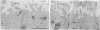Abstract
We studied the surface delivery pathways followed by newly synthesized plasma membrane proteins in intestinal cells. To this end, we developed an assay and characterized an epithelial cell line (SK-CO-15) derived from human colon adenocarcinoma. Polarized confluent monolayers (2000 omega.cm2), grown on polycarbonate filter chambers, were pulsed with radioactive methionine/cysteine and, at different times of chase, the protein fraction reaching the apical or basolateral surface was recovered by domain-selective biotinylation, immunoprecipitation, and immobilized streptavidin precipitation. Both an apical and a basolateral marker were found to be delivered vectorially to the respective surface, with a sorting efficiency of 50:1 for the basolateral marker and 14:1 for the apical marker.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartles J. R., Feracci H. M., Stieger B., Hubbard A. L. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987 Sep;105(3):1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartles J. R., Hubbard A. L. Plasma membrane protein sorting in epithelial cells: do secretory pathways hold the key? Trends Biochem Sci. 1988 May;13(5):181–184. doi: 10.1016/0968-0004(88)90147-8. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bretscher M. S., Lutter R. A new method for detecting endocytosed proteins. EMBO J. 1988 Dec 20;7(13):4087–4092. doi: 10.1002/j.1460-2075.1988.tb03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Anderson H. C., Palade G. E., Jamieson J. D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986 Aug 15;46(4):623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Compton T., Ivanov I. E., Gottlieb T., Rindler M., Adesnik M., Sabatini D. D. A sorting signal for the basolateral delivery of the vesicular stomatitis virus (VSV) G protein lies in its luminal domain: analysis of the targeting of VSV G-influenza hemagglutinin chimeras. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4112–4116. doi: 10.1073/pnas.86.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M. Biosynthesis of intestinal microvillar proteins. Evidence for an intracellular sorting taking place in, or shortly after, exit from the Golgi complex. Eur J Biochem. 1985 Oct 15;152(2):493–499. doi: 10.1111/j.1432-1033.1985.tb09223.x. [DOI] [PubMed] [Google Scholar]
- Dudouet B., Robine S., Huet C., Sahuquillo-Merino C., Blair L., Coudrier E., Louvard D. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29-18 clones. J Cell Biol. 1987 Jul;105(1):359–369. doi: 10.1083/jcb.105.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers U., Klumperman J., Hauri H. P. Nocodazole, a microtubule-active drug, interferes with apical protein delivery in cultured intestinal epithelial cells (Caco-2). J Cell Biol. 1989 Jan;108(1):13–22. doi: 10.1083/jcb.108.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feracci H., Bernadac A., Gorvel J. P., Maroux S. Localization by immunofluorescence and histochemical labeling of aminopeptidase N in relation to its biosynthesis in rabbit and pig enterocytes. Gastroenterology. 1982 Feb;82(2):317–324. [PubMed] [Google Scholar]
- Fransen J. A., Ginsel L. A., Hauri H. P., Sterchi E., Blok J. Immuno-electronmicroscopical localization of a microvillus membrane disaccharidase in the human small-intestinal epithelium with monoclonal antibodies. Eur J Cell Biol. 1985 Jul;38(1):6–15. [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Bosc-Biern I., Reggio H. Characterization of a glycoprotein expressed on the basolateral membrane of human intestinal epithelial cells and cultured colonic cell lines. Eur J Cell Biol. 1988 Apr;46(1):113–120. [PubMed] [Google Scholar]
- Le Bivic A., Hirn M., Reggio H. Apical membrane marker is expressed early in colonic epithelial cells. Biol Cell. 1987;60(3):209–216. doi: 10.1111/j.1768-322x.1987.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Hirn M., Reggio H. HT-29 cells are an in vitro model for the generation of cell polarity in epithelia during embryonic differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(1):136–140. doi: 10.1073/pnas.85.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Sargiacomo M., Graeve L., Saltiel A. R., Rodriguez-Boulan E. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9557–9561. doi: 10.1073/pnas.85.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzsonn V., Korsmo H., Olsen W. A. Localization of sucrase-isomaltase in the rat enterocyte. Gastroenterology. 1987 Jan;92(1):98–105. doi: 10.1016/0016-5085(87)90844-4. [DOI] [PubMed] [Google Scholar]
- Massey D., Feracci H., Gorvel J. P., Rigal A., Soulié J. M., Maroux S. Evidence for the transit of aminopeptidase N through the basolateral membrane before it reaches the brush border of enterocytes. J Membr Biol. 1987;96(1):19–25. doi: 10.1007/BF01869331. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J Cell Biol. 1984 Dec;99(6):2131–2139. doi: 10.1083/jcb.99.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S. The sorting of proteins to the plasma membrane in epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2565–2568. doi: 10.1083/jcb.103.6.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misek D. E., Bard E., Rodriguez-Boulan E. Biogenesis of epithelial cell polarity: intracellular sorting and vectorial exocytosis of an apical plasma membrane glycoprotein. Cell. 1984 Dec;39(3 Pt 2):537–546. doi: 10.1016/0092-8674(84)90460-4. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Deitcher D. L. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986 Aug 15;46(4):613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S., Fuller S. D., Simons K. Intracellular sorting and basolateral appearance of the G protein of vesicular stomatitis virus in Madin-Darby canine kidney cells. J Cell Biol. 1985 Aug;101(2):470–476. doi: 10.1083/jcb.101.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Kirsch K., Weiser M. M. Synthesis of membrane glycoproteins in rat small-intestinal villus cells. Redistribution of L-[1,5,6-3H]fucose-labelled membrane glycoproteins among Golgi, lateral basal and microvillus membranes in vivo. Biochem J. 1979 Jul 15;182(1):203–212. doi: 10.1042/bj1820203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggio H., Webster P., Louvard D. Use of immunocytochemical techniques in studying the biogenesis of cell surfaces in polarized epithelia. Methods Enzymol. 1983;98:379–395. doi: 10.1016/0076-6879(83)98166-1. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Ivanov I. E., Plesken H., Sabatini D. D. Polarized delivery of viral glycoproteins to the apical and basolateral plasma membranes of Madin-Darby canine kidney cells infected with temperature-sensitive viruses. J Cell Biol. 1985 Jan;100(1):136–151. doi: 10.1083/jcb.100.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E. Polarized assembly of enveloped viruses from cultured epithelial cells. Methods Enzymol. 1983;98:486–501. doi: 10.1016/0076-6879(83)98176-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Salas P. J. External and internal signals for epithelial cell surface polarization. Annu Rev Physiol. 1989;51:741–754. doi: 10.1146/annurev.ph.51.030189.003521. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M., Lisanti M., Graeve L., Le Bivic A., Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989 Mar;107(3):277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Stieger B., Matter K., Baur B., Bucher K., Höchli M., Hauri H. P. Dissection of the asynchronous transport of intestinal microvillar hydrolases to the cell surface. J Cell Biol. 1988 Jun;106(6):1853–1861. doi: 10.1083/jcb.106.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]