Abstract
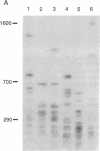
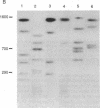
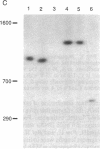
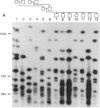
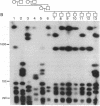
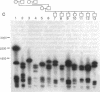
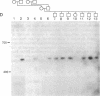
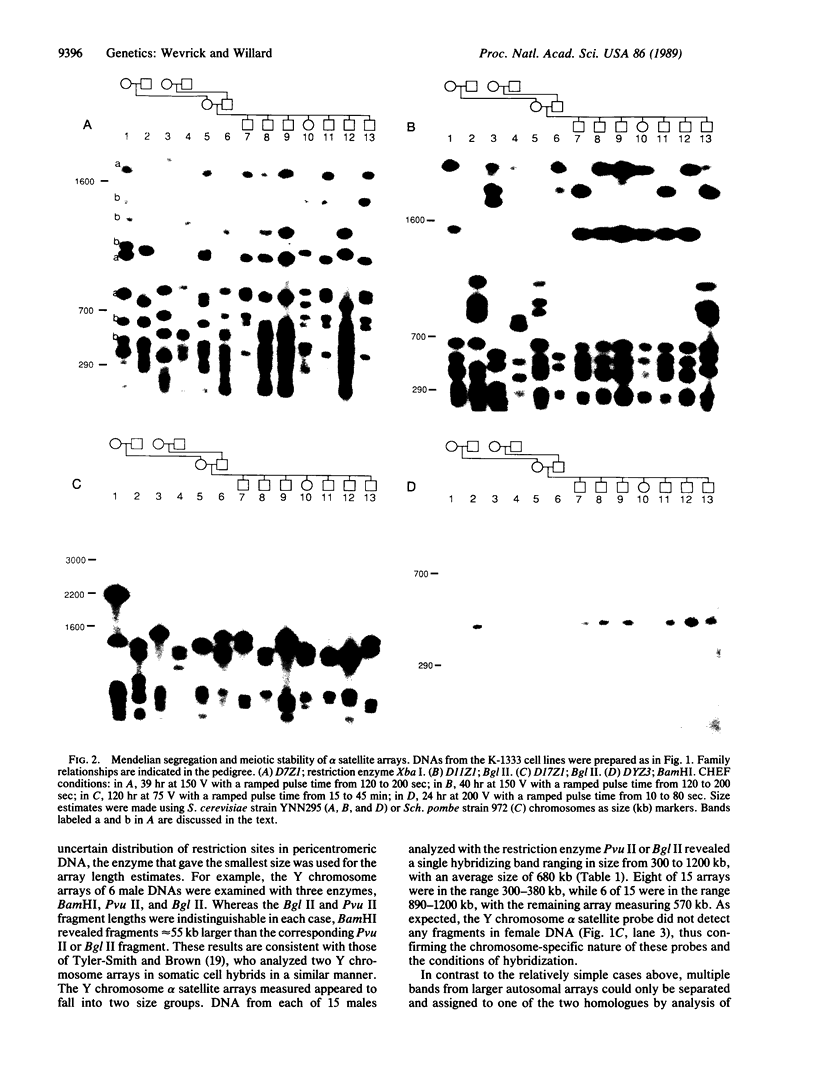
The long-range organization of arrays of alpha satellite DNA at the centromeres of human chromosomes was investigated by pulsed-field gel electrophoresis techniques. Both restriction-site and array-length polymorphisms were detected in multiple individuals and their meiotic segregation was observed in three-generation families. Such variation was detected in all of the alpha satellite arrays examined (chromosomes 1, 3, 7, 10, 11, 16, 17, X, and Y) and thus appears to be a general feature of human centromeric DNA. The length of individual centromeric arrays was found to range from an average of approximately 680 kilobases (kb) for the Y chromosome to approximately 3000 kb for chromosome 11. Furthermore, individual arrays appear to be meiotically stable, since no changes in fragment lengths were observed. In total, we analyzed 84 meiotic events involving approximately 191,000 kb of alpha satellite DNA from six autosomal centromeres without any evidence for recombination within an array. High-frequency array length variation and the potential to detect meiotic recombination within them allow direct comparisons of genetic and physical distances in the region of the centromeres of human chromosomes. The generation of primary consensus physical maps of alpha satellite arrays is a first step in the characterization of the centromeric DNA of human chromosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armour J. A., Patel I., Thein S. L., Fey M. F., Jeffreys A. J. Analysis of somatic mutations at human minisatellite loci in tumors and cell lines. Genomics. 1989 Apr;4(3):328–334. doi: 10.1016/0888-7543(89)90338-8. [DOI] [PubMed] [Google Scholar]
- Barker D., Wright E., Nguyen K., Cannon L., Fain P., Goldgar D., Bishop D. T., Carey J., Baty B., Kivlin J. Gene for von Recklinghausen neurofibromatosis is in the pericentromeric region of chromosome 17. Science. 1987 May 29;236(4805):1100–1102. doi: 10.1126/science.3107130. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. The structure and function of yeast centromeres. Annu Rev Genet. 1985;19:29–55. doi: 10.1146/annurev.ge.19.120185.000333. [DOI] [PubMed] [Google Scholar]
- Devilee P., Kievits T., Waye J. S., Pearson P. L., Willard H. F. Chromosome-specific alpha satellite DNA: isolation and mapping of a polymorphic alphoid repeat from human chromosome 10. Genomics. 1988 Jul;3(1):1–7. doi: 10.1016/0888-7543(88)90151-6. [DOI] [PubMed] [Google Scholar]
- Durfy S. J., Willard H. F. Molecular analysis of a polymorphic domain of alpha satellite from the human X chromosome. Am J Hum Genet. 1987 Sep;41(3):391–401. [PMC free article] [PubMed] [Google Scholar]
- Fishel B., Amstutz H., Baum M., Carbon J., Clarke L. Structural organization and functional analysis of centromeric DNA in the fission yeast Schizosaccharomyces pombe. Mol Cell Biol. 1988 Feb;8(2):754–763. doi: 10.1128/mcb.8.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnenberger K. M., Baum M. P., Polizzi C. M., Carbon J., Clarke L. Construction of functional artificial minichromosomes in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1989 Jan;86(2):577–581. doi: 10.1073/pnas.86.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultén M. A., Palmer R. W., Laurie D. A. Chiasma derived genetic maps and recombination fractions: chromosome 1. Ann Hum Genet. 