg/ml) as compared to the other methods using high calcium and thrombin (mean concentration 273 - 548 pg/ml), but the release of other growth factors were lowered (Fig. 1A). All platelet agonists caused a similar production of PMP except for Triton-X, what dissolved the platelet membrane (Fig. 1B).
Fig. 1.
A: Release of growth factors measured with ELISA. Platelet-derived growth factor (upper left), transforming growth factor-beta (upper right), basic fibroblast-growth factor (lower left), vascular endothelial growth factor (lower right). B: Production of platelet microparticles (PMPs) detected with Western blot. Standard; molecular weight standard of GPIIb α(125kD). Group A; activation with 10% CaCl2 and platelet-poor plasma (PPP), Group B; lysis with 0.1% Triton-X, Group C; activation with 142.8 U/mL thrombin and 4.3 mg/ml CaCl2, Group D; activation with shear force, 20 mg collagen, 10 U/ml thrombin and 2 mM CaCl2. Values represent mean and standard deviation.
2. Effect of different PRPs on cellular behaviors
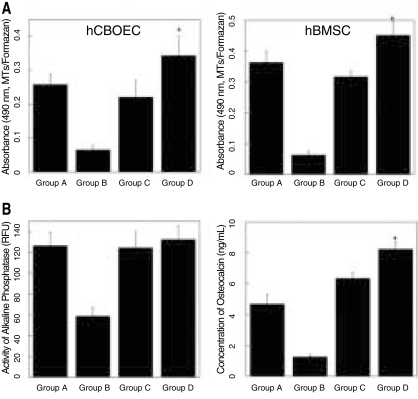
PRP supernatants from the modified method (Group D) were significantly more mitogenic in both hCBOECs and hBMSCs than all control groups (Group A to C, P < .05). Because of cell toxicity of Triton-X, cell proliferation was significantly inhibited by even small amounts of Triton-X (0.1%) (Fig. 2A). In vascular endothelial cell-assisted osteogenic differentiation of MSCs, ALP activity was not different between the groups, except for the group treated with Triton-X. In contrast, the OCN concentration was significantly higher in the group cultured with the modified PRP supernatant (Group D, Fig. 2B, P < .05).
Fig. 2.
A: Proliferation of cord blood-derived outgrowth endothelial-like cells (left) and human bone marrow stromal cells (hBMSCs) (right) as indicated by absorbance of formazan at 490 nm after 48 hrs. B: Osteogenic differentiation of hBMSCs modulated by human cord blood-derived outgrowth endothelial-like cells (hCBOECs). Activity of alkaline phosphatase (left, unit: intensity of fluorescence). Production of osteocalcin (right, ng/mL). hBMSCs (1 × 104/cm2) and hCBOECs (1 × 104/cm2) were co-cultured with IMDM media, 1% FBS, 10-7 M dexamethasone and each PRP (100 µl/ml). Group A; activation with 10% CaCl2 and plateletpoor plasma (PPP), Group B; lysis with 0.1% Triton-X, Group C; activation with 142.8 U/ml thrombin and 4.3 mg/ml CaCl2, Group D; activation with shear force, 20 mg/ml collagen, 10 U/ml thrombin and 2 mM CaCl2. Values represent mean and standard deviation.
