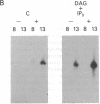
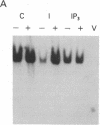
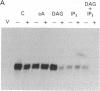
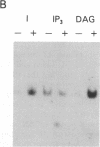
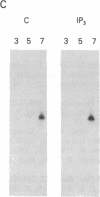
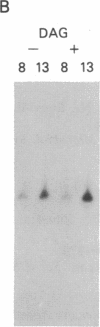
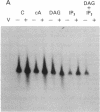
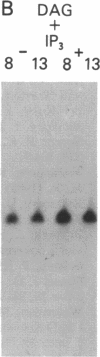
Abstract
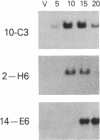
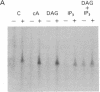
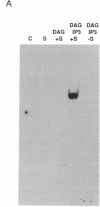
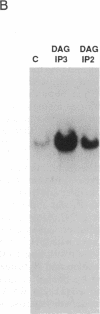
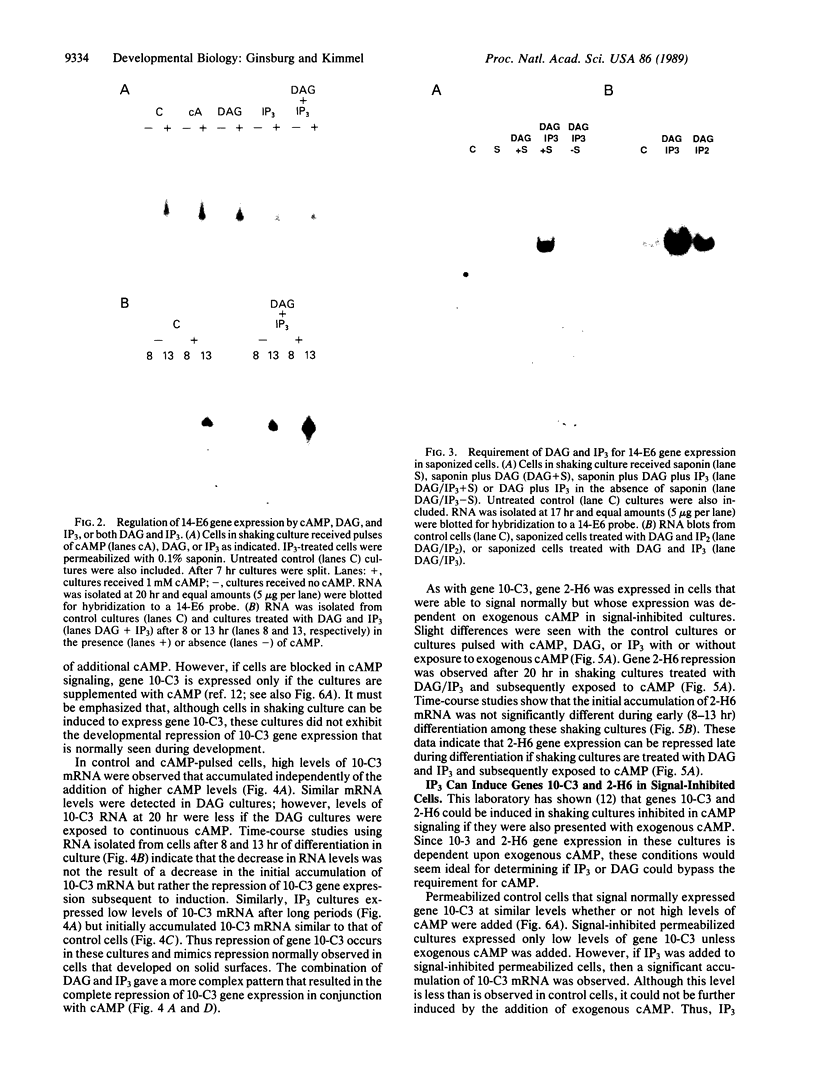
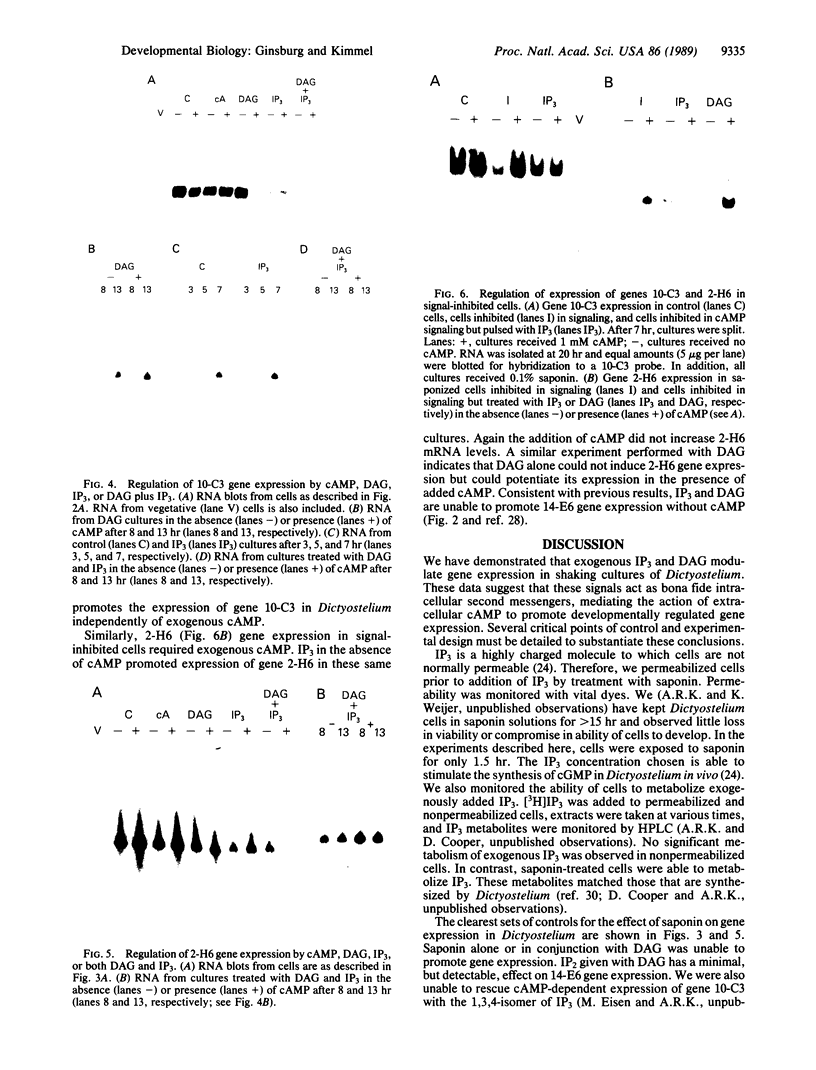
We have previously shown that several genes expressed during Dictyostelium development could be induced in shaking culture by exogenous cAMP, even though the accumulation of intracellular cAMP was inhibited. The use of selected cAMP analogs indicated that the exogenous cAMP functioned by activating the cell surface cAMP receptor and not by interacting with the regulatory subunit of the intracellular cAMP-dependent protein kinase. Although some genes in Dictyostelium appear to be regulated by intracellular cAMP, these data suggest that this is not the case for all genes regulated by cAMP. Intracellular second messengers other than cAMP may, therefore, promote the expression of these other genes. Here, we have examined inositol trisphosphate and diacylglycerol as candidates for such mediators of signal transduction. We have studied three genes that exhibit disparate modes of temporal and spatial expression during development of Dictyostelium. In shaking cultures, maximal levels of expression of each are dependent on the accumulation of or exposure to extracellular cAMP. We show that the addition of inositol trisphosphate and/or diacylglycerol to cells in shaking culture has distinct effects on the expression of each gene and, under specific conditions, can bypass the requirement for extracellular cAMP. These data suggest that extracellular cAMP interacting with its cell surface receptor may promote synthesis of inositol trisphosphate and diacylglycerol to regulate gene expression and aspects of differentiation in Dictyostelium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Yanagisawa K. A new class of rapidly developing mutants in Dictyostelium discoideum: implications for cyclic AMP metabolism and cell differentiation. Dev Biol. 1983 Jan;95(1):200–210. doi: 10.1016/0012-1606(83)90018-0. [DOI] [PubMed] [Google Scholar]
