Abstract
Urate oxidase, or uricase (EC 1.7.3.3), is a peroxisomal enzyme that catalyzes the oxidation of uric acid to allantoin in most mammals. In humans and certain other primates, however, the enzyme has been lost by some unknown mechanism. To identify the molecular basis for this loss, urate oxidase cDNA clones were isolated from pig, mouse, and baboon, and their DNA sequences were determined. The mouse urate oxidase open reading frame encodes a 303-amino acid polypeptide, while the pig and baboon urate oxidase cDNAs encode a 304-amino acid polypeptide due to a single codon deletion/insertion event. The authenticity of this single additional codon was confirmed by sequencing the mouse and pig genomic copies of the gene. The urate oxidase sequence contains a domain similar to the type 2 copper binding motif found in other copper binding proteins, suggesting that the copper ion in urate oxidase is coordinated as a type 2 structure. Based upon a comparison of the NH2-terminal peptide and deduced sequences, we propose that the maturation of pig urate oxidase involves the posttranslational cleavage of a six-amino acid peptide. Two nonsense mutations were found in the human urate oxidase gene, which confirms, at the molecular level, that the urate oxidase gene in humans is nonfunctional. The sequence comparisons favor the hypothesis that the loss of urate oxidase in humans is due to a sudden mutational event rather than a progressive mutational process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Christen P., Peacock W. C., Christen A. E., Wacker W. E. Urate oxidase in primate phylogenesis. Eur J Biochem. 1970 Jan;12(1):3–5. doi: 10.1111/j.1432-1033.1970.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Conley T. G., Priest D. G. Purification of uricase from mammalian tissue. Prep Biochem. 1979;9(2):197–203. doi: 10.1080/00327487908061683. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Putnam F. W. Internal duplication and evolution of human ceruloplasmin. Proc Natl Acad Sci U S A. 1981 May;78(5):2805–2809. doi: 10.1073/pnas.78.5.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman T. B., Polanco G. E., Appold J. C., Mayle J. E. On the loss of uricolytic activity during primate evolution--I. Silencing of urate oxidase in a hominoid ancestor. Comp Biochem Physiol B. 1985;81(3):653–659. doi: 10.1016/0305-0491(85)90381-5. [DOI] [PubMed] [Google Scholar]
