Abstract
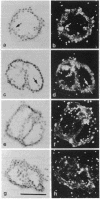
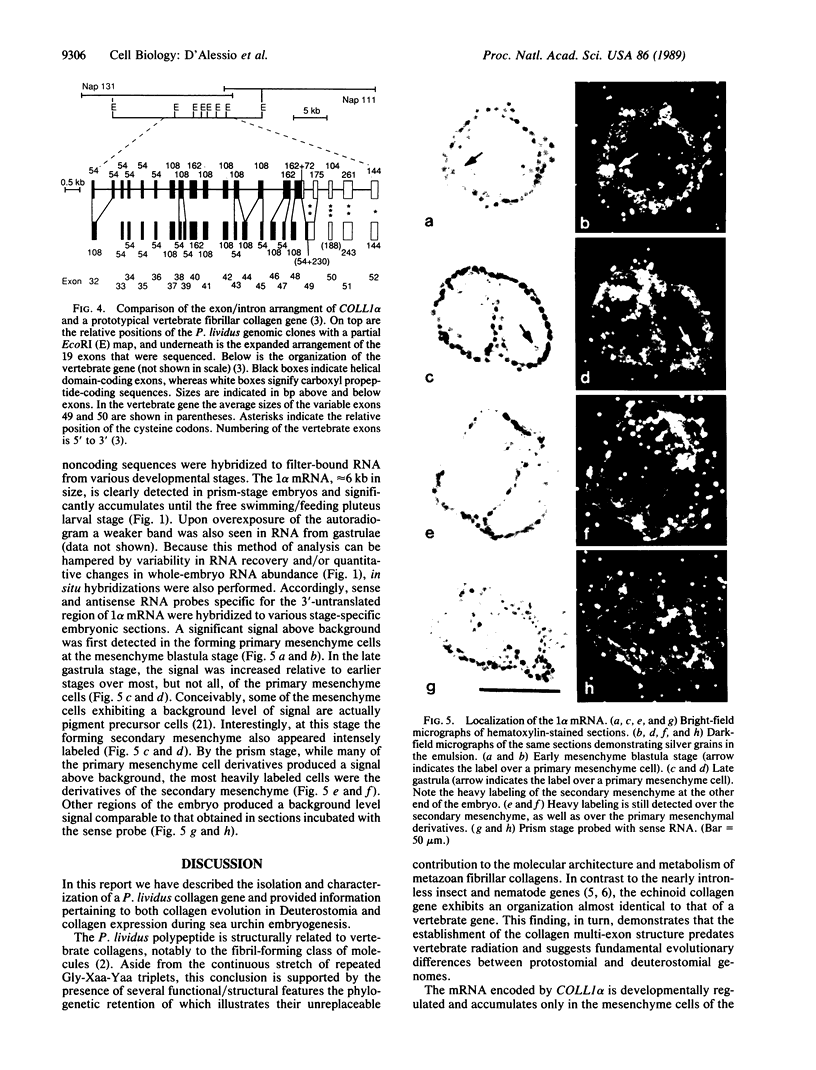
We have isolated and characterized cDNA and genomic clones that specify a Paracentrotus lividus procollagen chain. The cDNAs code for 160 uninterrupted Gly-Xaa-Yaa triplets and a 252-amino acid carboxyl propeptide. Analysis of the deduced amino acid sequences indicated that the sea urchin polypeptide exhibits structural features that are characteristic of the fibril-forming class of collagen molecules. Partial characterization of two genomic recombinants revealed that the 3' end of the echinoid gene displays a complex organization that closely resembles that of a prototypical vertebrate fibrillar collagen gene. In situ and Northern (RNA) blot hybridizations established the size, time of appearance, and tissue distribution of the collagen transcripts in the developing sea urchin embryo. Collagen mRNA, approximately equal to 6 kilobases in size, is first detected in the forming primary mesenchyme cells of late blastulae where it progressively accumulates until the free swimming/feeding pluteus larval stage. Interestingly, collagen transcripts are also detected in the forming secondary mesenchyme cells of late gastrulae, and by the prism stage, their derivatives appear to be the most intensively labeled cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Chambers S. A., Yang Q., Venkatesan M., Angerer R. C., Simpson R. T. Expression of a collagen gene in mesenchyme lineages of the Strongylocentrotus purpuratus embryo. Genes Dev. 1988 Feb;2(2):239–246. doi: 10.1101/gad.2.2.239. [DOI] [PubMed] [Google Scholar]
- Benson-Chanda V., Su M. W., Weil D., Chu M. L., Ramirez F. Cloning and analysis of the 5' portion of the human type-III procollagen gene (COL3A1). Gene. 1989 May 30;78(2):255–265. doi: 10.1016/0378-1119(89)90228-x. [DOI] [PubMed] [Google Scholar]
- Bernard M., Yoshioka H., Rodriguez E., Van der Rest M., Kimura T., Ninomiya Y., Olsen B. R., Ramirez F. Cloning and sequencing of pro-alpha 1 (XI) collagen cDNA demonstrates that type XI belongs to the fibrillar class of collagens and reveals that the expression of the gene is not restricted to cartilagenous tissue. J Biol Chem. 1988 Nov 15;263(32):17159–17166. [PubMed] [Google Scholar]
- Blankenship J., Benson S. Collagen metabolism and spicule formation in sea urchin micromeres. Exp Cell Res. 1984 May;152(1):98–104. doi: 10.1016/0014-4827(84)90233-7. [DOI] [PubMed] [Google Scholar]
- Blumberg B., MacKrell A. J., Fessler J. H. Drosophila basement membrane procollagen alpha 1(IV). II. Complete cDNA sequence, genomic structure, and general implications for supramolecular assemblies. J Biol Chem. 1988 Dec 5;263(34):18328–18337. [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., Angerer L. M., Lee J. J., Davidson E. H., Angerer R. C. Cell lineage-specific programs of expression of multiple actin genes during sea urchin embryogenesis. J Mol Biol. 1986 Mar 20;188(2):159–172. doi: 10.1016/0022-2836(86)90301-3. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Lineage-specific gene expression and the regulative capacities of the sea urchin embryo: a proposed mechanism. Development. 1989 Mar;105(3):421–445. doi: 10.1242/dev.105.3.421. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Myers J. C. COOH-terminal propeptides of the major human procollagens. Structural, functional and genetic comparisons. J Mol Biol. 1987 Jan 5;193(1):127–143. doi: 10.1016/0022-2836(87)90632-2. [DOI] [PubMed] [Google Scholar]