3. In vivo animal experiments
1) Angiogenesis and Perfusion
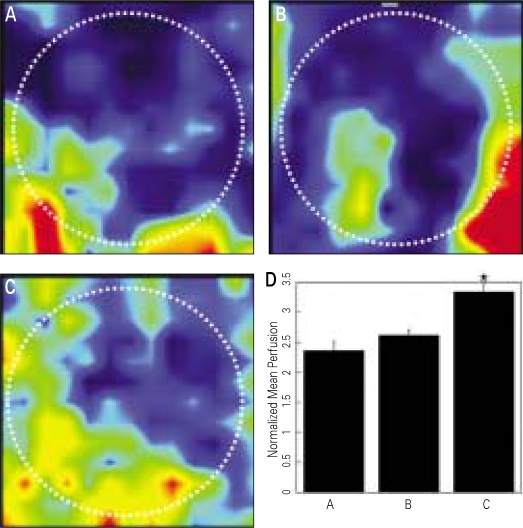
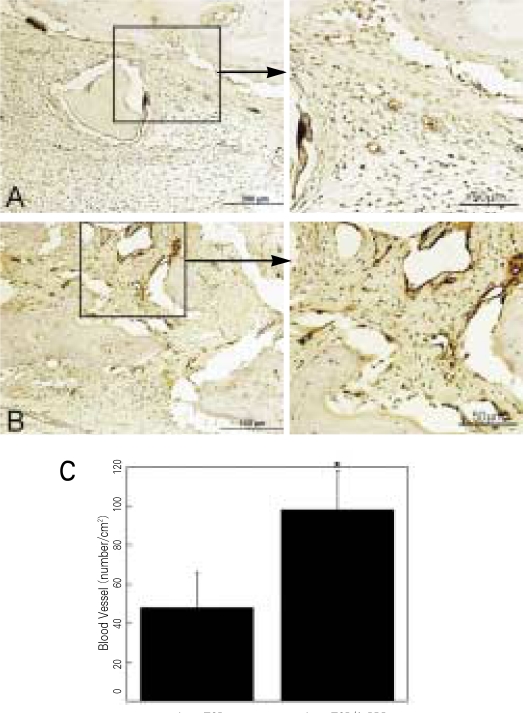
β-TCP without or with various types of human PRPs were implanted into the cranial defects of athymic rats to determine the effects of human PRP treatment on wound angiogenesis (Fig. 3). A significant increase of blood perfusion was observed in the defects treated with angiogenic factors-enriched PRP at 2 weeks, compared to other conditions (31 - 42% improvement, P < .05). The blood vessel densities within the defects compared to the β-TCP only grafts (Group A, 48 ± 18 vessels/cm2), showed significantly higher neovascularization in the experimental group (Group D, 98 ± 20 vessel/cm2; P < .05) (Fig. 4A-C).
Fig. 3.
A-C: Laser Doppler perfusion images of periosteal region of the cranial defect at 2 weeks after graft procedures. A. β-TCP only, B. β-TCP and conventional PRP activated with 142.8 U/ml thrombin and 4.3 mg/ml CaCl2, C. β-TCP and angiogenic factor-enriched PRP (PRP activated with shear force, 20 mg/ml collagen, 10 U/ml thrombin and 2 mM CaCl2, and containing peripheral blood mononuclear cells), D. Mean perfusion of each group (P < .05). The mean blood flows are normalized to the perfusion measured in the tail of the same animal (unit: voltage). White dotted circles manifest the original defects. Increased blood flow was indicated as color change from dark red to navy blue.
Fig. 4.
Staining for vWF in critical size (8 mm) rat craniotomy defects following implantation of β-TCP only (A) and β-TCP + angiogenic factorsenriched PRP (A-PRP, B) at 2 weeks. Blood vessels are identified as circular structure with dark brown color: Quantitative analysis of the number of blood vessels in both groups (C). Values represent the mean and standard deviation.
2) New bone formation
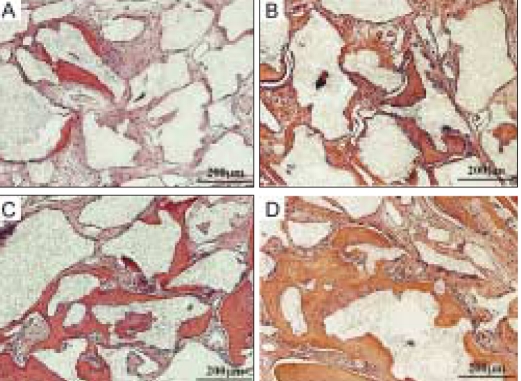
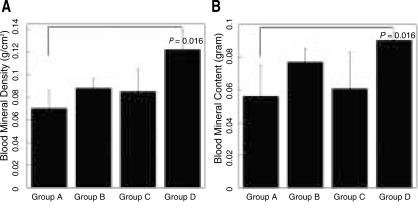
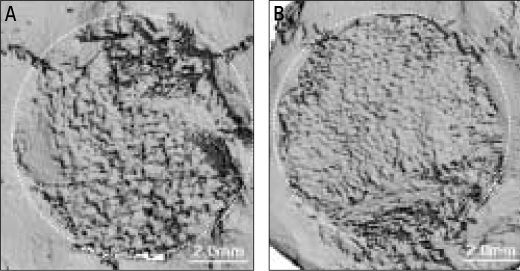
Analysis of cranial implants indicated that all groups showed favorable tissue response without significant inflammatory reaction (Fig. 5). Human angiogenic factors-enriched PRP (Group D) led to significantly increased BMC and BMD versus control defects grafted with β-TCP only (Group A, P < .05) (Fig. 6). Although some control groups with PRPs (Group B and C) showed higher BMC and BMD values compared to the group grafted with β-TCP only (Group A), they were not statistically significant. These findings were confirmed by 3D µCT imaging, which revealed the formation of a continuous bone mass across the defects (Fig. 7). The control condition without any PRPs demonstrated significantly decreased bone formation.
