Abstract
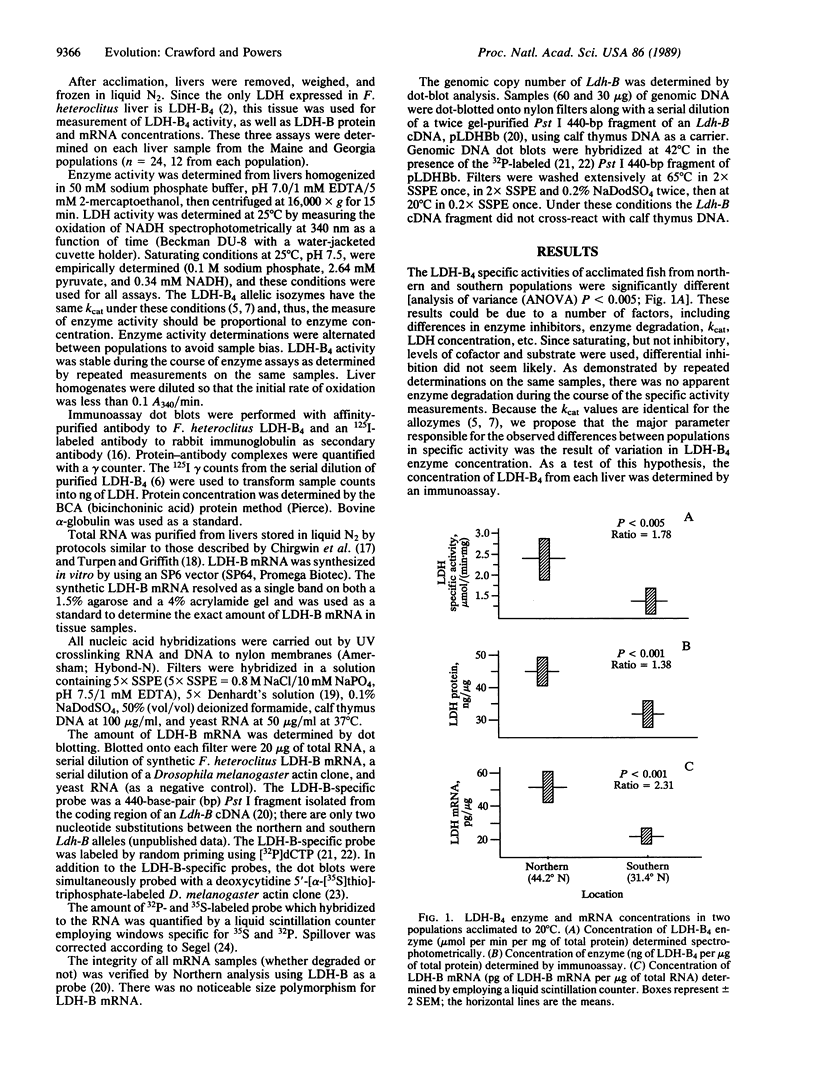
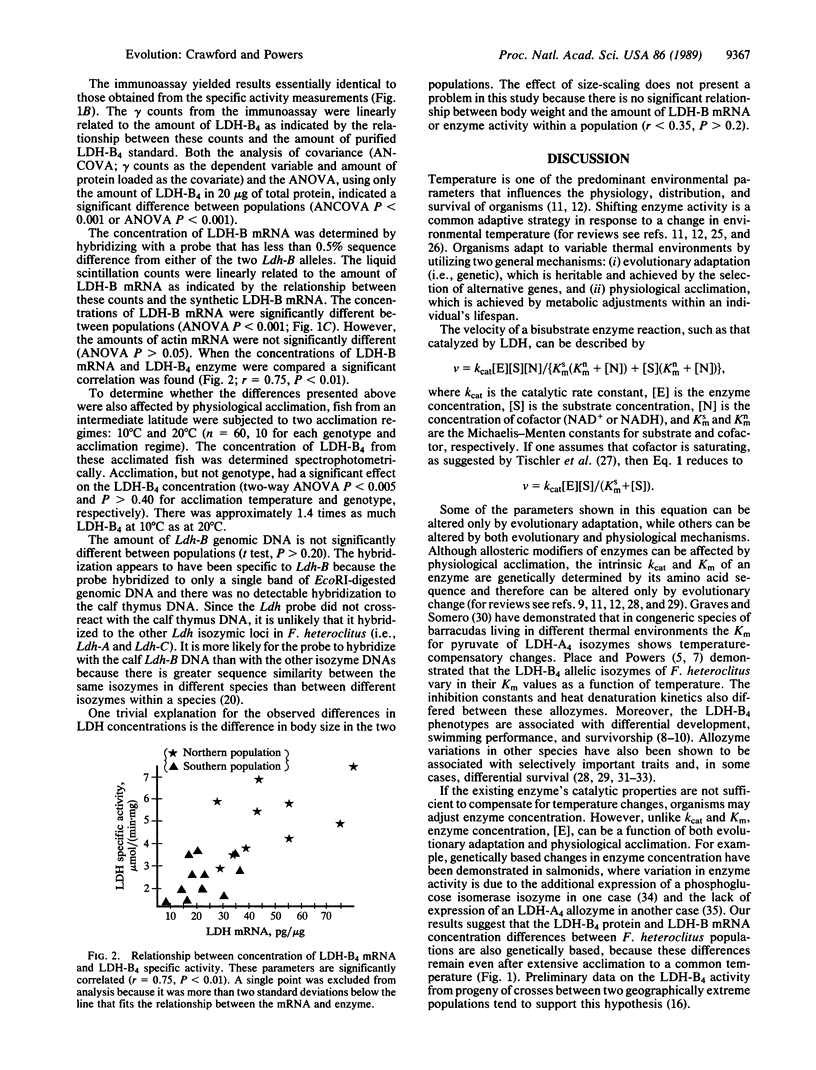
At the extremes of its natural distribution, populations of the common killifish Fundulus heteroclitus experience a difference of more than 15 degrees C in mean annual temperature. These populations are virtually fixed for two different codominant alleles at the heart-type lactate dehydrogenase locus (Ldh-B) which code for allozymes with different and adaptive kinetic responses to temperature. Two populations near the extremes of the species range (i.e., Maine and Georgia) were further studied for thermal adaptation at this locus. In the absence of any kinetic differences one would predict that to maintain a constant reaction velocity, 2 to 3 times as much enzyme would be required for each 10 degrees C decrease in environmental temperature. Consistent with this adaptive strategy and in addition to the adaptive kinetic characteristics, the LDH-B4 enzyme (EC 1.1.1.27) concentration and its mRNA concentration were approximately twice as great in the northern population as in the southern population. Acclimation experiments allow us to conclude that these differences are due to a combination of fixed genetic traits (evolutionary adaptation) and plastic responses to temperature (physiological acclimation). Furthermore, our calculations show that the LDH-B4 reaction velocities are essentially equivalent for these two populations, even though they live in significantly different thermal environments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allendorf F. W., Knudsen K. L., Leary R. F. Adaptive significance of differences in the tissue-specific expression of a phosphoglucomutase gene in rainbow trout. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1397–1400. doi: 10.1073/pnas.80.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. M., McDonald J. F. Biochemical and molecular analysis of naturally occurring Adh variants in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4798–4802. doi: 10.1073/pnas.80.15.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R. S., Feldman M. W. Physiological effects of an allozyme polymorphism: glutamate-pyruvate transaminase and response to hyperosmotic stress in the copepod Tigriopus californicus. Biochem Genet. 1983 Apr;21(3-4):239–251. doi: 10.1007/BF00499136. [DOI] [PubMed] [Google Scholar]