- Ettensohn C. A., McClay D. R. Cell lineage conversion in the sea urchin embryo. Dev Biol. 1988 Feb;125(2):396–409. doi: 10.1016/0012-1606(88)90220-5. [DOI] [PubMed] [Google Scholar]
- Gibson A. W., Burke R. D. The origin of pigment cells in embryos of the sea urchin Strongylocentrotus purpuratus. Dev Biol. 1985 Feb;107(2):414–419. doi: 10.1016/0012-1606(85)90323-9. [DOI] [PubMed] [Google Scholar]
- Golob R., Chetsanga C. J., Doty P. The onset of collagen synthesis in sea urchin embryos. Biochim Biophys Acta. 1974 Apr 27;349(1):135–141. doi: 10.1016/0005-2787(74)90017-3. [DOI] [PubMed] [Google Scholar]
- Greenburg G., Hay E. D. Cytodifferentiation and tissue phenotype change during transformation of embryonic lens epithelium to mesenchyme-like cells in vitro. Dev Biol. 1986 Jun;115(2):363–379. doi: 10.1016/0012-1606(86)90256-3. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Kramer J. M., Cox G. N., Hirsh D. Comparisons of the complete sequences of two collagen genes from Caenorhabditis elegans. Cell. 1982 Sep;30(2):599–606. doi: 10.1016/0092-8674(82)90256-2. [DOI] [PubMed] [Google Scholar]
- Nemer M., Harlow P. Sea-urchin RNAs displaying differences in developmental regulation and in complementarity to a collagen exon probe. Biochim Biophys Acta. 1988 Sep 7;950(3):445–449. doi: 10.1016/0167-4781(88)90143-1. [DOI] [PubMed] [Google Scholar]
- Saitta B., Buttice G., Gambino R. Isolation of a putative collagen-like gene from the sea urchin Paracentrotus lividus. Biochem Biophys Res Commun. 1989 Feb 15;158(3):633–639. doi: 10.1016/0006-291x(89)92768-x. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Hough-Evans B. R., Franks R. R., Britten R. J., Davidson E. H. A regulatory domain that directs lineage-specific expression of a skeletal matrix protein gene in the sea urchin embryo. Genes Dev. 1988 Oct;2(10):1238–1250. doi: 10.1101/gad.2.10.1238. [DOI] [PubMed] [Google Scholar]
- Swalla B. J., Upholt W. B., Solursh M. Analysis of type II collagen RNA localization in chick wing buds by in situ hybridization. Dev Biol. 1988 Jan;125(1):51–58. doi: 10.1016/0012-1606(88)90057-7. [DOI] [PubMed] [Google Scholar]
- Venkatesan M., de Pablo F., Vogeli G., Simpson R. T. Structure and developmentally regulated expression of a Strongylocentrotus purpuratus collagen gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3351–3355. doi: 10.1073/pnas.83.10.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel G. M., Marchase R. B., McClay D. R. Ontogeny of the basal lamina in the sea urchin embryo. Dev Biol. 1984 May;103(1):235–245. doi: 10.1016/0012-1606(84)90025-3. [DOI] [PubMed] [Google Scholar]
- Wessel G. M., McClay D. R. Gastrulation in the sea urchin embryo requires the deposition of crosslinked collagen within the extracellular matrix. Dev Biol. 1987 May;121(1):149–165. doi: 10.1016/0012-1606(87)90148-5. [DOI] [PubMed] [Google Scholar]