Fig. 5.
Photomicrographs of histological sections from critical size 8 mm rat craniotomy defects following implantation of β-TCP (Synthograft®) and several types of PRP at 7 weeks. A: β-TCP only, B: β-TCP and 0.1% triton-X treated PRP, C: β-TCP and PRP activated with 142.8 U/ml thrombin and 4.3 mg/ml CaCl2, D: β-TCP and angiogenic factorsenriched PRP (activated with shear force, 20 mg/ml collagen, 10 U/ml thrombin and 2 mM CaCl2 and containing peripheral blood mononuclear cells).
Fig. 6.
Bone mineral density (A) and bone mineral content (B) of critical size (8 mm) rat craniotomy defects following implantation of β-TCP (Synthograft®) and several types of human PRP in athymic rats at 7 weeks. Group A. β-TCP only, Group B. β-TCP and 0.1% triton-X treated PRP, Group C. β-TCP and PRP activated with 142.8 U/ml thrombin and 4.3 mg/ml CaCl2, Group D. β-TCP and angiogenic factors-enriched PRP (activated with shear deformation, 20 mg/ml collagen, 10 U/ml thrombin and 2 mM CaCl2 and containing peripheral blood mononuclear cells). Values represent mean and standard deviation.
Fig. 7.
MicroCT image reconstruction of critical size (8 mm) rat craniotomy defects at 7 weeks. A: β-TCP only, B: β-TCP and human angiogenic factors-enriched PRP in athymic rat. It shows positive effect of PRP in our experimental group.
DISCUSSION
Primary hypothesis of this study was the rapid establishment of vascular network. It may play the key role for reproducibility of PRP effect in bone regeneration. Our previous study showed increased initial angiogenesis by animal PRP enhanced bony regeneration in the critical size defect without any graft material.22 In this study authors checked whether human PRP showed the same effect around acellular synthetic graft material before clinical trial. Also our hypothesis can be supported by other recent study.23 Although VEGF is potent angiogenic factor and positive correlation was observed between VEGF levels and bone formation, its absolute amounts in platelet is very low, therefore it may have minimal effect on osteogenesis. For increasing the angiogenic potential, other factors should be considered. In the present study, three methods such as a control of the concentration of thrombin, increasing PMP production and including only PBMNCs among white blood cell components, were used for that purpose.
Although calcium and thrombin induce immediate growth factor release from platelet in a dose-dependent manner,24 high concentrations of thrombin could present an adverse effect on migration and proliferation of vascular endothelial cells.14,15 Also thrombin has a threshold effect in relation to VEGF release, and high concentrations of thrombin decrease the release of VEGF.25 Because the exact threshold mechanism and concentration of thrombin are unknown, we set the concentration of thrombin and calcium optimal for vascular endothelial cell proliferation. In the present study, incorporation of other platelet activators, along with collagen and mechanical stimulus (shear force), increased the initial secretion of VEGF as compared to activation of 10 U/1 × 109 platelets with thrombin under 2 mM CaCl2 only (Data are not shown). Collagen and shear stimuli were intended to compensate for the decrease of other growth factors and increase the production of PMPs, but they also further increased the VEGF secretion. This may be explained by a significant release of VEGF from platelets under a combination of low concentration of each agonist that causes platelet aggregation induced by shear stress.20 Combination of these three platelet activators results in significant VEGF release (2.3 - 4.6 times), compared to other activation methods described previously.
It is interesting that Triton X, a solubilizer of platelet membrane did not increase the release of VEGF as compared to other platelet growth factors. The result implicates that VEGF might have a different releasing mechanism and some interactions with Triton-X.
