Abstract
Chronic analgesic abuse has been shown to induce severe renal injury characterized by renal papillary necrosis (RPN), an injury detectable at late stage. While direct toxicity of the drug may exist, the molecular mechanisms underlying analgesics induction of RPN remain unknown. A major limitation to study the pathogenesis of RPN is the required chronic exposure before detection of injury. Here, we employed 2-bromoethanamine (BEA) to simulate rapid papillary toxicity using inner medullary collecting duct (IMCD3) cells. Although exposure to 10μM BEA had no effect on cellular viability under isotonic conditions, a 50% loss in cell viability was observed in the first 24 h when cells were subjected to sublethal hypertonic stress and nearly complete cell death after 48 h suggesting that BEA exerts cytotoxicity only under hypertonic conditions. Because TonEBP is a transcription factor critical for cell survival during hypertonic conditions, we undertook experiments to examine the effect of BEA on TonEBP expression and activity. Exposure of cells to 10μM BEA resulted in a substantial reduction in TonEBP protein expression after 24 h. In addition, TonEBP was not translocated to the nucleus in BEA-treated IMCD3 cells under acute hypertonic stress for transcription of target genes essential for osmolyte accumulation. Finally, we found a substantial decrease in TonEBP expression in medullary kidney tissues of mice injected with a single ip dose of BEA. Our data suggest that TonEBP is a potential target for BEA leading to the process of papillary necrosis in the settings of hypertonic stress.
Keywords: Renal papillary necrosis, 2-bromoetanamine, TonEBP, Hypertonic stress
The prolonged use of analgesics (e.g. aspirin, paracetamol, and phenacetin) may result in chronic analgesic nephropathy (AN), a slowly progressive renal disease characterized by papillary sclerosis/necrosis/calcifications, sclerosis, renal cortical atrophy, and chronic interstitial nephritis (De Broe and Elseviers, 1998, 2009). This disease is enhanced by caffeine, codeine, and/or barbiturates, which may lead to psychological dependence and overuse. The incidence of the disease is probably underestimated by the lack of reliable data, but some studies employing computerized tomography (CT) scanning without contrast medium have confirmed a substantial number of patients with AN with unknown cause or interstitial nephritis (Elseviers et al., 1995).
The major limitation in the study of the pathogenesis of this disease is the lack of animal models, made difficult by the need to employ long-term treatment (weeks and months) with mixtures of analgesics in order to cause papillary lesions (Molland, 1978; Shelley, 1978). Therefore, new strategies are being developed which employ compounds that induce renal papillary necrosis (RPN) mimicking the injury induced during AN within hours or days. Among these compounds, 2-bromoethanamine (BEA) is the most frequently employed drug to induce RPN (Garrod et al., 2001; Holmes et al., 1992, 1995). Previous studies have reported that BEA administration (150 mg/kg) in rats induced kidney toxicity characterized by sustained increase in urinary flow rate, electrolyte losses, enzymuria, and a marked decrease in urinary osmolality (Holmes et al., 1995; Yuan et al., 2009). Also, urine analysis by 1H magic angle spinning nuclear magnetic resonance revealed depletion in urinary concentrations of tricarboxylic acid cycle intermediates, excretion of glutaric acid (GTA) and perturbation in the concentration of renal osmolytes. Furthermore, this technique was applied to intact kidney tissues from mice injected with BEA revealing an early increase of GTA and later marked depletion in intracellular accumulation of osmolytes within the renal papilla (Garrod et al., 2001).
In humans, the inner medulla and the papilla are the only tissues exposed to hypertonicity under physiological conditions. This is mainly due to the high salinity and urea content of the interstitial fluid that is generated by the urine concentrating mechanism, which may reach up to 1200 mOsm/kgH2O at the tip of the papilla. In order to survive this environment, the cells of the inner medulla of the kidney accumulate in their cytoplasm organic compatible osmolytes to compensate for extracellular tonicity. These osmolytes include sorbitol, betaine, taurine, myo-inositol, and glycerophosphocholine (GPC) (Beck and Neuhofer, 2005; Burg, 2002; Garcia-Perez and Burg, 1991). The intracellular accumulation of these osmolytes is dependent on the increased expression of specific enzymes and transporters such as aldose reductase (sorbitol), the betaine/gamma amino butiric acid transporter (BGT1, betaine), the taurine transporter (TauT, taurine), the sodium/myo-inositol transporter 1 (SMIT1, myo-inositol), and the neuropathy target esterase (GPC). The transcriptional activity of these genes is mediated by the transcription factor associated with hypertonicity, TonEBP (also known as nuclear factor activated T cell 5) (Jeon et al., 2006; Woo and Kwon, 2002). Besides these genes, TonEBP also activates the transcription of Hsp70, a protein that is crucial in repairing double-stranded DNA breaks induced by high sodium concentrations (Woo et al., 2002). Under hypertonic stress, TonEBP is activated through two main mechanisms: an increase in its protein expression and increased nuclear localization (Andres-Hernando et al., 2008; Jeon et al., 2006).
Absence of TonEBP expression or activity leads to lack of tonicity-responsive gene expression and renal atrophy (Lopez-Rodriguez et al., 2004). TonEBP is also present in the cytoplasm of cells at isotonic conditions (300 mOsm/kgH2O). However, under hypertonic conditions, TonEBP is rapidly translocated to the nucleus where it activates the transcription of these osmoprotective genes (Andres-Hernando et al., 2008). We have previously demonstrated that failure of TonEBP nuclear expression under hypertonic stress conditions dramatically diminishes the osmotic stress response and cell survival (Andres-Hernando et al., 2008).
The present studies were undertaken to examine the effect of BEA on TonEBP expression, and its possible role in causing the papillary necrosis observed with this agent.
MATERIALS AND METHODS
Materials.
Cell culture medium, fetal calf serum (FCS), and antibiotics were from Gibco (Rockville, MD). Antibody to TonEBP was a kind gift from Dr Moo Kwon at University of Maryland. Antibodies to Nup88, Hsp70, and β-actin were obtained from BD/Clontech (Mount View, CA), Stressgen (Ann Arbor, MI), and Cell signaling (Danvers, MA), respectively. All other chemicals including 2-bromoethanamine hydrobromide, myo-inositol, and betaine were ultrapure grade and purchased from Sigma (St Louis, MO).
Plasmids.