- Barklis E., Lodish H. F. Regulation of dictyostelium discoideum mRNAs specific for prespore or prestalk cells. Cell. 1983 Apr;32(4):1139–1148. doi: 10.1016/0092-8674(83)90297-0. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Nairn A. C., Kuo J. F. Protein kinases 1988: a current perspective. FASEB J. 1988 Nov;2(14):2957–2969. doi: 10.1096/fasebj.2.14.2972578. [DOI] [PubMed] [Google Scholar]
- Blumberg D. D., Comer J. F., Higinbotham K. G. A Ca2+-dependent signal transduction system participates in coupling expression of some cAMP-dependent prespore genes to the cell surface receptor. Dev Genet. 1988;9(4-5):359–369. doi: 10.1002/dvg.1020090417. [DOI] [PubMed] [Google Scholar]
- Chu D. T., Granner D. K. The effect of phorbol esters and diacylglycerol on expression of the phosphoenolpyruvate carboxykinase (GTP) gene in rat hepatoma H4IIE cells. J Biol Chem. 1986 Dec 25;261(36):16848–16853. [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Inositol 1,4,5-trisphosphate induces cyclic GMP formation in Dictyostelium discoideum. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1115–1122. doi: 10.1016/0006-291x(85)91731-0. [DOI] [PubMed] [Google Scholar]
- Gomer R. H., Armstrong D., Leichtling B. H., Firtel R. A. cAMP induction of prespore and prestalk gene expression in Dictyostelium is mediated by the cell-surface cAMP receptor. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8624–8628. doi: 10.1073/pnas.83.22.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D., Peacey M. J., von Strandmann R. P. Plasma membrane proton pump inhibition and stalk cell differentiation in Dictyostelium discoideum. Differentiation. 1988 Jul;38(2):91–98. doi: 10.1111/j.1432-0436.1988.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Haribabu B., Dottin R. P. Pharmacological characterization of cyclic AMP receptors mediating gene regulation in Dictyostelium discoideum. Mol Cell Biol. 1986 Jul;6(7):2402–2408. doi: 10.1128/mcb.6.7.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Janssens P. M., Van Haastert P. J. Molecular basis of transmembrane signal transduction in Dictyostelium discoideum. Microbiol Rev. 1987 Dec;51(4):396–418. doi: 10.1128/mr.51.4.396-418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermyn K. A., Berks M., Kay R. R., Williams J. G. Two distinct classes of prestalk-enriched mRNA sequences in Dictyostelium discoideum. Development. 1987 Aug;100(4):745–755. doi: 10.1242/dev.100.4.745. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R., Carlisle B. A gene expressed in undifferentiated vegetative Dictyostelium is repressed by developmental pulses of cAMP and reinduced during dedifferentiation. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2506–2510. doi: 10.1073/pnas.83.8.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R. Different molecular mechanisms for cAMP regulation of gene expression during Dictyostelium development. Dev Biol. 1987 Jul;122(1):163–171. doi: 10.1016/0012-1606(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R., Eisen M. Regulation of gene expression by the intracellular second messengers IP3 and diacylglycerol. Dev Genet. 1988;9(4-5):351–358. doi: 10.1002/dvg.1020090416. [DOI] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., Devreotes P. N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988 Sep 16;241(4872):1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Mehdy M. C., Ratner D., Firtel R. A. Induction and modulation of cell-type-specific gene expression in Dictyostelium. Cell. 1983 Mar;32(3):763–771. doi: 10.1016/0092-8674(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Newell P. C., Europe-Finner G. N., Small N. V., Liu G. Inositol phosphates, G-proteins and ras genes involved in chemotactic signal transduction of Dictyostelium. J Cell Sci. 1988 Feb;89(Pt 2):123–127. doi: 10.1242/jcs.89.2.123. [DOI] [PubMed] [Google Scholar]
- Oyama M., Blumberg D. D. Interaction of cAMP with the cell-surface receptor induces cell-type-specific mRNA accumulation in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4819–4823. doi: 10.1073/pnas.83.13.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic J., Haribabu B., Dottin R. P. Transmembrane signal transduction regulates gene expression in Dictyostelium discoideum. Dev Genet. 1988;9(4-5):371–382. doi: 10.1002/dvg.1020090418. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Saxe C. L., 3rd, Firtel R. A. Analysis of gene expression in rapidly developing mutants of Dictyostelium discoideum. Dev Biol. 1986 Jun;115(2):407–414. doi: 10.1016/0012-1606(86)90260-5. [DOI] [PubMed] [Google Scholar]
- Saxe C. L., 3rd, Klein P., Sun T. J., Kimmel A. R., Devreotes P. N. Structure and expression of the cAMP cell-surface receptor. Dev Genet. 1988;9(4-5):227–235. doi: 10.1002/dvg.1020090405. [DOI] [PubMed] [Google Scholar]
- Schaap P., van Driel R. V. Induction of post-aggregative differentiation in Dictyostelium discoideum by cAMP. Evidence of involvement of the cell surface cAMP receptor. Exp Cell Res. 1985 Aug;159(2):388–398. doi: 10.1016/s0014-4827(85)80012-4. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Sussman J. J., Merćep M., Saito T., Germain R. N., Bonvini E., Ashwell J. D. Dissociation of phosphoinositide hydrolysis and Ca2+ fluxes from the biological responses of a T-cell hybridoma. Nature. 1988 Aug 18;334(6183):625–628. doi: 10.1038/334625a0. [DOI] [PubMed] [Google Scholar]