Significant positive correlation was observed between angiogenic factor level and cell behaviors. Proliferation of both hCBOECs and hBMSCs were influenced by the preparation method of platelets (Fig. 2A). Although it is not clear whether the concentration of thrombin and calcium or the composition of release molecules is responsible for this effect, the mitogenic activity of platelet supernatant generated by the modified method (Group D) was higher than that of platelets activated with high thrombin and calcium. The relatively high proliferation in platelets activated with 10% calcium was likely due to additional release of bFGF and it might partially compensate for the inhibition by the high concentration of calcium. Triton-X, even in a low concentration, showed an inhibitory effect on cell proliferation. Osteogenic differentiation of hBMSCs supported by hCBOECs also was influenced by different levels of angiogenic factors (Fig. 2B). Activated platelet supernatants were previously reported to hold a potent mitogenic and chemotactic activity for mesenchymal progenitor cells, however, to decrease osteogenic differentiation.26 ECs, in contrast, enhance the osteogenic potential of bone marrow stromal cells (BMSCs) grown in co-culture in vitro, likely via BMP-2 production.27 In this study, increased angiogenecity of the PRP significantly enhanced the late stage osteogenic differentiation of hBMSCs co-cultured with hCBOECs. Although it has been reported that thrombin increases proliferation and migration of bone marrow stromal cells.15 These effects was found only up to 1 U/ml thrombin. This report may explain why the proliferation rate and osteogenic differentiation of hBMSCs in the PRP with a high concentration of thrombin is low.
The in vivo findings reflected the in vitro results. For maximizing angiogenic potential of the PRP, only PBMNCs were included among white blood cells, usually contained during the PRP preparation. The experimental group grafted with β-TCP and angiogenic factor-enriched PRP showed a 59% higher periosteal perfusion than those grafted with β-TCP only (Fig. 3). Considering the importance of the periosteal blood supply in fracture healing,28 the periosteal perfusion above a defect might reflect overall re-vascularization of the defects after surgery. Vessel densities in the defects were in accordance with the LDPI findings (Fig. 4). PBMNCs are known to include the source of cells of monocyte lineage secreting angiogenic factors, but PMNs likely show antiangiogenic effect due to the release of neutrophile elastase.29,30 Therefore, the additive effects of PBMNCs and modified PRP could explain the enhanced early angiogensis and subsequent osteogenesis in this study.
Bone density was improved to average of 71% by angiogenic factor-enriched PRP, compared to the control group grafted with only β-TCP (Fig. 6). This finding suggests that the enhanced angiogenic activity contributed to later osteogenesis. However, long-term animal experiments are needed to confirm these findings.
CONCLUSION
These results confirm that enhancing the angiogenic potential of PRP can be advantageous when grafted with non-cellular alloplastic graft material. This combination resulted in faster and more extensive new bone formation, likely due to enhanced angiogenesis. However, the release mechanisms of platelet-derived growth factors are complicated, and many factors should be considered and harmonized to maximize the functions of those growth factors. Although, additional studies should be performed to confirm the findings of this report, these findings provide optimistic prospects for bone graft procedures.
Acknowledgements
The authors acknowledge generous financial support from the Chungnam National University Industry Collaboration Foundation (2008-2212) for this work.