Cloning into the pAcGFP-N1 vector (Clontech, Mountain View, CA) of the first 800 bp of TonEBP that includes its regulatory part was previously described (Andres-Hernando et al., 2008). Insertion of the regulatory part of TonEBP lacking its nuclear export signal (NES) and auxiliary export domain (AED) was performed as described employing the following primers for directional cloning: sense 5′-GACCTCGAGATGGATAACAGTCGGATGTCCTGCCAG-3′, antisense 5′-GACGAATTCTGGCACTGTCGGCATCAAAT-3′ (XhoI and EcoRI restriction sites are underlined).
Cell culture and cell transfection.
The established murine inner medullary collecting duct (IMCD3) cell line originally developed by Rauchman et al. (1993) was previously provided by Dr Steve Gullans (Boston, MA). Cell stocks were frozen in liquid N2 and propagated in 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 nutrient mixture supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin. In experiments involving hypertonic stress, the media in culture dishes were exchanged for that with added NaCl to the specified osmolality depending on the experiment. Osmolality was determined with an Advanced Instruments Micro-Osmometer (model 3300; Advanced Instruments, Norwood, MA). In BEA experiments, cells were first maintained with 2% FCS during 24 h. IMCD3 cultures were transfected with the pAcGFP-TonEBP constructs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as described by the manufacturer.
Cell viability experiments.
Cell viability experiments were determined by cell counts following incubation at osmotic stress. Here, viability experiments were performed with minimal serum medium (2% FCS) as higher levels of serum containing hormones/growth factors complement and potential endotoxins can mask the effect of the stress. Cells were grown in 24-well flat bottom tissue culture plates (#35-3047; Falcon BD Labware, Franklin Lakes, NJ) with each experimental time point performed in triplicate. At the defined time point, medium was removed, wells were then washed vigorously with 2 ml of new media and the media removed and 1 ml of trypsin added, and incubated at 37°C for 10 min. An additional 1.5 ml of media was then added to each well and the cells resuspended and a 20 μl aliquot of cell suspension was diluted 1:1 with Trypan Blue (Gibco) and bright/non-blue cells counted using a hemocytometer (Fisher, Pittsburg, PA). Data from cell counts were similar to that obtained using the Cell Titer 96 assay (MTS reagent; Promega, Madison, WI) albeit with greater reproducibility.
Mouse kidney tissues.
C57/B6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were injected with a single ip dose of 150 mg/kg (in water) BEA. At the conclusion of the experiment, mice were killed by cervical dislocation and urine samples collected from the bladder for osmolality analysis and kidneys removed and papilla and cortex tissues dissected, and snap frozen in liquid nitrogen. Tissues were either homogenized using a glass tissue grinder on ice with mitogen activated protein kinase lysis buffer (Lanaspa et al., 2007) or analyzed by immunohistochemistry. Mice were maintained on a standard diet and water was made freely available. All experiments were conducted with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Animal Care and Use Committee of the University of Colorado.
RNA extraction, analysis, and message quantification.
Total RNA was isolated from both confluent cultures and kidney tissues using the RNeasy kit (Qiagen, Valencia, CA). Prior to quantitative PCR (QPCR), RNA integrity was assessed by capillary electrophoresis using an Agilent Bioanalyzer (model 2100; Foster City, CA [using the 28 S to 18 S ribosomal RNA [rRNA] ratio]). Ribosomal RNA was converted to complementary DNA (cDNA) using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) as described by the manufacturer. QPCR primers specific to mouse TonEBP, aldose reductase, BGT1, and Hsp70 were designed using Beacon Designer 5.0 software (Premier Biosoft International, Palo Alto, CA). QPCR was performed using the primers depicted in Table 1 (70nM each) and the SYBR JumpStart Taq Readymix qPCR kit (Sigma) on a Bio-Rad I-Cycler. QPCR runs were analyzed by agarose gel electrophoresis and melt curve to verify that the correct amplicon was produced. β-Actin RNA was used as internal control, and the amount of RNA was calculated by the comparative CT method as recommended by the manufacturer.
TABLE 1.
Primer sets employed for QPCR
| Gene | Accession number | Primer forward | Primer reverse |
| α subunit Na/K ATPase | NM_144900 | GGATTGTTGGCTCTGATG | CGGAATTGTTACTGGTTAGG |
| AR | NM_009658 | CTATTTCCCACTGGATGCCTCAG | TTTCACCAAACCTTCATCCACTAG |
| β-Actin | NM_007393 | CTCTCCCTCACGCCATCC | GTAACAGTCCGCCTAGAAGC |
| BGT1 | NM_133661 | AAGGCAAGGACCAGGTGAAGG | CACACTCCCCTGGCTGCTATAC |
| Hsp70 | NM_010479 | GGTCTAAACGTGCTGCGGATC | AGTCCTCCCCTCCCAGGTG |
| SMIT | NM_017391 | CCTACCGTGCTCCTGAGTGTG | GTGGGAGGTGGTGTGAGAAGAC |
| TonEBP | NM_133957 | CCTGTAGTTCTCTGCTTCATAG | TTGCTGTCGGTGACTGAG |
Protein extraction and western blotting.
Cell protein lysates were prepared from confluent cell cultures in 100 × 20 mm tissue culture dishes as previously described (Lanaspa et al., 2007). Sample protein content was then determined by the BCA protein assay (Pierce, Rockford, IL). Seventy micrograms of total protein was loaded per lane for SDS-polyacrylamide gel electrophoresis (10% wt/vol) analysis and then transferred to polyvinylidene fluoride membranes. Membranes were incubated with primary antibody and visualized using a horseradish peroxidase secondary antibody and Immunstar HRP chemiluminescence kit (Bio-Rad) as described by the manufacturer. Chemiluminescence was recorded with an Image Station 440CF and results analyzed with the 1D Image Software (Kodak Digital Science, Rochester, NY). Blots were also analyzed for β-actin as a loading control.
Sorbitol determination.
Cells kept at isotonic conditions or hypertonically stressed were harvested and extracted using the perchloric acid method as described previously (Lanaspa et al., 2009), and 20 μl of extract was analyzed using an SDH-based enzymatic kit (Megazyme International Ireland Ltd., Wicklow, Ireland) according to the manufacturer's protocol.
Confocal fluorescence microscopy.
IMCD3 cells were grown to confluence in eight-well glass slides (# 177402; NUNC, Rochester, NY). TonEBP-green fluorescent protein (GFP) fusion constructs were imaged with a ×40 water immersion objective using a laser scanning confocal microscope (model LSM510; Zeiss, Thornwood, NY). Data were analyzed with LSM Image analyzer postacquisition software (Zeiss).