- Sylvia V., Curtin G., Norman J., Stec J., Busbee D. Activation of a low specific activity form of DNA polymerase alpha by inositol-1,4-bisphosphate. Cell. 1988 Aug 26;54(5):651–658. doi: 10.1016/s0092-8674(88)80009-6. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Fink J. S., Mandel G., Goodman R. H. Identification of a region in the human vasoactive intestinal polypeptide gene responsible for regulation by cyclic AMP. J Biol Chem. 1987 Jun 25;262(18):8743–8747. [PubMed] [Google Scholar]
- Van Haastert P. J., De Vries M. J., Penning L. C., Roovers E., Van der Kaay J., Erneux C., Van Lookeren Campagne M. M. Chemoattractant and guanosine 5'-[gamma-thio]triphosphate induce the accumulation of inositol 1,4,5-trisphosphate in Dictyostelium cells that are labelled with [3H]inositol by electroporation. Biochem J. 1989 Mar 1;258(2):577–586. doi: 10.1042/bj2580577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J. Determination of inositol 1,4,5-trisphosphate levels in Dictyostelium by isotope dilution assay. Anal Biochem. 1989 Feb 15;177(1):115–119. doi: 10.1016/0003-2697(89)90024-9. [DOI] [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Wang M., Spek W., Peters D., Schaap P. Lithium respecifies cyclic AMP-induced cell-type specific gene expression in Dictyostelium. Dev Genet. 1988;9(4-5):589–596. doi: 10.1002/dvg.1020090435. [DOI] [PubMed] [Google Scholar]
- Waterman M., Murdoch G. H., Evans R. M., Rosenfeld M. G. Cyclic AMP regulation of eukaryotic gene transcription by two discrete molecular mechanisms. Science. 1985 Jul 19;229(4710):267–269. doi: 10.1126/science.2990047. [DOI] [PubMed] [Google Scholar]
- Williams G. B., Elder E. M., Sussman M. Modulation of the cAMP relay in Dictyostelium discoideum by ammonia and other metabolites: possible morphogenetic consequences. Dev Biol. 1984 Oct;105(2):377–388. doi: 10.1016/0012-1606(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Williams J. G. The role of diffusible molecules in regulating the cellular differentiation of Dictyostelium discoideum. Development. 1988 May;103(1):1–16. [PubMed] [Google Scholar]
- Williams J. G., Tsang A. S., Mahbubani H. A change in the rate of transcription of a eukaryotic gene in response to cyclic AMP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7171–7175. doi: 10.1073/pnas.77.12.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]