References
- 1.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 2.Butterfield KJ, Bennett J, Gronowicz G, Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005;63:370–376. doi: 10.1016/j.joms.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Pryor ME, Polimeni G, Koo KT, Hartman MJ, Gross H, April M, Safadi FF, Wikesjö UM. Analysis of rat calvaria defects implanted with a platelet-rich plasma preparation: histologic and histometric observations. J Clin Periodontol. 2005;32:966–972. doi: 10.1111/j.1600-051X.2005.00772.x. [DOI] [PubMed] [Google Scholar]
- 4.Raghoebar GM, Schortinghuis J, Liem RS, Ruben JL, van der Wal JE, Vissink A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16:349–356. doi: 10.1111/j.1600-0501.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 5.Fürst G, Gruber R, Tangl S, Zechner W, Haas R, Mailath G, Sanroman F, Watzek G. Sinus grafting with autogenous platelet-rich plasma and bovine hydroxyapatite. A histomorphometric study in minipigs. Clin Oral Implants Res. 2003;14:500–508. doi: 10.1034/j.1600-0501.2003.00859.x. [DOI] [PubMed] [Google Scholar]
- 6.Grageda E. Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dent. 2004;13:301–309. doi: 10.1097/01.id.0000148555.91063.06. [DOI] [PubMed] [Google Scholar]
- 7.Suba Z, Takacs D, Gyulai-Gaal S, Kovacs K. Facilitation of beta-tricalcium phosphate-induced alveolar bone regeneration by platelet-rich plasma in beagle dogs: a histologic and histomorphometric study. Int J Oral Maxillofac Implants. 2004;19:832–838. [PubMed] [Google Scholar]
- 8.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 9.Chow KM, Rabie AB. Vascular endothelial growth pattern of endochondral bone graft in the presence of demineralized intramembranous bone matrix--quantitative analysis. Cleft Palate Craniofac J. 2000;37:385–394. doi: 10.1597/1545-1569_2000_037_0385_vegpoe_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 10.Rabie AB, Chow KM, Wong RW. Demineralized intramembranous bone matrix augments the healing of endochondral bone graft. Int J Adult Orthodon Orthognath Surg. 1999;14:185–197. [PubMed] [Google Scholar]
- 11.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Günther KP, Dehio C, Puhl W, Brenner RE. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–477. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 13.Schlegel KA, Zimmermann R, Thorwarth M, Neukam FW, Klongnoi B, Nkenke E, Felszeghy E. Sinus floor elevation using autogenous bone or bone substitute combined with platelet-rich plasma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e15–e25. doi: 10.1016/j.tripleo.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Borrelli V, Sterpetti AV, Coluccia P, Randone B, Cavallaro A, Santoro D' Angelo L, Cucina A. Bimodal concentration-dependent effect of thrombin on endothelial cell proliferation and growth factor release in culture. J Surg Res. 2001;100:154–160. doi: 10.1006/jsre.2001.6231. [DOI] [PubMed] [Google Scholar]
- 15.Karp JM, Tanaka TS, Zohar R, Sodek J, Shoichet MS, Davies JE, Stanford WL. Thrombin mediated migration of osteogenic cells. Bone. 2005;37:337–348. doi: 10.1016/j.bone.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horstman LL, Ahn YS. Platelet microparticles: a wide-angle perspective. Crit Rev Oncol Hematol. 1999;30:111–142. doi: 10.1016/s1040-8428(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 19.Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, Kojima H, Iwasaka T. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106:2019–2025. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88:3456–3464. [PubMed] [Google Scholar]
- 21.Holme PA, Solum NO, Brosstad F, Roger M, Abdelnoor M. Demonstration of platelet-derived microvesicles in blood from patients with activated coagulation and fibrinolysis using a filtration technique and western blotting. Thromb Haemost. 1994;72:666–671. [PubMed] [Google Scholar]
- 22.Park EJ, Kim ES, Weber H-P, Wright RF, Mooney DJ. Improved bone healing by angiogenic-facotr enriched platelet-rich plasma and its synergistic enhancement by bone morphogenic protein-2. Int J Oral Maxillofac Impl. 2008;23:818–826. [PMC free article] [PubMed] [Google Scholar]
- 23.Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res. 2007;18:639–648. doi: 10.1111/j.1600-0501.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 24.Martineau I, Lacoste E, Gagnon G. Effect of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004;25:4489–4502. doi: 10.1016/j.biomaterials.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Maloney JP, Sillimann CC, Ambruso DR, Wang J, Tuder RM, Voelkel NF. In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol. 1998;275:H1054–H1061. doi: 10.1152/ajpheart.1998.275.3.H1054. [DOI] [PubMed] [Google Scholar]
- 26.Gruber R, Karreth F, Kandler B, Fuerst G, Rot A, Fischer MB, Watzek G. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 2004;15:29–35. doi: 10.1080/09537100310001643999. [DOI] [PubMed] [Google Scholar]
- 27.Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665–667. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 28.Kowalski MJ, Schemitsch EH, Kregor PJ, Senft D, Swiontkowski MF. Effect of periosteal stripping on cortical bone perfusion: a laser Doppler study in sheep. Calcif Tissue Int. 1996;59:24–26. doi: 10.1007/s002239900080. [DOI] [PubMed] [Google Scholar]
- 29.Zammaretti P, Zisch AH. Adult 'endothelial progenitor cells'. Renewing vasculature. Int J Biochem Cell Biol. 2005;37:493–503. doi: 10.1016/j.biocel.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–H2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]