Statistics and data analysis.
All data are presented as the mean ± SEM. Data graphics and statistical analysis were performed using Instat (version 3.0) and Prism 4 (both GraphPad Software, San Diego, CA). Independent replicates for each data point (n) are identified in figure legends. For in vivo studies, multiple comparisons were analyzed by ANOVA. P values <0.05 were recognized as statistically significant.
RESULTS
BEA is Toxic to IMCD3 Only Under Hypertonic Stress Conditions by Inhibiting Intracellular Osmolyte Accumulation
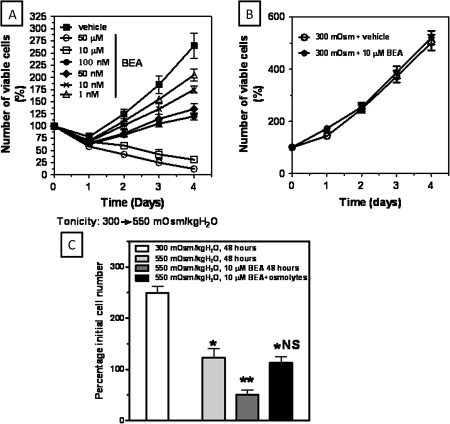
In order to assess the role of tonicity in the RPN reported by previous studies in rats injected with BEA, we initially evaluated in vitro the osmotic stress response of IMCD3 cells following preincubation for 24 h with vehicle or increasing concentrations of BEA (1nM–50μM). IMCD3 cells were then exposed to acute hypertonic stress (550 mOsm/kgH2O). Cell viability of IMCD3 cells was analyzed during the ensuing 4 days. As shown in Figure 1A, we found a marked dose-dependent reduction in cell survival when BEA was added to the medium. At day 4 following hypertonic stress, the reduction was significant with all concentrations employed when compared with vehicle: (24.2 ± 3.2 and 35.5 ± 5.8% reduction with 1nM and 10nM BEA, respectively, p < 0.05 and 54.8 ± 5.5, 58.7 ± 6.3, 87.8 ± 3.3, and 93.3 ±7.7% reduction with 50nM, 100nM, 10μM, and 50μM, respectively, p < 0.01). BEA concentrations greater or equal to 10μM were considered lethal for IMCD3 cells under hypertonic conditions.
FIG. 1.
BEA is toxic to IMCD3 cells only under hypertonic conditions. (A) Cell viability determination in IMCD3 cells after acute hypertonic stress (550 mOsm/kgH2O) following exposure to increasing concentrations of BEA (range 1nM–50μM). Concentrations greater than 10μM were considered lethal under acute hypertonic stress. (B) Cell viability determination in IMCD3 cells maintained at isotonic conditions exposed to vehicle or 10μM BEA for 4 days. (C) Addition of osmolytes (myo-inositol and betaine, 500μM each) to hypertonically stressed IMCD3 cells rescues cell viability in the presence of 10μM BEA for 48 h (*p < 0.05 as compared with isotonic, **p < 0.01 as compared with isotonic. NS means no statistical significance as compared with non-BEA–exposed hypertonic cells; results are obtained from three different experiments with three replicates per condition).
To determine whether the observed effect on cell survival was due to BEA or was dependent on changes in tonicity, we incubated IMCD3 cells with 10μM BEA at isotonic conditions (300 mOsm/kgH2O) for 4 days and cell survival in these cells was compared versus untreated IMCD3 cells. As depicted in Figure 1B, no significant difference in cell survival was found between treated and untreated cells maintained at isotonic conditions (p > 0.05) indicating that BEA toxicity is observed only in hypertonic stress conditions.
Because the marked decrease in cell survival occurred only under hypertonic stress and in view of the previous reports demonstrating a significant decrease in cellular osmolyte accumulation in the renal papilla after BEA treatment (Garrod et al., 2001), we evaluated whether we could rescue cell survival in BEA-exposed cells by addition of different osmolytes to the medium. To this end, we incubated hypertonically stressed cells during 4 days with either BEA alone or in combination with a mixture of 500μM myo-inositol and 500μM betaine. As shown in Figure 1C and as compared with isotonically maintained IMCD3 cells, a significant decrease in survival (48.2% reduction, p < 0.01) was observed when cells were exposed to acute sublethal hypertonic stress (550 mOsm/KgH2O) for 48 h. This reduction in cell survival was decreased further significantly when cells were treated with 10μM BEA (76.7%, p < 0.01 as compared with isotonic cells and 58.4% reduction, p < 0.01 when compared with hypertonically non-treated cells). In contrast, when hypertonically exposed cells treated with BEA were co-incubated with the mixture of osmolytes, cell survival increased to levels similar to non-BEA–treated cells (p > 0.05 between non-treated cells and BEA- and osmolyte-treated cells). This data therefore suggests that treatment of BEA impairs the intracellular accumulation of osmolytes during hypertonic stress.
BEA Impairs TonEBP Expression and Normal Upregulation of TonEBP-Target Genes Under Acute Hypertonic Stress in IMCD3 Cells
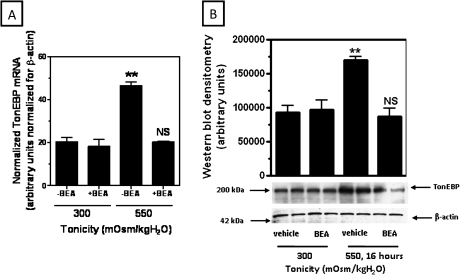
Since TonEBP is the major transcription factor responsible for the upregulation of genes involved in the osmotic stress response, we wanted to determine if TonEBP is affected by BEA treatment in IMCD3 cells. TonEBP messenger RNA (mRNA) expression was evaluated by QPCR employing specific primers. As shown in Figure 2A, TonEBP mRNA expression is significantly increased when IMCD3 cells are acutely exposed for 8 h to sublethal hypertonic stress (550 mOsm/kgH2O) (2.2 ± 0.3-fold, p < 0.01 as compared with IMCD3 cells kept at isotonic conditions). In contrast, no statistically significant upregulation in TonEBP expression was observed when IMCD3 cells were incubated with 10μM BEA in hypertonic conditions (p > 0.05 as compared with IMCD3 cells kept at isotonic conditions). Since the same TonEBP mRNA expression levels were found in both BEA-treated cells under hypertonic stress and in isotonically maintained cells, we studied whether this amount of mRNA was sufficient to upregulate TonEBP protein expression. As shown in Figure 2B, there is no difference in TonEBP protein expression between BEA-treated and non-treated IMCD3 cells maintained at isotonic conditions (p > 0.05). In contrast, after 16 h of acute hypertonic stress, non-treated cells demonstrated a significant increase in TonEBP protein (1.7 ± 0.3-fold increase, p < 0.01), whereas there was no upregulation of TonEBP protein expression when cells were treated with BEA as compared with cells maintained at isotonic conditions (p > 0.05).