- Cashon R. E., Van Beneden R. J., Powers D. A. Biochemical genetics of Fundulus heteroclitus (L.). IV. Spatial variation in gene frequencies of Idh-A, Idh-B, 6-Pgdh-A, and Est-S. Biochem Genet. 1981 Aug;19(7-8):715–728. doi: 10.1007/BF00484004. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crawford D. L., Constantino H. R., Powers D. A. Lactate dehydrogenase-B cDNA from the teleost Fundulus heteroclitus: evolutionary implications. Mol Biol Evol. 1989 Jul;6(4):369–383. doi: 10.1093/oxfordjournals.molbev.a040559. [DOI] [PubMed] [Google Scholar]
- DiMichele L., Powers D. A. LDH-B genotype-specific hatching times of Fundulus heteroclitus embryos. Nature. 1982 Apr 8;296(5857):563–564. doi: 10.1038/296563a0. [DOI] [PubMed] [Google Scholar]
- DiMichele L., Powers D. A. Physiological basis for swimming endurance differences between LDH-B genotypes of Fundulus heteroclitus. Science. 1982 May 28;216(4549):1014–1016. doi: 10.1126/science.7079747. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferguson M. M., Knudsen K. L., Danzmann R. G., Allendorf F. W. Developmental rate and viability of rainbow trout with a null allele at a lactate dehydrogenase locus. Biochem Genet. 1988 Feb;26(1-2):177–189. doi: 10.1007/BF00555498. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Hilbish T. J., Koehn R. K. Dominance in physiological phenotypes and fitness at an enzyme locus. Science. 1985 Jul 5;229(4708):52–54. doi: 10.1126/science.4012310. [DOI] [PubMed] [Google Scholar]
- King J. J., McDonald J. F. Post-translational control of alcohol dehydrogenase levels in Drosophila melanogaster. Genetics. 1987 Apr;115(4):693–699. doi: 10.1093/genetics/115.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie C. C., Stam L. F. Quantitative analysis of RNA produced by slow and fast alleles of Adh in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5161–5165. doi: 10.1073/pnas.85.14.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. F., Ayala F. J. Gene regulation in adaptive evolution. Can J Genet Cytol. 1978 Jun;20(2):159–175. doi: 10.1139/g78-018. [DOI] [PubMed] [Google Scholar]
