Abstract
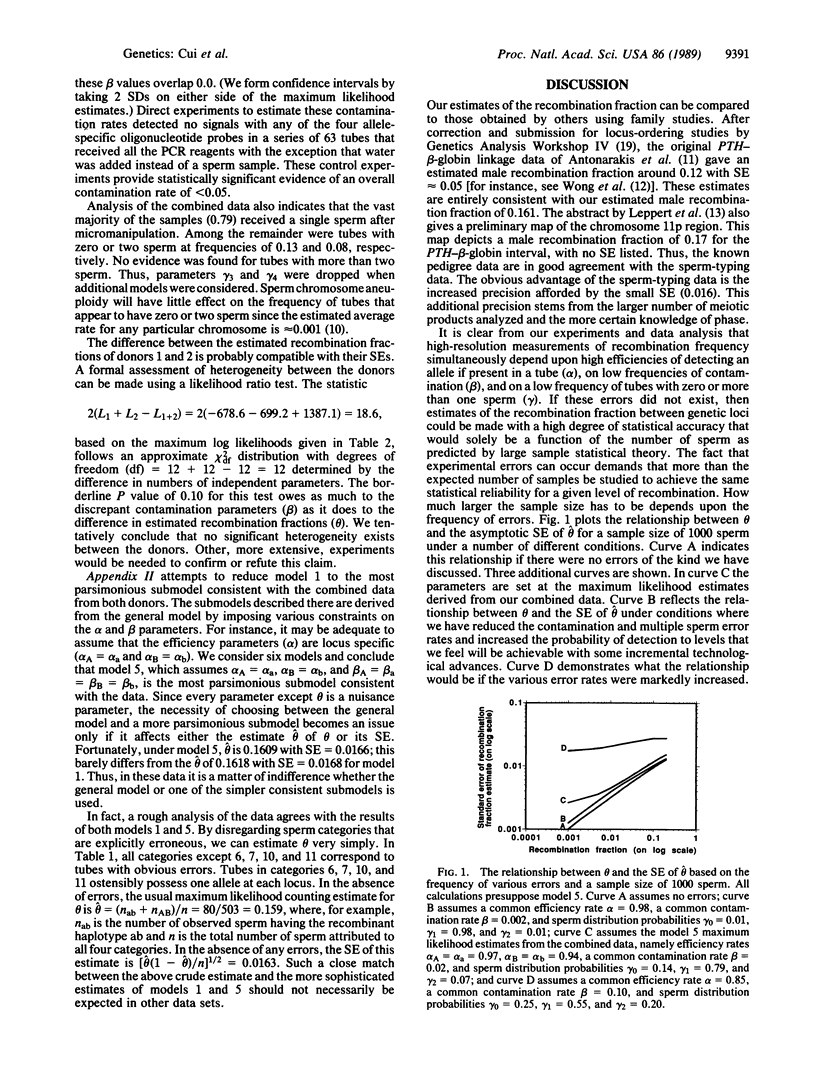
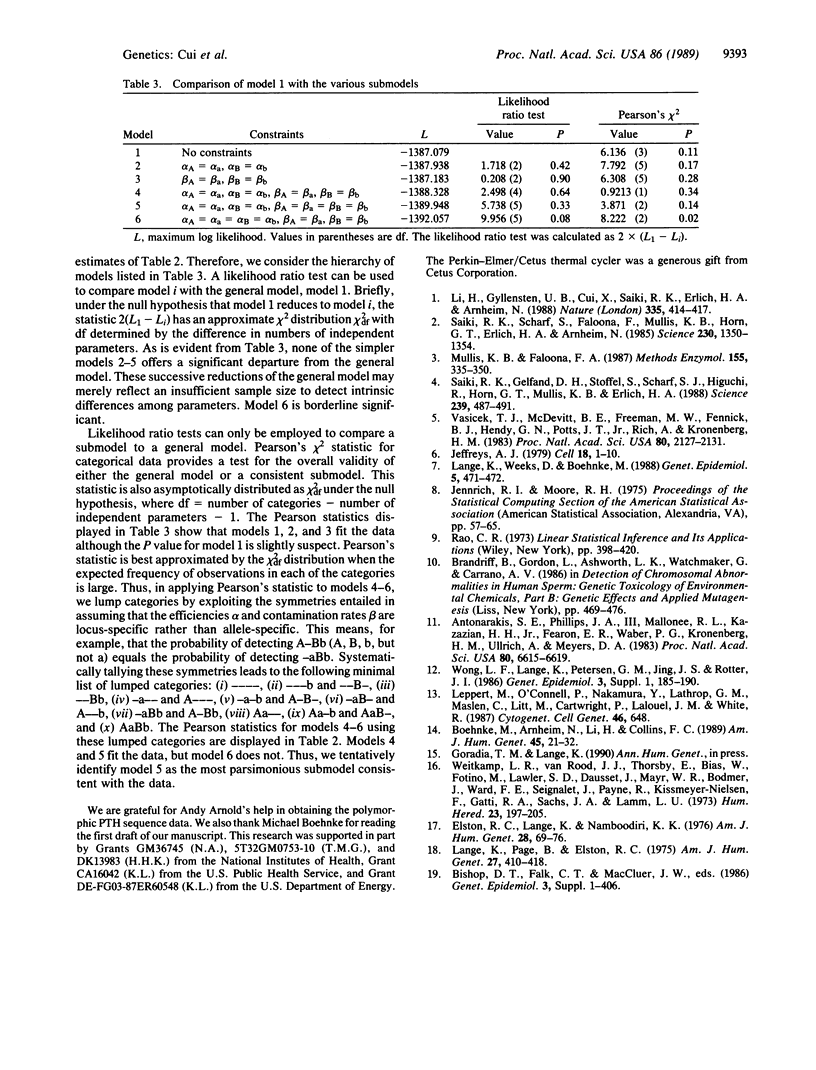
The frequency of recombination between the G gamma-globin (HBG2) and parathyroid hormone (PTH) loci on the short arm of human chromosome 11 was estimated by typing greater than 700 single-sperm samples from two males. The sperm-typing technique employed involves the polymerase chain reaction and allele-specific oligonucleotide hybridization. Our maximum likelihood recombination fraction estimate of 0.16 (95%) confidence interval, 0.13-0.19) falls well within previous estimates based on family studies. With current technology and a sample size of 1000 sperm, recombination fractions down to approximately 0.009 can be estimated with statistical reliability; with a sample size of 5000 sperm, this value drops to about 0.004. Reasonable technological improvements could result in the detection of recombination frequencies less than 0.001.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Phillips J. A., 3rd, Mallonee R. L., Kazazian H. H., Jr, Fearon E. R., Waber P. G., Kronenberg H. M., Ullrich A., Meyers D. A. Beta-globin locus is linked to the parathyroid hormone (PTH) locus and lies between the insulin and PTH loci in man. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6615–6619. doi: 10.1073/pnas.80.21.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehnke M., Arnheim N., Li H., Collins F. S. Fine-structure genetic mapping of human chromosomes using the polymerase chain reaction on single sperm: experimental design considerations. Am J Hum Genet. 1989 Jul;45(1):21–32. [PMC free article] [PubMed] [Google Scholar]
- Brandriff B., Gordon L., Ashworth L. K., Watchmaker G., Carrano A. V. Detection of chromosomal abnormalities in human sperm. Prog Clin Biol Res. 1986;209B:469–476. [PubMed] [Google Scholar]