FIG. 2.
BEA inhibits TonEBP upregulation under hypertonic stress at (A) mRNA and (B) protein level. IMCD3 cells were incubated in the presence or absence of vehicle or 10μM BEA for 8 (mRNA) or 16 (protein) h and then harvested for message and protein expression (**p < 0.01 as compared with isotonic and BEA in hypertonically exposed cells. NS means no statistical significance as compared with isotonic conditions; results are obtained from three different experiments with three replicates per condition).
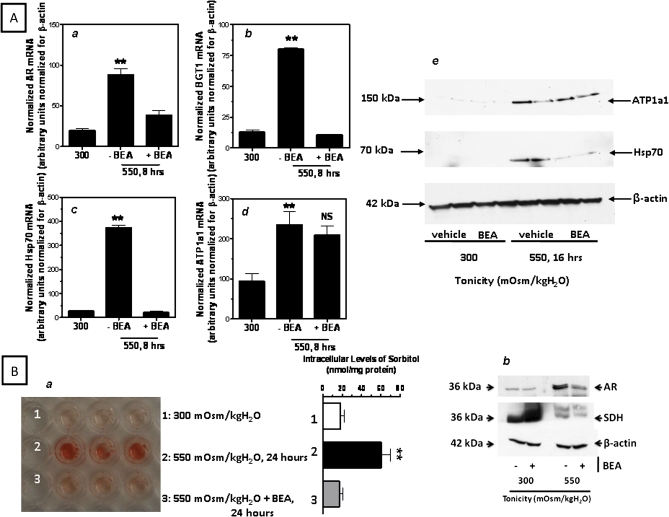
We then examined the effect of BEA-dependent blunting in TonEBP upregulation caused in the transcription of its downstream osmoprotective genes. As depicted in Figure 3A, message levels of TonEBP-target genes AR, BGT1, and Hsp70 under hypertonic stress in BEA-treated cells are unchanged from isotonic levels as compared with the upregulation in non-treated cells (68.3 ± 4.8% average reduction, p < 0.01). No significant change was found in the mRNA expression of a TonEBP-independent osmotic stress response gene, the α subunit of the Na+/K+-ATPase (ATP1a1). The effect observed in mRNA was verified by Western blot analysis. As shown in the figure, Hsp70 protein expression was significantly downregulated in IMCD3 cells exposed to BEA for 16 h as compared with non-treated cells, whereas no change in expression was found in ATP1a1. This data suggests that BEA interrupts the hypertonic stress-induced transcriptional activity of TonEBP target genes.
FIG. 3.
BEA inhibits the expression of TonEBP target genes during the osmotic stress response. (A) Analysis of the mRNA (left) of TonEBP target genes AR (a), BGT1 (b), and Hsp70 (c) and protein (right) for Hsp70 (e) expression in hypertonically stress IMCD3 cells treated to BEA as compared with non-treated cells. The expression of the α subunit of the Na/K-ATPase (d and e) is shown for comparison. (B, a) Hypertonically induced intracellular osmolyte accumulation of sorbitol is significantly decreased in IMCD3 cells exposed to BEA suggesting a reduction in AR activity. (B, b) Protein expression of AR and SDH in IMCD3 cells treated with BEA and maintained at isotonic conditions or acutely stressed to hypertonic stress depicts reduced AR expression, whereas SDH expression does not change significantly (**p < 0.01 as compared with isotonic IMCD3 cells, NS, non significant as compared with hypertonically BEA exposed cells; results are obtained from three different experiments with three replicates per condition).
To determine if this reduction in the expression of osmoprotective genes was associated with a decrease in intracellular osmolyte accumulation, we analyzed sorbitol levels (a function of AR activity) by an enzymatic assay. As shown in Figure 3B (a), the amount of sorbitol produced in BEA-treated IMCD3 cells was significantly lower than in non-treated cells indicating that BEA impairs sorbitol accumulation under hypertonic stress (78.3 ± 4.4% reduction, p < 0.01). Because changes in sorbitol accumulation by BEA treatment may not only be due to decreased AR levels but also to an increase in the expression of sorbitol dehydrogenase (SDH) that reduces sorbitol, we examined the protein expression of these two enzymes under BEA treatment conditions. As shown in Figure 3B (b), the expression of AR protein is significantly lower under hypertonic stress conditions when cells were treated with 10μM BEA as compared with non-treated cells (65.5 ± 7.8% AR reduction, p < 0.01). On the other hand, no significant change was observed in SDH expression with BEA under hypertonic conditions pointing to the lack of sorbitol accumulation in the presence of BEA as being due primarily to a blunting of AR upregulation rather than to an increase in SDH.