- Ghosh M. K., Hajra A. K. A rapid method for the isolation of peroxisomes from rat liver. Anal Biochem. 1986 Nov 15;159(1):169–174. doi: 10.1016/0003-2697(86)90323-4. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Ito M., Suzuki M., Takagi Y. Nucleotide sequence of cDNA and predicted amino acid sequence of rat liver uricase. Eur J Biochem. 1988 Apr 15;173(2):459–463. doi: 10.1111/j.1432-1033.1988.tb14021.x. [DOI] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., HUBSCHER G., BAUM R. Studies on uricase. I. Preparation, purification, and properties of a cuproprotein. J Biol Chem. 1955 Oct;216(2):625–641. [PubMed] [Google Scholar]
- Motojima K., Kanaya S., Goto S. Cloning and sequence analysis of cDNA for rat liver uricase. J Biol Chem. 1988 Nov 15;263(32):16677–16681. [PubMed] [Google Scholar]
- Nalbantoglu J., Phear G., Meuth M. DNA sequence analysis of spontaneous mutations at the aprt locus of hamster cells. Mol Cell Biol. 1987 Apr;7(4):1445–1449. doi: 10.1128/mcb.7.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts O. M., Priest D. G., Fish W. W. Uricase. Subunit composition and resistance to denaturants. Biochemistry. 1974 Feb 26;13(5):888–892. doi: 10.1021/bi00702a009. [DOI] [PubMed] [Google Scholar]
- Reddy P. G., Nemali M. R., Reddy M. K., Reddy M. N., Yuan P. M., Yuen S., Laffler T. G., Shiroza T., Kuramitsu H. K., Usuda N. Isolation and sequence determination of a cDNA clone for rat peroxisomal urate oxidase: liver-specific expression in the rat. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9081–9085. doi: 10.1073/pnas.85.23.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. S., Thomas K. A., Richardson D. C. Alpha-carbon coordinates for bovine Cu,Zn superoxide dismutase. Biochem Biophys Res Commun. 1975 Apr 21;63(4):986–992. doi: 10.1016/0006-291x(75)90666-x. [DOI] [PubMed] [Google Scholar]
- Richardson J., Thomas K. A., Rubin B. H., Richardson D. C. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349–1353. doi: 10.1073/pnas.72.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H., Joysey K. A., Friday A. E. Molecular evolution of myoglobin and the fossil record: a phylogenetic synthesis. Nature. 1973 Dec 14;246(5433):389–395. doi: 10.1038/246389a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout J. T., Caskey C. T. The Lesch-Nyhan syndrome: clinical, molecular and genetic aspects. Trends Genet. 1988 Jun;4(6):175–178. doi: 10.1016/0168-9525(88)90024-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
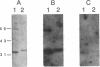
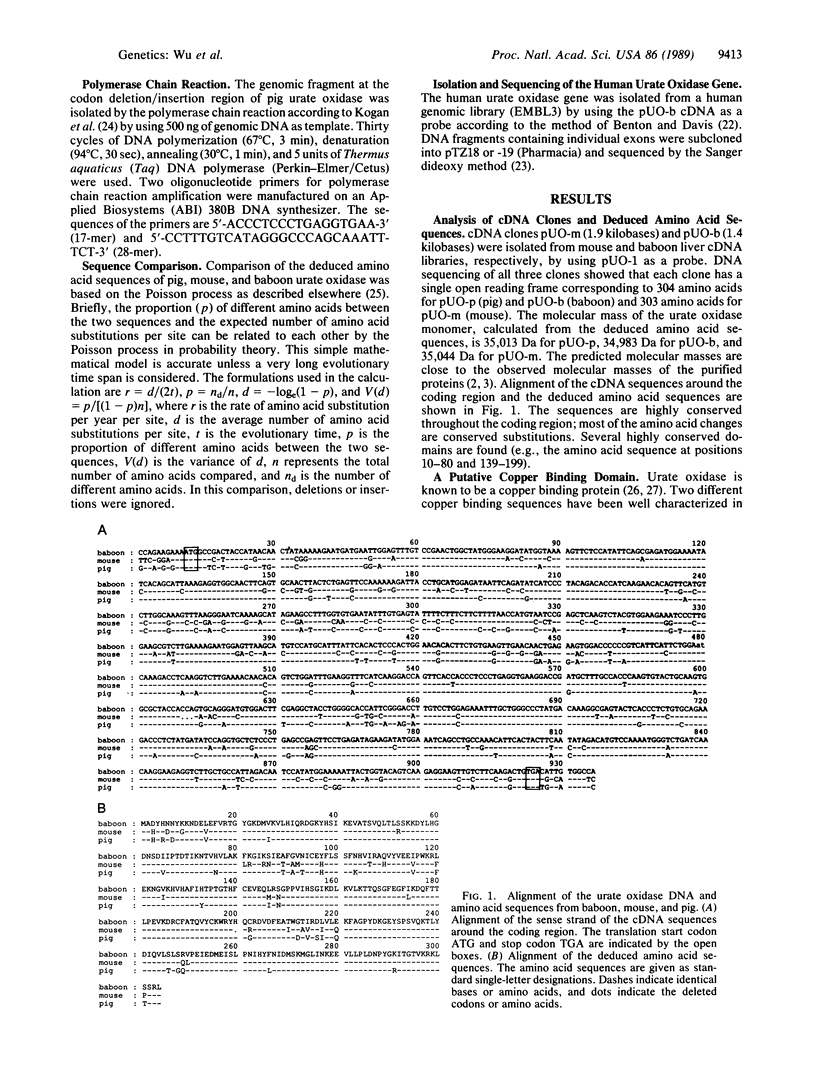
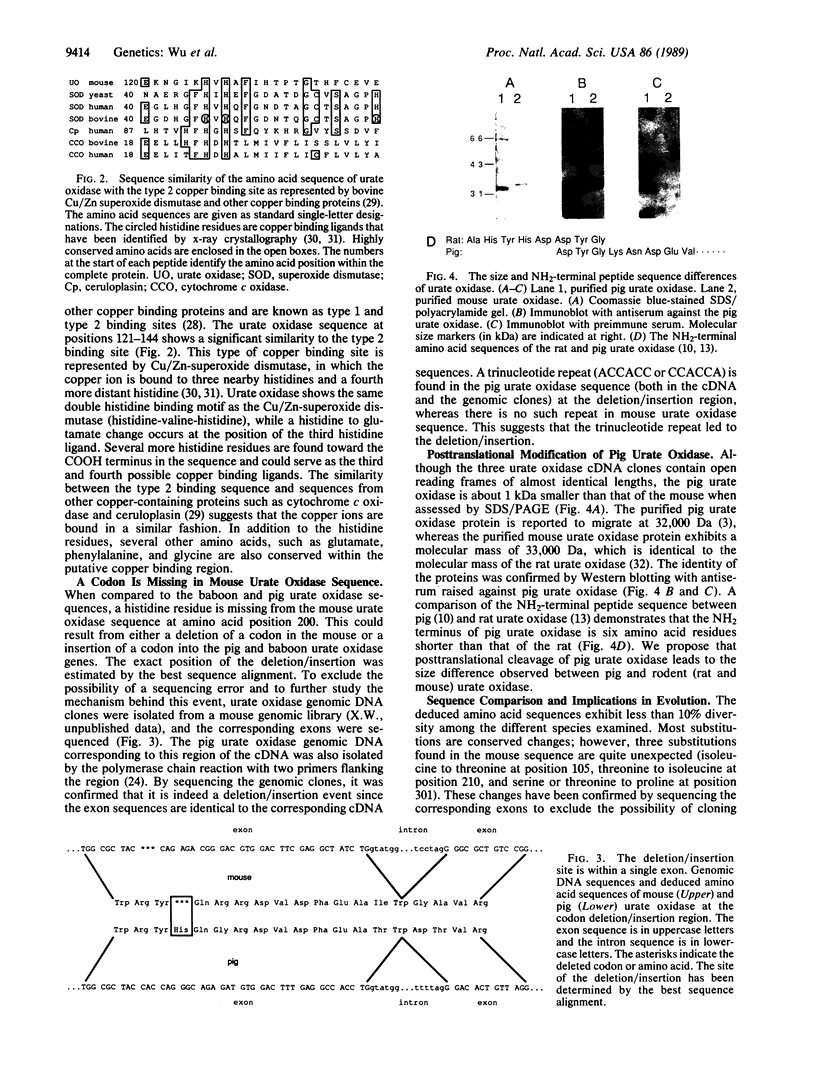
- Usuda N., Reddy M. K., Hashimoto T., Rao M. S., Reddy J. K. Tissue specificity and species differences in the distribution of urate oxidase in peroxisomes. Lab Invest. 1988 Jan;58(1):100–111. [PubMed] [Google Scholar]