- McDonald J. F., Ayala F. J. Genetic and biochemical basis of enzyme activity variation in natural populations. I. Alcohol dehydrogenase in Drosophila melanogaster. Genetics. 1978 Jun;89(2):371–388. doi: 10.1093/genetics/89.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. F., Chambers G. K., David J., Ayala F. J. Adaptive response due to changes in gene regulation: a study with Drosophila. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4562–4566. doi: 10.1073/pnas.74.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place A. R., Powers D. A. Genetic variation and relative catalytic efficiencies: lactate dehydrogenase B allozymes of Fundulus heteroclitus. Proc Natl Acad Sci U S A. 1979 May;76(5):2354–2358. doi: 10.1073/pnas.76.5.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place A. R., Powers D. A. Kinetic characterization of the lactate dehydrogenase (LDH-B4) allozymes of Fundulus heteroclitus. J Biol Chem. 1984 Jan 25;259(2):1309–1318. [PubMed] [Google Scholar]
- Place A. R., Powers D. A. Purification and characterization of the lactate dehydrogenase (LDH-B4) allozymes of Fundulus heteroclitus. J Biol Chem. 1984 Jan 25;259(2):1299–1308. [PubMed] [Google Scholar]
- Powers D. A., Place A. R. Biochemical genetics of Fundulus heterolitus (L.). I. Temporal and spatial variation in gene frequencies of Ldh-B, Mdh-A, Gpi-B, and Pgm-A. Biochem Genet. 1978 Jun;16(5-6):593–607. doi: 10.1007/BF00484222. [DOI] [PubMed] [Google Scholar]
- Shaklee J. B., Christiansen J. A., Sidell B. D., Prosser C. L., Whitt G. S. Molecular aspects of temperature acclimation in fish: contributions of changes in enzyme activities and isozyme patterns to metabolic reorganization in the green sunfish. J Exp Zool. 1977 Jul;201(1):1–20. doi: 10.1002/jez.1402010102. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Friedrichs D., Coll K., Williamson J. R. Pyridine nucleotide distributions and enzyme mass action ratios in hepatocytes from fed and starved rats. Arch Biochem Biophys. 1977 Nov;184(1):222–236. doi: 10.1016/0003-9861(77)90346-0. [DOI] [PubMed] [Google Scholar]
- Van Beneden R. J., Cashon R. E., Powers D. A. Biochemical genetics of Fundulus heteroclitus (L.). III. Inheritance of isocitrate dehydrogenase (Idh-A and Idh-B), 6-phosphogluconate dehydrogenase (6-Pgdh-A), and serum esterase (Est-S) polymorphisms. Biochem Genet. 1981 Aug;19(7-8):701–714. doi: 10.1007/BF00484003. [DOI] [PubMed] [Google Scholar]