BEA Inhibits the Nuclear Import of TonEBP Under Hypertonic Stress Conditions
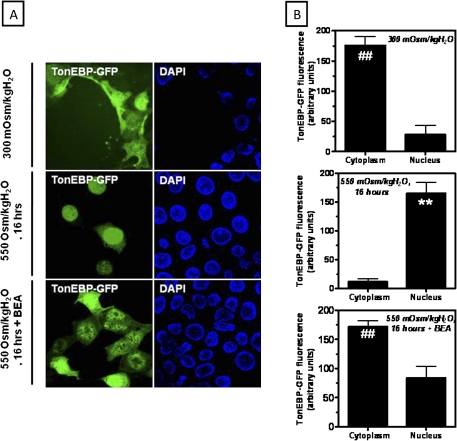
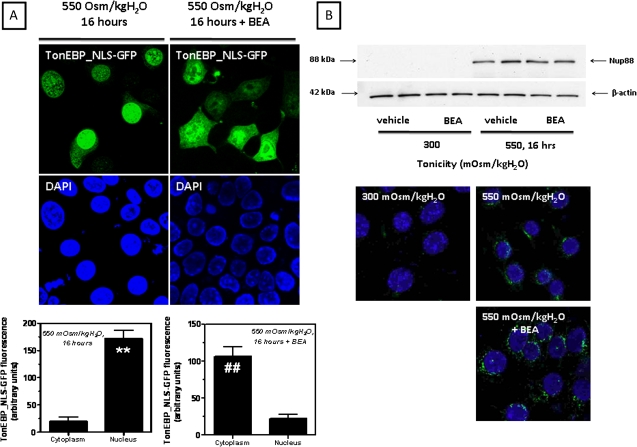
To analyze the localization of TonEBP in live IMCD3 cells, we have first cloned the trafficking regulatory domain of TonEBP that includes its nuclear localization signal (NLS), NES, and the AED upstream the GFP. IMCD3 cells were transfected with this construct and TonEBP localization was analyzed by confocal fluorescence microscopy. Figure 4A depicts a representative confocal image of TonEBP in IMCD3 cells. As shown, TonEBP (green) is located mostly in the cytosol of IMCD3 cells maintained at isotonic conditions with only minor nuclear expression (top panel). After 16 h of acute hypertonic stress (550 mOsm/kgH2O), TonEBP is fully translocated to the nucleus where it colocalizes with the nuclear marker 4′,6-diamidino-2-phenylindole (blue) (middle panel). In contrast, when IMCD3 cells were treated with 10μM BEA and hypertonically stressed, TonEBP remains in the cytoplasm with minimal nuclear localization (bottom panel) denoting that BEA impairs TonEBP nuclear retention under hypertonic stress. Fluorescence intensity analysis of TonEBP expression in the cytosol versus its expression in the nucleus is shown in Figure 4B. As depicted, in IMCD3 cells, the translocation of TonEBP to the nucleus under hypertonic stress was found to be 10.2-fold greater than cytosolic expression. On the other hand, in BEA-treated cells, TonEBP expression in the nucleus is a 3.6-fold lower (p < 0.01). To further evaluate whether the loss of nuclear expression of TonEBP with BEA was due to a decrease in its nuclear import or to increased export out of the nucleus, we eliminated the NES and AED from the TonEBP-GFP construct (TonEBP_NLS-GFP) leaving only the NLS before transfection of IMCD3 cells. As shown in Figure 5A left panel, the TonEBP_NLS-GFP construction is retained within the nucleus of hypertonically stressed IMCD3 cells (left column). In contrast, when cells were preincubated with 10μM BEA, this construct was mainly located in the cytoplasm (right column). Fluorescence intensity analysis shown in Figure 5A (bottom panel) revealed that the cytosolic expression of TonEBP-NLS was 5.3 ± 0.7 greater than the nuclear expression in BEA-treated cells (p < 0.01). Data indicate that BEA impairs the normal import of TonEBP to the nucleus during hypertonic stress. We have previously reported that TonEBP export out of the nucleus was mediated by the expression in the nuclear membrane of nucleoporin 88 (Nup88). Nup88 silenced IMCD3 cells failed to retain TonEBP in the nucleus, which was swiftly exported to the cytoplasm (Andres-Hernando et al., 2008). To evaluate whether the normal machinery that controls TonEBP nuclear export was affected by BEA, we analyzed Nup88 expression under acute hypertonic stress in BEA-treated and non-treated cells. As shown in Figure 5B, Nup88 expression is markedly upregulated in IMCD3 cells after hypertonic shock confirming our previous results. This expression correlates with localization of Nup88 to the nuclear envelope, as no significant difference in Nup88 expression was observed between BEA-treated and non-treated cells further suggesting that the export machinery of TonEBP is not affected by BEA.
FIG. 4.
BEA prevents the translocation of TonEBP from the cytoplasm to the nucleus under acute hypertonic conditions. (A) Immunofluorescence of the construct TonEBP-GFP in IMCD3 cells demonstrating cytosolic staining (green) in isotonically maintained IMCD3 cells. The TonEBP-GFP construct is located in the nucleus under acute hypertonic stress (550 mOsm/kgH2O, 16 h) where it colocalizes with the nucear marker 4′,6-diamidino-2-phenylindole (blue). In contrast, in BEA-treated cells, the construct remains in the cytoplasm. (B) Fluorescence analysis of the localization of the TonEBP-GFP construct by confocal microscopy (Data analysis is for 10 high-power fields counting > 50 cells total) (**p < 0.01 as compared with cytoplasmic expression of TonEBP, ##p < 0.01 as compared with nuclear expression of TonEBP, n = 50 different cells from three independent experiments).
FIG. 5.
BEA impairs nuclear import of TonEBP under hypertonicity. (A) Immunofluorescence of the construct TonEBP-GFP_NLS in IMCD3 cells with or without 10μM BEA demonstrating cytosolic staining after acute hypertonic stress (top, 550 mOsm/kgH2O, 16 h) and fluorescence analysis by confocal microscopy (bottom). (B) Expression and localization of nucleoporin 88 (Nup88) under hypertonic stress conditions is not affected by BEA (**p < 0.01 as compared with cytoplasmic expression of TonEBP, ##p < 0.01 as compared with nuclear expression of TonEBP, n = 50 different cells from three independent experiments).
BEA Inhibits TonEBP and TonEBP-Target Genes Expression in the Inner Medulla of the Kidney
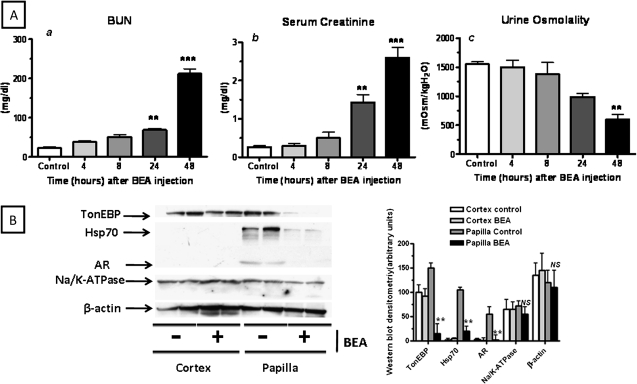
To evaluate the effects of BEA on TonEBP activity in vivo, we analyzed its expression as well as the expression of its target genes on kidney lysates from BEA-treated mice (150 mg/kg). Intraperitoneal injection of BEA led to increased serum creatinine and blood urea nitrogen levels within the first 72 h (Figure 6A). This increase was significant at 48 and 72 h as compared with controls indicating that BEA exerts renal toxicity. Urinary osmolality was found to be significantly lower in BEA-injected mice (1500 ± 135 before versus 515 ± 52 mOsm/kgH2O 48 h after BEA injection, p < 0.001) reflecting a reduction in urinary concentrating ability in these animals presumably due to papillary necrosis (Fig. 6A). Protein and mRNA expression of TonEBP as well as TonEBP target genes is shown in Figure 6B. As depicted, although TonEBP was found in both cortical and papillary regions of normal mice, BEA significantly decreased its expression in the hypertonic papilla (90.2 ± 3 and 84.5 ± 5% decrease for mRNA and protein respectively, p < 0.001) with no effect in the isotonic cortex. The observed downregulation in TonEBP expression was accompanied by a significant decrease in protein expression of any well-established TonEBP target gene analyzed. The papillary protein expression of Hsp70 decreased by 78 ± 10% (p < 0.001), whereas the papillary protein expression of AR was reduced by 90 ± 3% (p < 0.001). In contrast, no changes in the expression of α subunit of the Na/K-ATPase were found in either the cortex or the papilla indicating that BEA affected specifically the osmotic response driven by TonEBP.