- Elston R. C., Lange E., Namboodiri K. K. Age trends in human chiasma frequencies and recombination fractions. II. Method for analyzing recombination fractions and applications to the ABO:nail-patella linkage. Am J Hum Genet. 1976 Jan;28(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J. DNA sequence variants in the G gamma-, A gamma-, delta- and beta-globin genes of man. Cell. 1979 Sep;18(1):1–10. doi: 10.1016/0092-8674(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Lange K., Page B. M., Elston R. C. Age trends in human chiasma frequencies and recombination fractions. I. Chiasma frequencies. Am J Hum Genet. 1975 May;27(3):410–418. [PMC free article] [PubMed] [Google Scholar]
- Lange K., Weeks D., Boehnke M. Programs for Pedigree Analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol. 1988;5(6):471–472. doi: 10.1002/gepi.1370050611. [DOI] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Vasicek T. J., McDevitt B. E., Freeman M. W., Fennick B. J., Hendy G. N., Potts J. T., Jr, Rich A., Kronenberg H. M. Nucleotide sequence of the human parathyroid hormone gene. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2127–2131. doi: 10.1073/pnas.80.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitkamp L. R., Van Rood J. J., Thorsby E., Bias W., Fotino M., Lawler S. D., Dausset J., Mayr W. R., Bodmer J., Ward F. E. The relation of parental sex and age to recombination in the HL-A system. Hum Hered. 1973;23(3):197–205. doi: 10.1159/000152574. [DOI] [PubMed] [Google Scholar]
- Wong F. L., Lange K., Petersen G. M., Jing J. S., Rotter J. I. Sex differences in recombination fraction estimates and their effect on ordering of chromosome 11 markers. Genet Epidemiol Suppl. 1986;1:185–190. doi: 10.1002/gepi.1370030729. [DOI] [PubMed] [Google Scholar]