1982 May;46(Pt 2):167–175. doi: 10.1111/j.1469-1809.1982.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Jabs E. W., Goble C. A., Cutting G. R. Macromolecular organization of human centromeric regions reveals high-frequency, polymorphic macro DNA repeats. Proc Natl Acad Sci U S A. 1989 Jan;86(1):202–206. doi: 10.1073/pnas.86.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs E. W., Meyers D. A., Bias W. B. Linkage studies of polymorphic, repeated DNA sequences in centromeric regions of human chromosomes. Am J Hum Genet. 1986 Mar;38(3):297–308. [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Kaback D. B., Steensma H. Y., de Jonge P. Enhanced meiotic recombination on the smallest chromosome of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 May;86(10):3694–3698. doi: 10.1073/pnas.86.10.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambie E. J., Roeder G. S. Repression of meiotic crossing over by a centromere (CEN3) in Saccharomyces cerevisiae. Genetics. 1986 Nov;114(3):769–789. doi: 10.1093/genetics/114.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtani M. M., Willard H. F. A primary genetic map of the pericentromeric region of the human X chromosome. Genomics. 1988 May;2(4):294–301. doi: 10.1016/0888-7543(88)90017-1. [DOI] [PubMed] [Google Scholar]
- Mather K. Crossing over and Heterochromatin in the X Chromosome of Drosophila Melanogaster. Genetics. 1939 Apr;24(3):413–435. doi: 10.1093/genetics/24.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyzis R. K., Albright K. L., Bartholdi M. F., Cram L. S., Deaven L. L., Hildebrand C. E., Joste N. E., Longmire J. L., Meyne J., Schwarzacher-Robinson T. Human chromosome-specific repetitive DNA sequences: novel markers for genetic analysis. Chromosoma. 1987;95(6):375–386. doi: 10.1007/BF00333988. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Julier C., Wolff R., Holm T., O'Connell P., Leppert M., White R. Characterization of a human 'midisatellite' sequence. Nucleic Acids Res. 1987 Mar 25;15(6):2537–2547. doi: 10.1093/nar/15.6.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaseko Y., Adachi Y., Funahashi S., Niwa O., Yanagida M. Chromosome walking shows a highly homologous repetitive sequence present in all the centromere regions of fission yeast. EMBO J. 1986 May;5(5):1011–1021. doi: 10.1002/j.1460-2075.1986.tb04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R. B. Mitosis. Adv Cell Biol. 1971;2:225–297. doi: 10.1007/978-1-4615-9588-5_5. [DOI] [PubMed] [Google Scholar]
- Renwick J. H. Progress in mapping human autosomes. Br Med Bull. 1969 Jan;25(1):65–73. doi: 10.1093/oxfordjournals.bmb.a070673. [DOI] [PubMed] [Google Scholar]
- Rieder C. L. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Spence J. E., Perciaccante R. G., Greig G. M., Willard H. F., Ledbetter D. H., Hejtmancik J. F., Pollack M. S., O'Brien W. E., Beaudet A. L. Uniparental disomy as a mechanism for human genetic disease. Am J Hum Genet. 1988 Feb;42(2):217–226. [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980 Apr 3;284(5755):426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]
- Tartof K. D. Unequal crossing over then and now. Genetics. 1988 Sep;120(1):1–6. doi: 10.1093/genetics/120.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler-Smith C., Brown W. R. Structure of the major block of alphoid satellite DNA on the human Y chromosome. J Mol Biol. 1987 Jun 5;195(3):457–470. doi: 10.1016/0022-2836(87)90175-6. [DOI] [PubMed] [Google Scholar]