FIG. 6.
BEA impairs renal function, urinary concentration and affects TonEBP expression and activity in the inner medulla of the kidney. (A) BEA (150 mg/kg ip) induces renal injury within 48 h as reflected by increased blood urea nitrogen (a) and serum creatinine (b). BEA markedly decreases urinary osmolality (c) in mice after 48 h. (B) Left, representative western blot showing that BEA inhibits medullary protein expression of TonEBP and its target genes AR, Hsp70 but not the expression of the α subunit of the Na/K-ATPase. Right, Western blot densitometry and statistical analysis NS means not significant as compared with papilla control, ***p < 0.001, **p < 0.01 as compared with papilla control, n = 4–6 mice per condition.
DISCUSSION
RPN is commonly found in patients with chronic AN generally as a result of frequent use of analgesics with central-acting dependence-inducing substances including caffeine, codeine, and/or barbiturates (De Broe and Elseviers, 1998, 2009). Other conditions including diabetes, sickle-cell disease, and pyelonephritis may cause RPN as well (Atta and Whelton, 1997; Groop et al., 1989). It is generally accepted that non-steroidal anti-inflammatory drugs induce hemodynamically mediated acute kidney injury as a consequence of the inhibition of vasodilatation in the clinical setting of a stimulated renin-angiotensin system. However, this renal disorder is usually reversible and may not be related to the disorders observed in the irreversible chronic AN. Diagnosis of RPN in humans is difficult because in the early stages, there are few specific symptoms and only validated test available to diagnose RPN is expensive and not used in everyone. The use of CT scanning without contrast medium has significantly increased diagnostic sensitivity and confirmed the underestimation of AN in several countries (Elseviers et al., 1995).
Although the effects of AN-induced RPN on the kidney are well characterized, the molecular mechanisms underlying this condition remain unknown. In this regard, the lack of a good and reliable animal model for the study of RPN has limited the study of this condition. The limitations comprise several factors including anatomical differences between human beings and most animal species, technical difficulties in studying the renal papilla, and the chronic treatment with mixtures of analgesics required in experimental animals, which may take weeks or months to cause papillary lesions (Molland, 1978; Shelley, 1978). Fortunately, the use of different compounds, especially BEA, has helped to characterize a good animal model of RPN (Garrod et al., 2001; Holmes et al., 1995; Yuan et al., 2009). A single ip injection of BEA (150–200 mg/kg) causes RPN in rats within hours as demonstrated by degeneration of interstitial medullary cells, necrosis of the renal papilla, and further tubular and glomerular damage. We did not observe significant injury in our cultured renal cells under isotonic conditions, in vivo, both renal cortical cells and liver cells are not immune from the toxicity of this compound (Garrod et al., 2001). Nonetheless, BEA is widely recognized as the prototypic model of RPN as described by Bach and Nguyen (1998). In careful time-course studies, injury to the medullary interstitial cells was seen as early as 4 h, whereas the renal cortex was morphologically normal at that time (Gregg et al., 1990). This is in line with the observation that renal damage caused by BEA is accompanied by a marked decrease in urinary osmolality, increased urinary volume, and free water clearance (Yuan et al., 2009). We must acknowledge, however, that observations of the present manuscript obtained with BEA, while an excellent model for acute papillary necrosis, may not be identical to those operant in the setting of AN.
Over the past several years, two major mechanisms have been proposed to explain the pathogenesis of RPN during AN. One proposed mechanism is ischemic injury through inhibition of cyclooxygenase-mediated production of prostaglandins. The other mechanism is direct toxicity in the medullary cells from reactive intermediates, acetylation of critical cellular proteins, or inhibition of cellular functions (Schnellmann, 1998). Probably, RPN is most likely a common end point for both mechanistic pathways. For example, it has been demonstrated that the NSAID rofecoxib triggers apoptosis of renal medullary cells in dehydrated rats by decreasing the expression of Hsp70 (Neuhofer et al., 2004), a well-established TonEBP target gene involved in repairing DNA breaks induced by high sodium concentrations (Dmitrieva and Burg, 2005; Woo et al., 2002). In this manuscript, we have demonstrated that the papillotoxic compound BEA exerts its toxicity in IMCD3 by impairing the normal intracellular accumulation of organic osmolytes in IMCD3 cells exposed to hypertonic stress. Physiologically, the papilla is hypertonic as compared with the rest of the kidney and papillary cells adapt and survive to hypertonicity by accumulating intracellular compatible organic osmolytes that compensate the high extracellular tonicity Accumulation of osmolytes is critical for survival of IMCD3 cells to hypertonicity because (1) BEA does not affect cell viability at isotonic conditions where no significant accumulation of osmolytes are produced and (2) addition of osmolytes restored cell survival under acute hypertonic conditions. Because the transcription factor associated with hypertonicity, TonEBP, is the key player in the transcription of genes involved in osmolyte accumulation during hypertonicity, we studied whether TonEBP is a target of BEA. To date, two major mechanisms are known to mediate TonEBP activation by hypertonic stress in the inner medulla, increased protein expression and increased nuclear translocation from the cytoplasm (Andres-Hernando et al., 2008; Woo and Kwon, 2002). In this manuscript, we demonstrate that BEA affects both mechanisms under acute hypertonic conditions. We have previously reported that one of the mechanisms that allow the nuclear localization of TonEBP under hypertonicity is inhibition of its export rate out of the nucleus. This inhibition is controlled by the expression of the nucleoporin 88 (Nup88) in the nuclear membrane that blocks the export of TonEBP through the nuclear pore complex (Andres-Hernando et al., 2008). Here, we determined that BEA does not affect the expression of Nup88 or its localization in the nuclear membrane supporting that the loss of nuclear expression of TonEBP is likely because of a decrease in its import rate rather than to an increased export. To further support this mechanism, we employed a TonEBP fluorescent construct that included its NLS but lacked its NESs. With this construct, we demonstrated that BEA specifically affects TonEBP nuclear import. Previously, it has been shown that BEA is able to form adducts and alkylate peptides rich in cysteine, lysine, and arginine residues, which constitute the backbone of the NLS of TonEBP (Andres-Hernando et al., 2008; Hopkins et al., 2002; Rehulkova et al., 2009). Adducts formed by BEA may therefore mask the NLS of TonEBP and therefore inhibit its nuclear translocation.
It is of interest to postulate one potential mechanism that may explain how NSAIDs may inhibit nuclear transport of TonEBP during the osmotic stress response thus leading to RPN. Several studies demonstrated that the activation of TonEBP by hypertonicity requires its previous cleavage by the proteasome (Kojima et al., 2004; Lammers et al., 2005; Woo et al., 2000). Therefore, inhibition of proteasome activity leads to decreased expression of several TonEBP target genes including BGT1 and SMIT (Lammers et al., 2005; Woo et al., 2000). Interestingly, in these reports, proteasome inhibition resulted in the loss of nuclear expression of TonEBP reflecting that its import rate under hypertonic conditions was decreased (Woo et al., 2000). Also, several studies have reported that NSAIDs inhibit proteasomal activity (Dikshit et al., 2006; Huang et al., 2002). Therefore, inhibition of proteasomal activity in the inner medulla as a result of chronic exposure of the cells to NSAIDs may block the activation and nuclear translocation of TonEBP by hypertonicity in vivo thereby leading to an inappropriate osmotic response and cell death.
Recently, it has been shown that BEA and its metabolites form adducts with a surprisingly large number of endogenous molecules (Shipkova, Vassallo, Aranibar, Hnatyshyn, Zhang, Clayton, Cantor, Sanders, Coen, Lindon, Holmes, Nicholson, and Lehman-McKeeman, submitted). Although not proven, it is likely that the adduction to BEA and its metabolites alters the function of some of these molecules. Thus, inhibition of TonEBP, although one plausible mechanism of BEA-induced RPN, is probably not the sole mechanism of BEA toxicity.
Previous studies reported that BEA significantly diminished the accumulation of osmolytes in cells of the inner medulla and papilla of the kidney of rats (Garrod et al., 2001; Holmes et al., 1992). We therefore analyzed if the effects observed in IMCD3 on TonEBP activity were applicable in vivo in mice kidneys. Western blot analysis reveals that the expression of TonEBP in the medullary and papillary regions is markedly downregulated by BEA. This downregulation is accompanied by decreased expression of its target genes reflecting an absence of osmotic response in these cells in vivo. To determine the specificity of BEA on TonEBP activity, we analyzed the expression of genes important in the osmotic response but not dependent of TonEBP. As an example, the expression of the α subunit of the Na/K-ATPase did not change significantly as compared with non-treated mice.
In summary, we provide strong evidence for a molecular mechanism that could lead to RPN in vivo, namely the inhibition of the activity of the transcription factor associated with hypertonicity TonEBP. This impairs the normal osmolyte accumulation of the cells of the inner medulla making them unable to remain viable in the hypertonic environment. It is attractive to postulate that the similar pathway underlies the medullar injury observed with analgesic abuse.
FUNDING
National Institutes of Health (DK-19928, DK-66544) to T.B.
References
- Andres-Hernando A, Lanaspa MA, Rivard CJ, Berl T. Nucleoporin 88 (Nup88) is regulated by hypertonic stress in kidney cells to retain the transcription factor tonicity enhancer-binding protein (TonEBP) in the nucleus. J. Biol. Chem. 2008;283:25082–25090. doi: 10.1074/jbc.M802381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta MG, Whelton A. Acute renal papillary necrosis induced by ibuprofen. Am. J. Ther. 1997;4:55–60. doi: 10.1097/00045391-199701000-00011. [DOI] [PubMed] [Google Scholar]
- Bach PH, Nguyen TK. Renal papillary necrosis—40 years on. Toxicol. Pathol. 1998;26:73–91. doi: 10.1177/019262339802600110. [DOI] [PubMed] [Google Scholar]
- Beck FX, Neuhofer W. Response of renal medullary cells to osmotic stress. Contrib. Nephrol. 2005;148:21–34. doi: 10.1159/000086041. [DOI] [PubMed] [Google Scholar]
- Burg MB. Response of renal inner medullary epithelial cells to osmotic stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002;133:661–666. doi: 10.1016/s1095-6433(02)00203-9. [DOI] [PubMed] [Google Scholar]
- De Broe ME, Elseviers MM. Analgesic nephropathy. N. Engl. J. Med. 1998;338:446–452. doi: 10.1056/NEJM199802123380707. [DOI] [PubMed] [Google Scholar]
- De Broe ME, Elseviers MM. Over-the-counter analgesic use. J. Am. Soc. Nephrol. 2009;20:2098–2103. doi: 10.1681/ASN.2008101097. [DOI] [PubMed] [Google Scholar]
- Dikshit P, Chatterjee M, Goswami A, Mishra A, Jana NR. Aspirin induces apoptosis through the inhibition of proteasome function. J. Biol. Chem. 2006;281:29228–29235. doi: 10.1074/jbc.M602629200. [DOI] [PubMed] [Google Scholar]
- Dmitrieva NI, Burg MB. Hypertonic stress response. Mutat. Res. 2005;569:65–74. doi: 10.1016/j.mrfmmm.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Elseviers MM, De Schepper A, Corthouts R, Bosmans JL, Cosyn L, Lins RL, Lornoy W, Matthys E, Roose R, Van Caesbroeck D, et al. High diagnostic performance of CT scan for analgesic nephropathy in patients with incipient to severe renal failure. Kidney Int. 1995;48:1316–1323. doi: 10.1038/ki.1995.416. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A, Burg MB. Role of organic osmolytes in adaptation of renal cells to high osmolality. J. Membr. Biol. 1991;119:1–13. doi: 10.1007/BF01868535. [DOI] [PubMed] [Google Scholar]