- Tyler-Smith C. Structure of repeated sequences in the centromeric region of the human Y chromosome. Development. 1987;101 (Suppl):93–100. [PubMed] [Google Scholar]
- Tyler-Smith C., Taylor L., Müller U. Structure of a hypervariable tandemly repeated DNA sequence on the short arm of the human Y chromosome. J Mol Biol. 1988 Oct 20;203(4):837–848. doi: 10.1016/0022-2836(88)90110-6. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W. Resolution of DNA molecules greater than 5 megabases by contour-clamped homogeneous electric fields. Nucleic Acids Res. 1987 Oct 12;15(19):7865–7876. doi: 10.1093/nar/15.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Creeper L. A., Willard H. F. Organization and evolution of alpha satellite DNA from human chromosome 11. Chromosoma. 1987;95(3):182–188. doi: 10.1007/BF00330349. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Durfy S. J., Pinkel D., Kenwrick S., Patterson M., Davies K. E., Willard H. F. Chromosome-specific alpha satellite DNA from human chromosome 1: hierarchical structure and genomic organization of a polymorphic domain spanning several hundred kilobase pairs of centromeric DNA. Genomics. 1987 Sep;1(1):43–51. doi: 10.1016/0888-7543(87)90103-0. [DOI] [PubMed] [Google Scholar]
- Waye J. S., England S. B., Willard H. F. Genomic organization of alpha satellite DNA on human chromosome 7: evidence for two distinct alphoid domains on a single chromosome. Mol Cell Biol. 1987 Jan;7(1):349–356. doi: 10.1128/mcb.7.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Chromosome specificity of satellite DNAs: short- and long-range organization of a diverged dimeric subset of human alpha satellite from chromosome 3. Chromosoma. 1989 May;97(6):475–480. doi: 10.1007/BF00295032. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Chromosome-specific alpha satellite DNA: nucleotide sequence analysis of the 2.0 kilobasepair repeat from the human X chromosome. Nucleic Acids Res. 1985 Apr 25;13(8):2731–2743. doi: 10.1093/nar/13.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Human beta satellite DNA: genomic organization and sequence definition of a class of highly repetitive tandem DNA. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6250–6254. doi: 10.1073/pnas.86.16.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Molecular analysis of a deletion polymorphism in alpha satellite of human chromosome 17: evidence for homologous unequal crossing-over and subsequent fixation. Nucleic Acids Res. 1986 Sep 11;14(17):6915–6927. doi: 10.1093/nar/14.17.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Structure, organization, and sequence of alpha satellite DNA from human chromosome 17: evidence for evolution by unequal crossing-over and an ancestral pentamer repeat shared with the human X chromosome. Mol Cell Biol. 1986 Sep;6(9):3156–3165. doi: 10.1128/mcb.6.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Waye J. S., Skolnick M. H., Schwartz C. E., Powers V. E., England S. B. Detection of restriction fragment length polymorphisms at the centromeres of human chromosomes by using chromosome-specific alpha satellite DNA probes: implications for development of centromere-based genetic linkage maps. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5611–5615. doi: 10.1073/pnas.83.15.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J., Darling S. M., Erickson R. P., Craig I. W., Buckle V. J., Rigby P. W., Willard H. F., Goodfellow P. N. Isolation and characterization of an alphoid centromeric repeat family from the human Y chromosome. J Mol Biol. 1985 Apr 20;182(4):477–485. doi: 10.1016/0022-2836(85)90234-7. [DOI] [PubMed] [Google Scholar]