- Garrod S, Humpher E, Connor SC, Connelly JC, Spraul M, Nicholson JK, Holmes E. High-resolution (1)H NMR and magic angle spinning NMR spectroscopic investigation of the biochemical effects of 2-bromoethanamine in intact renal and hepatic tissue. Magn. Reson. Med. 2001;45:781–790. doi: 10.1002/mrm.1106. [DOI] [PubMed] [Google Scholar]
- Gregg NJ, Courtauld EA, Bach PH. High resolution light microscopic morphological and microvascular changes in an acutely induced renal papillary necrosis. Toxicol. Pathol. 1990;18:47–55. doi: 10.1177/019262339001800107. [DOI] [PubMed] [Google Scholar]
- Groop L, Laasonen L, Edgren J. Renal papillary necrosis in patients with IDDM. Diabetes Care. 1989;12:198–202. doi: 10.2337/diacare.12.3.198. [DOI] [PubMed] [Google Scholar]
- Holmes E, Bonner FW, Nicholson JK. Comparative studies on the nephrotoxicity of 2-bromoethanamine hydrobromide in the Fischer 344 rat and the multimammate desert mouse (Mastomys natalensis) Arch. Toxicol. 1995;70:89–95. doi: 10.1007/BF02733668. [DOI] [PubMed] [Google Scholar]
- Holmes E, Bonner FW, Sweatman BC, Lindon JC, Beddell CR, Rahr E, Nicholson JK. Nuclear magnetic resonance spectroscopy and pattern recognition analysis of the biochemical processes associated with the progression of and recovery from nephrotoxic lesions in the rat induced by mercury(II) chloride and 2-bromoethanamine. Mol. Pharmacol. 1992;42:922–930. [PubMed] [Google Scholar]
- Hopkins CE, O'Connor PB, Allen KN, Costello CE, Tolan DR. Chemical-modification rescue assessed by mass spectrometry demonstrates that gamma-thia-lysine yields the same activity as lysine in aldolase. Protein Sci. 2002;11:1591–1599. doi: 10.1110/ps.3900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Chuang LY, Hung WC. Mechanisms underlying nonsteroidal anti-inflammatory drug-induced p27(Kip1) expression. Mol. Pharmacol. 2002;62:1515–1521. doi: 10.1124/mol.62.6.1515. [DOI] [PubMed] [Google Scholar]
- Jeon US, Kim JA, Sheen MR, Kwon HM. How tonicity regulates genes: story of TonEBP transcriptional activator. Acta Physiol. (Oxf.) 2006;187:241–247. doi: 10.1111/j.1748-1716.2006.01551.x. [DOI] [PubMed] [Google Scholar]
- Kojima R, Randall JD, Ito E, Manshio H, Suzuki Y, Gullans SR. Regulation of expression of the stress response gene, Osp94: identification of the tonicity response element and intracellular signalling pathways. Biochem. J. 2004;380:783–794. doi: 10.1042/BJ20040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers PE, Beck JA, Chu S, Kempson SA. Hypertonic upregulation of betaine transport in renal cells is blocked by a proteasome inhibitor. Cell Biochem. Funct. 2005;23:315–324. doi: 10.1002/cbf.1241. [DOI] [PubMed] [Google Scholar]
- Lanaspa MA, Almeida NE, Andres-Hernando A, Rivard CJ, Capasso JM, Berl T. The tight junction protein, MUPP1, is up-regulated by hypertonicity and is important in the osmotic stress response in kidney cells. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13672–13677. doi: 10.1073/pnas.0702752104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanaspa MA, Andres-Hernando A, Rivard CJ, Dai Y, Li N, Berl T. ZAC1 is up-regulated by hypertonicity and decreases sorbitol dehydrogenase expression, allowing accumulation of sorbitol in kidney cells. J. Biol. Chem. 2009;284:19974–19981. doi: 10.1074/jbc.M109.001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, Bronson RT, Igarashi P, Rao A, Olson EN. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2392–2397. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molland EA. Experimental renal papillary necrosis. Kidney Int. 1978;13:5–14. doi: 10.1038/ki.1978.2. [DOI] [PubMed] [Google Scholar]
- Neuhofer W, Holzapfel K, Fraek ML, Ouyang N, Lutz J, Beck FX. Chronic COX-2 inhibition reduces medullary HSP70 expression and induces papillary apoptosis in dehydrated rats. Kidney Int. 2004;65:431–441. doi: 10.1111/j.1523-1755.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am. J. Physiol. 1993;265:F416–424. doi: 10.1152/ajprenal.1993.265.3.F416. [DOI] [PubMed] [Google Scholar]
- Rehulkova H, Marchetti-Deschmann M, Pittenauer E, Allmaier G, Rehulka P. Improved identification of hordeins by cysteine alkylation with 2-bromoethylamine, SDS-PAGE and subsequent in-gel tryptic digestion. J. Mass Spectrom. 2009;44:1613–1621. doi: 10.1002/jms.1675. [DOI] [PubMed] [Google Scholar]
- Schnellmann RG. Analgesic nephropathy in rodents. J. Toxicol. Environ. Health B Crit. Rev. 1998;1:81–90. doi: 10.1080/10937409809524544. [DOI] [PubMed] [Google Scholar]
- Shelley JH. Pharmacological mechanisms of analgesic nephropathy. Kidney Int. 1978;13:15–26. doi: 10.1038/ki.1978.3. [DOI] [PubMed] [Google Scholar]
- Woo SK, Kwon HM. Adaptation of kidney medulla to hypertonicity: role of the transcription factor TonEBP. Int. Rev. Cytol. 2002;215:189–202. doi: 10.1016/s0074-7696(02)15009-1. [DOI] [PubMed] [Google Scholar]
- Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol. Cell. Biol. 2002;22:5753–5760. doi: 10.1128/MCB.22.16.5753-5760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SK, Maouyo D, Handler JS, Kwon HM. Nuclear redistribution of tonicity-responsive enhancer binding protein requires proteasome activity. Am. J. Physiol. Cell Physiol. 2000;278:C323–330. doi: 10.1152/ajpcell.2000.278.2.C323. [DOI] [PubMed] [Google Scholar]
- Yuan K, Jin X, Gao S, Shah A, Kim SY, Kim SZ, Kim SH. Osmoregulation of natriuretic peptide receptors in bromoethylamine-treated rat kidney. Peptides. 2009;30:1137–1143. doi: 10.1016/j.peptides.2009.02.012. [DOI] [PubMed] [Google Scholar]